Abstract
Objective:
To identify developmental trajectories of adiposity from birth until early adulthood, and to investigate how they relate with cardiometabolic risk factors at 21 years of age.
Methods:
Participants’ weight and height measurements were obtained using the EPITeen cohort protocol at 13, 17 and 21 years of age, and extracted from child health books as recorded during health routine evaluations since birth. Blood pressure, triglycerides, cholesterol and insulin resistance (HOMA-IR) were assessed at 21 years. Trajectories were defined using 719 participants contributing 11 459 measurements. The individual growth curves were modelled using mixed-effects fractional polynomial, and the trajectories were estimated using normal mixture modelling for model-based clustering. Differences in cardiometabolic risk factors at 21 years according to adiposity trajectories were estimated through analysis of covariance (ANCOVA), and adjusted means are presented.
Results:
Two trajectories—‘Average body mass index (BMI) growth’ (80.7%) and ‘Higher BMI growth’ (19.3%)—were identified. Compared with those in ‘Average BMI growth’, ‘Higher BMI growth’ participants were more frequently delivered by caesarean section, mothers were younger and had higher BMI, and parental education was lower; and at 21 years showed higher adjusted mean systolic (111.6 vs 108.3 mm Hg, P<0.001) and diastolic blood pressure (71.9 vs 68.4 mm Hg, P<0.001), and lower high-density lipoprotein cholesterol (53.3 vs 57.0 mg dl−1, P=0.001). As there was a significant interaction between trajectories and sex, triglycerides and HOMA-IR were stratified by sex and we found significantly higher triglycerides, in males, and higher HOMA-IR in both sexes in ‘Higher BMI growth’ trajectory. All the differences were attenuated after adjustment for BMI at 21 years.
Conclusions:
In this long-term follow-up, we were able to identify two adiposity trajectories, statistically related to the BMI and cardiometabolic profile in adulthood. Our results also suggest that the impact of the adiposity trajectory on cardiometabolic profile is mediated by current BMI.
Similar content being viewed by others
Introduction
During the last decades, the prevalence of obesity reached an epidemic dimension worldwide. Although a levelling-off of obesity trends has been reported in some countries, such as in the US and in the UK adult population,1 and in Sweden for pediatric ages,2 the prevalence of obesity is still a major public health concern. According to a systematic analysis for the Global Burden of Disease Study 2013, the age-standardized regional prevalence estimates of overweight and obesity combined for Western Europe were 61.3% (60.5–62.2) in men and 47.6% (46.8–48.4) in women in 2013.3 For children and adolescents (<20 years), the western region presents the highest prevalence of combined overweight and obesity in Europe, being the estimates 24.2% (23.1–25.2) for boys and 22.0% (21.0–23.0) for girls.3
It is well established that childhood obesity tends to track into adulthood: the risk of overweight children to become overweight adults is at least twice as high compared with normal-weight children.4 The prenatal period, the adiposity rebound in childhood and puberty are critical for the development of obesity,5 but less is known about their effects on obesity tracking. Most studies on tracking of obesity were based on few points in time (some studies using only two) and ignored the dynamics of body mass index (BMI) across the pediatric age. The same occurred when addressing the association between childhood or adolescent obesity and future disease in adulthood.
Childhood obesity has been associated with increased risk of disease later in life, namely cardiometabolic morbidity, and increased overall mortality.6 However, trajectories of obesity across pediatric age and the independent effect of adult adiposity were not usually considered.7 As the same BMI at a given moment in two individuals might result from different accrual paces, different health outcomes can possibly result from apparently similar exposures at a certain moment. Therefore, approaches that take cumulative exposure to adiposity into account, beyond the information on the amount of adiposity at a specific moment, may overcome the limitations of using static analyses of adiposity/BMI. The identification of developmental trajectories of adiposity could add relevant information to describe how obesity evolves over time and to identify groups following similar developmental patterns across age.8
We aimed to identify trajectories of adiposity, from birth until early adulthood (21 years) in a Portuguese general population sample, and to investigate its association with cardiometabolic risk factors at 21 years of age.
