Abstract
A major challenge in tendon injury is the weak intrinsic healing capacity of tendon that may cause rupture of the repair after surgery. Growth factors are believed to be critical during tendon healing. This study aimed to investigate the effects of vascular endothelial growth factor (VEGF) genes delivered by adeno-associated virus (AAV) vectors on tendon healing and molecular events involved in a chicken model. A total of 128 deep flexor tendons in the long toes of chickens were completely transected and injected with 2 × 109 particles of AAV2-VEGF or saline before surgically repaired. At postoperative 4, 6 and 8 weeks, the gliding excursions of tendon were recorded and adhesions around the repair site scored. At 2, 4, 6 and 8 weeks, the ultimate strengths of the healing tendons were tested. Terminal deoxynucleotide transferase dUTP nick end labeling assay were performed to detect cellular apoptosis and immunofluorescence staining to detect type III collagen and matrix metalloprotease-2 (MMP2) expression in tendon tissues. The gliding excursion and adhesion score were similar between AAV2-VEGF-treated tendons and the control tendons. Delivery of AAV2-VEGF significantly increased ultimate strength of the healing tendons at postoperative 4, 6 and 8 weeks (P<0.05). Apoptotic reaction was inhibited from postoperative 2 to 8 weeks in tendon core area or surface area. Type III collagen expression was enhanced at 2, 4, 6 and 8 weeks and MMP2 expression enhanced at 2 and 4 weeks after AAV2-VEGF transfection. The current study confirms the therapeutic efficacy of AAV2-VEGF in improving healing strength of tendon without aggravating adhesion formation after tendon injury, shedding light on the application of molecular therapy in modulating tendon healing.
Similar content being viewed by others
Introduction
Tendon is a kind of tissue that has weak intrinsic healing capacity if injured, causing deficiency in strength gain during healing period, especially in the early period after tendon surgery.1 Therefore, gap formation or re-rupture of the repaired tendon can occur when performing postoperative movements. The poor healing capacity of tendon may be largely because of low cellular activity, disruption of nutrition from blood vessels or synovial fluid after injury and supply–demand imbalance of growth factors critical to tendon self-repair.2 Approaches aiming to improve ultimate strength of tendon are generally based on two aspects: surgical repair and biological modulation. The former includes increasing the number of core sutures, performing peripheral repair, setting a proper core suture purchase and tension and so on.3, 4 These methods could strengthen tendon repair to a certain extent but their effects on tendon healing in vivo have yet to be thrashed out. In recent years, biological modulations of injured tendon have been demonstrated to yield encouraging results and have proven to be promising.
Many efforts have been made to improve tendon healing by delivering exogenous growth factor genes to injured tendons. Tang et al.5 reported that transfection of basic fibroblast growth factor gene by adeno-associated viral vector-2 (AAV2) could significantly increase the healing strength of flexor tendon without increasing adhesion formation in a chicken model. Suwalski et al.6 proved that platelet-derived growth factor gene delivery with mesoporous silica nanoparticles significantly accelerated Achilles tendon healing in rat. Transfecting insulin-like growth factor-I complementary DNA by ultrasound-targeted destruction significantly increased the maximum load, stiffness and ultimate stress of wounded Achilles tendon in rat, indicating enhancement in tendon regeneration.7
Vascular endothelial growth factor (VEGF) is a potent mediator of angiogenesis, with direct mitogenic activity on cells of endothelial origin. Administration of exogenous VEGF in the transfected Achilles tendon significantly increased the tensile strength of the repaired tendon at postoperative 1 and 2 weeks when plantaris tendon was preserved.8 A local injection of VEGF-111 significantly increased the healing strength at days 15 and 30 in rat Achilles tendons after a surgical section.9 These studies, however, used VEGF protein solution directly in tendon at repair site. Little is known regarding the effect of VEGF gene transfection on tendon healing in vivo. In the current study we transferred VEGF gene by AAV2 vectors to injured flexor tendon of chicken toes to investigate its effects on tendon healing and adhesion formation up to 8 weeks after surgery. Biomechanical tests of gliding excursion and ultimate strength of the healing tendons were performed. Tendon healing status was observed and adhesion scores recorded. Cellular apoptosis and expression of type III collagen and matrix metalloprotease-2 (MMP2) were detected by immunofluorescence staining. In addition, the effect of double-stranded AAV2 (dsAAV2) vectors was also compared.
Results
Animal grouping
A total number of 128 long toes of 64 chickens were included (Table 1). The flexor digitorum profundus (FDP) tendons in the toes were transected completely and subdivided to different groups. First, 82 toes were divided into the AAV2-VEGF (38 toes) and control groups (44 toes). The gliding excursion of tendon and adhesions around the tendon repair site were recorded at postoperative 4, 6 and 8 weeks. The ultimate strength of the healing tendon was tested at 2, 4, 6 and 8 weeks. After the biomechanical tests, the tendon samples were harvested for terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay and immunofluorescence staining of type III collagen and MMP2. Meanwhile, dsAAV2-VEGF was also included in the current study. We tried to find out whether this kind of vector is superior to the AAV2 vectors in our chicken tendon injury models. Thus, the other 46 toes were divided into the dsAAV2-VEGF group (28 toes) and AAV2-VEGF group (18 toes). The ultimate strength was tested at 1, 2 and 3 weeks. The AAV2-VEGF-treated samples at 2 weeks here were obtained from the first part.