Subjects and methods
Participants
Data were collected as part of the EPITeen study—Epidemiological Health Investigation of Teenagers in Porto, a population-based cohort that recruited 13-year-old adolescents born in 1990 and enrolled at public and private schools of Porto, Portugal, during 2003–2004, as previously described.9 A second evaluation took place in 2007–2008 and a third evaluation in 2011–2013.
The Ethic Committee of Hospital S. João approved this project and appropriate standard procedures were developed to guarantee data confidentiality and protection. Parents and adolescents received written and oral information explaining the purpose and design of the study and written informed consent was obtained from both.
In the first and second study waves, participants were evaluated at schools and information was collected through self-administered questionnaires. In the third study wave, participants were invited to complete the evaluation at our University department where face-to-face interviews and biological measurements took place, and self-administered questionnaires were completed. All procedures were standardized and performed by a team of trained health professionals. At the third study wave, participants were also asked to provide their child health book and data regarding all measurements of weight and length/height were extracted.
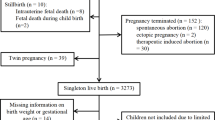
At the recruitment, 2786 eligible participants were identified and 2159 (77.5%) agreed to participate. In the second wave, we were able to revaluate 1716 participants (79.5%) and a new group of 783 adolescents were integrated in the cohort as they moved to the area schools, and were evaluated for the first time. In the third study wave, 1764 participants were revaluated and among these 764 have provided their child health books and were thus eligible for the analyses. We compared the characteristics of the participants included and those excluded from this study, and both groups were similar with regard to parental education, sex, practice of sports and BMI categories at 21 years of age. Regarding participants’ education, a higher proportion of those included in the study presented higher education.
Measures
Anthropometrics
At the study site weight and height were obtained with the subject in light indoor clothes and no shoes. Weight was measured in kilograms, to the nearest tenth, using a digital scale and height was measured in centimetres, to the nearest tenth, using a stadiometer, according to standardized procedures.10 The weight and length/height extracted from the child health book records were performed and recorded by a pediatrician or a nurse as part of the medical routine following similar procedures.
Self-reported weight and height of the parents were collected in the first and second study waves and their BMI was categorized according to WHO (World Health Organization).11 As some participants entered the study only in the second study wave, we created a single variable of mother’s BMI, combining the data collected at both moments, to minimize missing data. For those with data at both study waves, we considered the highest category; when data was available only for one moment, we considered that information. For mothers with available data for both waves, we found a high correlation between the two evaluations (r=0.842) and when using the variable in categories (normal BMI or underweight; overweight; obese) the agreement was moderate (Kappa=0.637).
Cardiometabolic risk factors
Cardiometabolic risk factors included in this analysis were assessed at 21 years of age. An overnight fast intravenous blood sample was taken from an antecubital vein. Routine chemistries, including glucose, triglycerides, total cholesterol and high-density lipoprotein cholesterol (HDL-C), were measured using conventional methods with an Olympus AU5400 automated clinical chemistry analyser (Beckman-Coulter, Brea, CA, USA). Insulin was measured by electrochemiluminescent immunoassay using a Cobas e411 automated analyser (Roche, Roche Diagnostics GmbH, Mannheim, Germany). All determinations took place in the Clinical Pathology Department of the São João Hospital Center, Porto.
Low-density lipoprotein cholesterol was calculated according to the Friedewald equation.12 Insulin resistance was assessed by the homeostasis model method (HOMA-IR), based on fasting glucose and insulin concentrations: HOMA-IR=insulin (μU ml−1) × glucose (mg dl−1)/405.13
Blood pressure was measured on a single occasion, according to the guidelines from the American and International Societies of Hypertension,14 using an oscillometric method (OMRON Blood Pressure Monitor). After 10 min of rest, two blood pressure measurements were taken, separately by at least 5 min, being a third measure taken when the difference between the first two was higher than 5 mm Hg. For analysis, the average of the two closest measurements was used.
Covariates
Perinatal information, such as weight and length at birth, gestational age and type of delivery, was extracted from child health book records. Mother’s smoking and smoking during pregnancy, breastfeeding and complementary feeding were assessed at the baseline evaluation of the cohort using self-reported questionnaires administered to the mothers. Exclusive breastfeeding (including water intake), total breastfeeding and timing of introduction of complementary foods were also assessed in the mothers’ questionnaire. Smoking status of the mother was asked regarding the current situation and also for the pregnancy period, and classified as: no smoker; smoker, but not during pregnancy; smoker during pregnancy.
Self-perceived social class was evaluated using the options: lower, lower middle, upper middle, upper and does not know/answer. For the present analysis, the categories upper middle and upper were combined. The parental educational level was measured as the number of successfully completed years of formal schooling and we considered the information from the parent with the highest education.
Family history of a medical diagnosis of diabetes, dyslipidaemia and hypertension was asked separately to the mother and the father. For each of these conditions, we calculated the variable family history combining information of both mother and father, classified as: positive, when at least one of the adolescent's parents had the diagnosis; negative, when both parents reported no diagnosis; or non-classifiable, when the available information showed no diagnosis, but, at least, one of the parents reported to ‘be unaware’ or did not answer.
Statistical analysis
Developmental trajectories of adiposity
For the analyses of developmental trajectories, we used measurements extracted from the child health books and measurements obtained during the EPITeen cohort evaluations (at 13, 17 and 21 years of age). We excluded participants presenting less than five measurements from birth until 21 years of age (n=45). The measurements varied considerably between participants, and were more frequent at earlier ages. Therefore, instead of using all single measurements, a 3-month average was computed for each participant to decrease the effect of auto-correlation of the errors. The final analyses included 719 participants with 11 459 measurements of weight and length/height (average of 16 measurements per subject).
Developmental trajectories were identified using growth mixture modelling, estimated in two steps. In the first step, the growth modelling, random effects were used to capture individual differences in development through a mixed-effect fractional polynomial;15 in the second step, the mixture modelling, we used normal mixture modelling for model-based clustering.16
Regarding the first step, instead of modelling BMI or BMI z-score, which leaves residual confounding of height, we used a mixed-effect fractional polynomial to linearize the effect of age and height on the weight. The quality of different models was compared using Bayesian Information Criterion, the correlation between the predicted and observed values and the Relative Squared Error.17 The Relative Squared Error is the error associated with the model prediction, relative to what it would have been if a simple predictor, such as the average of the actual values, had been used instead. The best fit was found for the model that can be written as:

where ln(weightij) is the logarithm of weight for individual i at age j; β are the estimated fixed coefficients, representing the average regression coefficients for the population; and b are the random coefficients, representing the deviation from the average estimates for individual i.
In the second step, the individual random effects estimated in the mixed effects model (b in the formula (1)) were used as response variables in the normal mixture modelling for model-based clustering to identify the patterns in the growth trajectories. The most appropriate models were considered those allowing for the most homogeneous grouping of the individual patterns of growth selected among those with the lowest Bayesian Information Criterion.
These analyses were run in R language and software environment for statistical computation (version 3.0.1, R Foundation for Statistical Computing, Austria, 2013), using the packages nlme18 and mclust,19 for mixed-model analysis and model-based clustering, respectively.
Trajectories characterization and association with cardiometabolic risk factors
For the characterization of the trajectories according to social and early life factors and the association with cardiometabolic risk factors, data are presented as counts and proportions for categorical variables, and median (25th–75th percentiles) for continuous variables, and comparisons were performed using Chi-square and Mann–Whitney test, respectively.
To test the differences of cardiometabolic risk factors at 21 years of age according to developmental trajectories of adiposity we used analysis of covariance (ANCOVA) to estimate adjusted means. The best transformation for skewed variables (HOMA-IR and triglycerides) was performed, and adjusted means were then retransformed to the original scale to improve data interpretation. For each cardiometabolic risk, an adjusted model was computed (including sex and family history of disease) and then a second model with additional adjustment for BMI at 21 years of age was performed to test the effect of the trajectories on cardiometabolic outcomes, independently of current BMI. For HOMA-IR and triglycerides, a statistically significant interaction was found between sex and developmental trajectory, and analyses were stratified by sex.
Statistical analysis was conducted using SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0; IBM Corp., Armonk, NY, USA) and the significance level was set at 0.05 for two-sided tests.
Results
Using a total of 11 459 measurements from 719 subjects, two developmental trajectories of adiposity were identified: ‘Average BMI growth’ that comprised 580 (80.7%) participants and ‘Higher BMI growth’ that included 139 (19.3%) participants.