Tendon gliding excursion
At postoperative 4, 6 and 8 weeks, the gliding excursions of the tendons at the pulling force of 15 N were measured. Compared with the control group, AAV2-VEGF-treated tendons had relatively greater gliding excursions at these time points (Table 2). However, there were no significant differences in the gliding excursion between the AAV2-VEGF and control group (P>0.05).
Tendon healing status and adhesion score
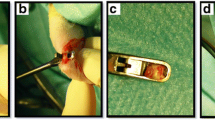
After testing the gliding excursion, we carefully dissected the toes and observed the healing status and adhesion formation. In the control tendons, the adhesions gradually occurred around the healing tendons and became severe with time (Figure 1). At 2 weeks, the adhesions were loose and limited to the repair site. After 4 weeks, the adhesions were moderately dense and extended over the repair site. The healing tendons seemed bulky because of the adhesions. At 6 and 8 weeks, dense and rigid adhesions formed around the tendons. The FDP tendon seemed to inosculate with the surrounding fibrous tissues, especially with the dorsal periosteum.
Healing status of the injured tendons in the AAV2-VEGF and control groups at postoperative 4, 6 and 8 weeks. The AAV2-VEGF-treated tendons had a smoother healing surface than the control tendons. The picture of the AAV2-VEGF-treated tendon at 8 weeks showed new vessel formation around the repair site.
The tendons treated with AAV2-VEGF had a relatively lower adhesion score than the control tendons (Table 2). However, no significant differences were found between these two groups at 4, 6 and 8 weeks. After separating the adhesions from the tendon, we observed that most of the tendons treated with AAV2-VEGF had a relatively smoother surface. The control tendons, in contrast, had a rough healing surface (Figure 1). At 8 weeks, new blood vessels formed around the healing tendon treated with AAV2-VEGF in some toes (Figure 1).
AAV2-VEGF improves healing strength of tendon
Finally, the tendons were separated from the surrounding tissues and the ultimate strength of tendon was tested. The healing strength of the AAV2-VEGF-treated tendons was significantly increased at postoperative 4 weeks (12.4±6.7 vs 5.7±1.1 N, P=0.037), 6 weeks (46.4±11.2 vs 27.1±8.8 N, P=0.006) and 8 weeks (73.5±15.1 vs 50.6±14.6 N, P=0.012) as compared with that of the control tendons (Figure 2).
AAV2-VEGF decreases cellular apoptosis
When all the biomechanical tests were finished, a 1.5 cm long tendon segment centered by the cut site was harvested. The tendon samples were processed to TUNEL and immunofluorescence staining. The apoptosis index of the AAV2-VEGF-treated tendon was decreased compared with that of the control tendons at all the observed time points (Figure 3). In the tendon surface area, the apoptosis index was significantly lower in the AAV2-VEGF group than in the control group at 2, 4 and 6 weeks (P<0.05). In the tendon core area, the apoptosis index was significantly lower in the AAV2-VEGF group than in the control group at 6 and 8 weeks (P<0.01).
Apoptosis index (AI) of the tendon tissues in the AAV2-VEGF and control groups at postoperative 2, 4, 6 and 8 weeks. The tendon surface and core areas were observed separately. The AI of the AAV2-VEGF-treated tendon was decreased compared with that of the control tendons at all the observed time points. In the tendon surface, the AI was significantly decreased in the AAV2-VEGF group at 2, 4 and 6 weeks (P<0.05). In the tendon core, the AI was significantly decreased in the AAV2-VEGF group at 6 and 8 weeks (P<0.01).
AAV2-VEGF influences expression of type III collagen and MMP2
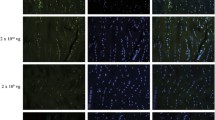
Immunofluorescence staining showed continuous increase in type III collagen expression of the control tendons from 2 to 8 weeks (Figure 4). With treatment of AAV2-VEGF, the tendons showed obviously stronger positive staining of type III collagen at all the time points that reached the highest level from 4 to 8 weeks. MMP2 expression in the control tendons peaked at 2 weeks and decreased thereafter (Figure 5). After being treated with AAV2-VEGF, MMP2 expression was notably enhanced at 2 and 4 weeks. Both groups showed very weak or no positive staining at 6 and 8 weeks.
dsAAV2-VEGF is not superior to AAV2-VEGF
The healing strengths of tendon were similar between dsAAV2-VEGF- and AAV2-VEGF-treated tendons at 1 week (7.6±0.8 vs 7.8±2.0 N), 2 weeks (8.1±1.8 vs 8.8±2.3 N) and 3 weeks (9.5±2.9 vs 10.5±2.5 N; P>0.05).