Characterization of weight and BMI velocity
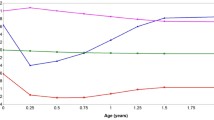
Figure 1 presents the mean values of predicted weight and predicted BMI, and respective velocities throughout age, for each trajectory identified. The weight gain presents velocity inflection points at similar ages for both trajectories (approximately at 4.5 years starts the increasing period and at 11 years the decreasing period). However, velocity in the trajectory ‘Higher BMI growth’ is higher than in the ‘Average BMI growth’ group (velocity of 3.0 vs 2.1 kg per year at 4.5 years; and of 5.4 vs 4.2 kg per year at 11 years, respectively, for the trajectories).
BMI velocity is affected by the rate of weight gain and of height gain, presenting the first inflection at the same age in both trajectories (about 4.5 years of age). At this inflection point, the trajectory ‘Average BMI growth’ reaches negative values (approximately −0.4 kg m−2 per year), which represents a decrease in BMI values, while trajectory ‘Higher BMI growth’ always presents a positive velocity with a minimum of 0.2 kg m−2 per year. The second inflexion point in BMI velocity happens earlier in the ‘Higher BMI growth’ (9.8 years vs 11.7 years), and with a higher velocity (0.9 kg m−2 per year vs 0.6 kg m−2 per year). These differences in BMI gain velocity result in increasing values of BMI across age and in higher adulthood mean BMI in ‘Higher BMI growth’, compared with the ‘Average BMI growth’ group. At 21 years of age, the prevalence of overweight and obesity combined is 71.8% in ‘Higher BMI growth’ and 12.1% in ‘Average BMI growth’.
Participants and family characteristics and trajectories
The ‘Higher BMI growth’ trajectory presented a lower proportion of participants reporting upper or upper middle self-perceived social class and a lower proportion of parents with higher education, although differences were not statistically significant (Table 1). Birth weight was similar between the two trajectories; however, participants from the ‘Higher BMI growth’ presented lower length at birth (49 vs 50 cm, P=0.049), and caesarean delivery was more frequent. Mothers of participants in the ‘Higher BMI growth’ trajectory were younger, and more frequently overweight (30.6 vs 27.9%) or obese (24.2 vs 9.6%; P<0.001).
Association between trajectories and cardiometabolic risk factors at 21 years of age
In the model adjusted for sex and family history of disease, higher mean systolic blood pressure (111.6 vs 108.3 mm Hg, P<0.001) and diastolic blood pressure (71.9 vs 68.4 mm Hg, P=0.001) were found in ‘Higher BMI growth’ group. On the contrary, lower HDL-C was found in the group ‘Higher BMI growth’ (53.3 vs 57.0 mg dl−1, P=0.001) (Table 2). However, differences in blood pressure and in HDL-C were no longer significant after adjustment for BMI at 21 years of age. As there was a significant interaction between trajectories and sex, triglycerides and HOMA-IR were stratified by sex (Table 3). In comparison with trajectory ‘Average BMI growth’, we found higher levels of triglycerides in participants from the trajectory ‘Higher BMI growth’, but only among males (89.0 vs 70.2 mg dl−1, P<0.001). Differences in adjusted means of HOMA-IR were also found when comparing trajectories, significant both in females and in males, but larger in males (2.41 vs 1.49, P<0.001) than in females (2.07 vs 1.67, P<0.001). After adjustment for BMI at 21 years of age, differences in triglycerides and HOMA-IR between trajectories were also attenuated and were no longer statistically significant.
We additionally computed the association between the trajectories and cardiometabolic factors at 13 years (Supplementary Table 1), and the results were similar to those found with the outcomes at 21 years, supporting that the associations are already present at 13 years of age.
Discussion
Using data from birth until early adulthood, we identified two developmental trajectories of adiposity. The ‘Average BMI growth’ trajectory was the most prevalent, presenting on average a BMI within normal ranges. The ‘Higher BMI growth’ trajectory is characterized by higher BMI mean values since birth, being the difference between the trajectories more pronounced since the period of adiposity rebound. At 21 years of age, almost three quarters of the individuals from this trajectory present overweight or obesity.
Developmental trajectories in children and adolescents were described in different settings but study comparisons are inappropriate due to differences in statistical solutions, growth modelling20, 21 versus growth mixture modelling (a group-based approach);22, 23, 24, 25, 26, 27 great heterogeneity in age ranges and differences in the outcome modelled, mostly using BMI25, 26, 27 or BMI z-score.20, 22, 23, 24
Adiposity differences between trajectories appeared in the first years of life and there was a low probability (about 10%) of changing trajectory from adolescence onwards (data not shown). These results suggest that the determinants of the two different trajectories are related to early events. We found that participants from trajectory ‘Higher BMI growth’ were born more frequently by caesarean section and their mothers were younger and more frequently overweight or obese. The direct association between parental BMI and childhood obesity have been extensively addressed and have been also found when analysing the association with BMI trajectories in childhood or youth.22, 23, 24, 26, 27, 28 The association between caesarean section and offspring overweight and obesity have been previously described,29 although some authors have argued that it is not causal, and may be due to uncontrolled confounding.30, 31 Maternal smoking during pregnancy, breastfeeding and complementary feeding was not different according to developmental trajectories. Limited accuracy of the information self-reported by the mothers when adolescents were 13 years of age without additional information from the second study wave, and no information for participants enrolled at that wave make inferences less reliable.
Parental education is often used as a proxy for socioeconomic status of the child or adolescent, influencing exposures since early stages of life. In our study, a higher proportion of participants in the trajectory ‘Higher BMI growth’ had parents with lower educational level. This finding is in accordance with other previous studies analysing developmental trajectories,27, 28 although some studies have reported non-significant associations.22, 26
Regarding the association between developmental trajectories and cardiometabolic risk factors in early adulthood, we found that participants from ‘Higher BMI growth’ trajectory presented a worse cardiometabolic profile, with higher levels of blood pressure, HOMA-IR and triglycerides, and lower levels of HDL-C. To our knowledge, few studies have evaluated the effect of pediatric adiposity trajectories on adult cardiometabolic outcomes, since the majority of studies are limited to childhood or adolescence. Ziyab et al.23 have studied BMI trajectories from 1 to 18 years in the Isle of Wight birth cohort, UK, and found that an early persistent obesity trajectory was associated with higher blood pressure at 18 years of age. The West Australian Pregnancy cohort showed three trajectories (birth to age 14) characterized by accelerating adiposity associated with increased risk of prehypertension or hypertension at 17 years of age.32 In a previous study, these trajectories were also related to higher insulin resistance at 14 years.33
In our study, we computed an additional model controlling for current BMI (at 21 years of age), and differences in the cardiometabolic outcomes were attenuated, or in some cases reverted. The adjustment for current BMI might be inappropriate, since it can be an intermediate step between the adiposity trajectory and the cardiometabolic outcome. This question has been discussed mainly with regard to analysis investigating the association between childhood obesity on adult disease, independently of adult BMI. Some authors argue that this adjustment may introduce over-adjustment biases,34 while others raise some questions about the interpretation of the results, because this adjustment controls for change in adiposity between measurements, not only for adult body size.35 Considering this difficulties, Lucas et al.35 recommend the presentation of different models, with and without adjustment for later adiposity, to make studies in this area comparable. In our study, we computed a model with additional adjustment for current BMI, and the attenuation of the results indicates that the association between adiposity trajectory and adult outcomes is mediated by current BMI. However, we are not able to fully disentangle this complex association with our analysis. Future studies using more sophisticated statistical techniques, such as structural equation modelling, are needed to clarify whether the effect of the adiposity trajectory is totally mediated by current BMI or not, and to quantify the direct and indirect effects of the trajectories on adult cardiometabolic outcomes.
Strengths and limitations
In addition to the anthropometric data assessed under the strict cohort exams, we also used data extracted from child health books, measured routinely by health professionals. Although we could not test the accuracy of the recorded information, weight and height extracted from child health individual books have been shown to present no systematic bias, supporting their use for research.36
Regarding the identification of the trajectories, we excluded participants having less than five measurements during the period under studied, to ensure a number of measurements higher than the number of random parameters in the model (four). We did not stratify the analysis of the trajectories by sex, but we tested a model including interaction terms between sex and all the other terms. Although the interaction terms were statistically significant, the goodness-of-fit of the model remained similar and the trajectories obtained from this analysis were similar to those obtained from the model without interaction for sex. Therefore, we chose the simplest model, without interaction for sex. This option was then supported by a similar sex distribution of the two trajectories identified. We also performed other sensitive analysis, such as the exclusion of the participants born pre-term (n=41). Trajectories identified without pre-terms were also similar to those identified in the total sample, and therefore we decided to use all the participants to enhance statistical power of the analysis, and to improve external validity of our results. The number of participants identified in the ‘Higher BMI growth’ trajectory was lower than in the other trajectory, but it was in accordance to the BMI distribution in a population setting. Despite this unbalanced sample size between trajectories, we were able to detect statistically significant differences on most of the cardiometabolic risk factors analysed.
The current analysis was performed in a sub-sample of the participants of the third study wave of the cohort. Participants included in the study presented higher education than those excluded and this difference may have led to an underestimation of the prevalence of the ‘Higher BMI growth’ trajectory, but it is not expected that it had influenced the definition of the trajectories.
Regarding the analysis of the association between the trajectories and the cardiometabolic outcomes, we also tested the effect of other potential confounders, such as parental and participants’ education, practice of sports and diet (total energy intake), but they were not considered in the final models, since they did not significantly changed the estimates.
The study is strengthened by using multiple anthropometric measures across the pediatric age (16 per subject, on average), which increases the accuracy of the trajectories identification. We have also tested different models, with different outcomes using either BMI or weight and height. When using BMI, a worse goodness-of-fit of the model was found suggesting it is a measure not equally adequate across the whole pediatric period. We also tested the use of different covariates in the model, as height and age. Our final model was the one presenting the best goodness-of-fit, using the logarithm of weight as outcome, and including height and age as covariates. Finally, our analysis covers the whole pediatric period and the early adulthood, and we were able to study the association of the adiposity trajectories on adult cardiometabolic outcomes, such as blood pressure, insulin resistance, cholesterol and triglycerides.
In this population-based cohort, we identified two trajectories of adiposity from birth until early adulthood. The trajectory ‘Higher BMI growth’ found in 19% of the population presented higher values of BMI in adulthood and led to a worse cardiometabolic profile. Our results also suggest that the impact of the adiposity trajectory on cardiometabolic profile is mediated by the effect on current BMI, but future studies are needed to clarify the direct and indirect effects of the adiposity trajectories on adult cardiometabolic outcomes.
References
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M . Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011; 378: 815–825.
Moraeus L, Lissner L, Sjoberg A . Stable prevalence of obesity in Swedish schoolchildren from 2008 to 2013 but widening socio-economic gap in girls. Acta Paediatr 2014; 103: 1277–1284.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781.
Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ . Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008; 9: 474–488.
Adair LS . Child and adolescent obesity: epidemiology and developmental perspectives. Physiol Behav 2008; 94: 8–16.
Reilly JJ, Kelly J . Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011; 35: 891–898.
Park MH, Falconer C, Viner RM, Kinra S . The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012; 13: 985–1000.
Nagin DS, Odgers CL . Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6: 109–138.
Ramos E, Barros H . Family and school determinants of overweight in 13-year-old Portuguese adolescents. Acta Paediatr 2007; 96: 281–286.
Gibson RS . Principles of Nutritional Assessment, 2nd edn. Oxford University Press: New York, 2005.
Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 1998; 68: 899–917.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014; 32: 3–15.
Royston P, Altman DG . Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Stat 1994; 43: 429–467.
Fraley C, Raftery AE . Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc 2002; 97: 611–631.
Witten IH, Frank E . Data Mining: Practical Machine Learning Tools and Techniques, Second Edition (Morgan Kaufmann Series in Data Management Systems). Morgan Kaufmann Publishers Inc.: San Francisco, CA, USA, 2005.
Pinheiro J, Bates D, DebRoy S, Sarkar D . Linear and nonlinear mixed effects models. R Package Version 2007; 3: 57.
Fraley C, Raftery AE, Murphy B, Scrucca L . Mclust Version 4 for R: Normal Mixture Modeling and Model-based Clustering, Classification, and Density Estimation. Technical Report No. 597. Department of Statistics, University of Washington: Washington, 2012.
Morrissey TW . Trajectories of growth in body mass index across childhood: Associations with maternal and paternal employment. Soc Sci Med 2013; 95: 60–68.
Howe LD, Tilling K, Matijasevich A, Petherick ES, Santos AC, Fairley L et al. Linear spline multilevel models for summarising childhood growth trajectories: a guide to their application using examples from five birth cohorts. Stat Methods Med Res 2013; pii: 0962280213503925 . e-pub ahead of print 9 October 2013.
Haga C, Kondo N, Suzuki K, Sato M, Ando D, Yokomichi H et al. Developmental trajectories of body mass index among Japanese children and impact of maternal factors during pregnancy. PLoS One 2012; 7: e51896.
Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH . Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health 2014; 68: 934–941.
Carter MA, Dubois L, Tremblay MS, Taljaard M, Jones BL . Trajectories of childhood weight gain: the relative importance of local environment versus individual social and early life factors. PLoS One 2012; 7: e47065.
Garden FL, Marks GB, Simpson JM, Webb KL . Body mass index (BMI) trajectories from birth to 11.5 years: relation to early life food intake. Nutrients 2012; 4: 1382–1398.
Pryor LE, Tremblay RE, Boivin M, Touchette E, Dubois L, Genolini C et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med 2011; 165: 906–912.
Nonnemaker JM, Morgan-Lopez AA, Pais JM, Finkelstein EA . Youth BMI trajectories: evidence from the NLSY97. Obesity (Silver Spring) 2009; 17: 1274–1280.
Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS . Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007; 15: 760–771.
Li HT, Zhou YB, Liu JM . The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2013; 37: 893–899.
Flemming K, Woolcott CG, Allen AC, Veugelers PJ, Kuhle S . The association between caesarean section and childhood obesity revisited: a cohort study. Arch Dis Child 2013; 98: 526–532.
Barros FC, Matijasevich A, Hallal PC, Horta BL, Barros AJ, Menezes AB et al. Cesarean section and risk of obesity in childhood, adolescence, and early adulthood: evidence from 3 Brazilian birth cohorts. Am J Clin Nutr 2012; 95: 465–470.
Huang RC, Burrows S, Mori TA, Oddy WH, Beilin LJ . Lifecourse adiposity and blood pressure between birth and 17 years old. Am J Hypertens 2015; 28: 1056–1063.
Huang RC, de Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care 2011; 34: 1019–1025.
Schisterman EF, Cole SR, Platt RW . Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009; 20: 488–495.
Lucas A, Fewtrell MS, Cole TJ . Fetal origins of adult disease-the hypothesis revisited. BMJ 1999; 319: 245–249.
Howe LD, Tilling K, Lawlor DA . Accuracy of height and weight data from child health records. Arch Dis Child 2009; 94: 950–954.
Acknowledgements
We gratefully acknowledge funding from the Portuguese Foundation for Science and Technology (FCOMP-01-0124-FEDER-015750, and SFRH/BD/78153/2011 to JA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Araújo, J., Severo, M., Barros, H. et al. Developmental trajectories of adiposity from birth until early adulthood and association with cardiometabolic risk factors. Int J Obes 39, 1443–1449 (2015). https://doi.org/10.1038/ijo.2015.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.128
- Springer Nature Limited
This article is cited by
-
Association between BMI trajectories from childhood to early adulthood and the carotid intima-media thickness in early adulthood: Tehran lipid and glucose study
BMC Public Health (2023)
-
Changes in sedentary behavior patterns during the transition from childhood to adolescence and their association with adiposity: a prospective study based on compositional data analysis
Archives of Public Health (2022)
-
Developmental trajectories of body mass index since childhood and health-related quality of life in young adulthood: Tehran Lipid and Glucose Study
Quality of Life Research (2022)
-
Resolving early obesity leads to a cardiometabolic profile within normal ranges at 23 years old in a two-decade prospective follow-up study
Scientific Reports (2021)
-
Maternal and infant prediction of the child BMI trajectories; studies across two generations of Northern Finland birth cohorts
International Journal of Obesity (2021)