Discussion
Molecular methods have been used to modulate tendon healing in basic research.5, 6, 7, 8, 9, 10 Previous in vitro and in vivo studies demonstrated that growth factor gene transfer could effectively improve flexor tendon healing. Exogenous platelet-derived growth factor or VEGF genes delivered by plasmids significantly increased the expression of type I collagen and transforming growth factor-β genes of cultured rat tenocytes.11, 12 With a more efficient delivery vehicle, AAV2, basic fibroblast growth factor gene has been successfully transferred to cultured rat intrasynovial tenocytes and injured flexor tendon of chicken.5, 13 These studies showed that AAV2 basic fibroblast growth factor significantly increased the expression of type I and III collagen genes of cultured tenocytes and enhanced the healing strength of injured tendons.
VEGF was chosen currently because tendon is insufficient in vascular supply and VEGF is regarded as an angiogenic marker that may be particularly essential in the healing process of tendon and ligment.14, 15, 16 Our results showed that VEGF gene transfection significantly increased healing strength of tendon at 4, 6 and 8 weeks and AAV2-VEGF-treated tendons healed with a relatively smoother surface than the control tendons. An unexpected finding is that new vessels formed around the tendon repair site in some toes at 8 weeks after VEGF gene transfection. All these indicated enhancement of tendon healing by introduction of exogenous VEGF genes. However, the tendon gliding and adhesion formation were not significantly affected although AAV2-VEGF-treated tendons showed a slight downward trend in adhesion score and upward trend in gliding excursion. The superior healing status has not been reflected in the biomechanical function of tendon gliding. The main cause may be that elimination of adhesions around the healing tendons as well as recovery of gliding function is a lengthy process.17 It would be a long term (far more than 8 weeks) before VEGF gene transfer could have noticeable beneficial effects on adhesion formation and then tendon gliding.
The visual observations and biomechanical evaluations led us to further investigate the mechanisms behind the modulation of AAV2-VEGF in tendon healing. Cellular apoptosis were first detected because apoptotic reaction has been proven to be an indispensable event lasting the whole process of tendon healing.18, 19, 20 Apoptotic peak during the early period of tendon healing was thought to be associated with the acute trauma and inflammatory reactions. High level of apoptosis in the late period was found to be responsible for eliminating extra cells and restoring cell-to-matrix ration in tendon remodeling. We separately observed the tendon surface and tendon core because these two areas react differently after tendon injury. Previous studies proved that cells from tendon surface are more reactive after tendon injury and these cells can track into the cut site and displace into the core substance in the early period of tendon healing.21 At present, the apoptotic reactions were notably lower in the AAV2-VEGF-treated tendon than in the control tendons. We speculate that VEGF gene transfer inhibited apoptosis of tenocytes, especially those in the tendon surface area, that somehow benefits cellular growth and migration. It may ultimately improve tendon healing.
Next, we examined the changes of type III collagen and MMP2 expression in tendon tissues. Type III collagen is the major collagen in healing tendon and is associated with granulation formation during the proliferative stage of tendon healing. After tendon injury, the cut site is initially bridged by granulation tissues composed primarily of type III collagen that is later remodeled to more organized, mature type I collagen that makes up the majority of tendon structure during homeostasis.1, 22 MMP2 is one of the members of MMPs family that have been found to play crucial roles in extracellular matrix (ECM) degradation and tissue remodeling.23 Loiselle et al.24 investigated the role of MMP2 during tendon healing and suggested that MMP2 facilitated the transition from early granulation tissue to a more organized collagen structure. As such, expression of type III collagen and MMP2 are important for characterizing remodeling of tendon substance as well as the synthesis and degradation of ECM. Our results showed that both type III collagen and MMP2 expression were enhanced after AAV2-VEGF transfection. The increase in type III collagen production may make up for the loss of collagen after tendon injury and aid in the resynthesis of ECM. The increased level of MMP2 may benefit not only cell migration, but also collagen deposition and rearrangement. The combined effect of the above two aspects would conduce to the enhancement of tendon healing.
Finally, the efficiency of dsAAV2 vectors was investigated in this study. The dsAAV vectors have been reported to achieve a more robust and stable transgenic expression than the AAV vectors.25, 26 We had expected a faster gene transfection and an earlier effect of VEGF on tendon healing. However, we did not see any superiority of dsAAV2 transfection over AAV2 during the first 3 weeks in our animal model. We did not observe a longer therapeutic effect of dsAAV2-VEGF because the postoperative 3 weeks is critical for tendon healing, during which tendon repair is vulnerable and easy to rupture.27, 28–29 After that, tendon healing accelerates and healing strength gains rapidly. Therefore, we look more favorably upon an earlier or faster efficiency of the transfection vehicle. In addition, we do not know the effect of direct application of VEGF protein on tendon healing, as demonstrated by previous studies.8, 9 It would be better to compare the effect of VEGF gene transfection with that of VEGF protein, and this seems to be a limitation of the current study. We also understand a number of recent efforts in enhancing tendon healing strength or decreasing the adhesions have not been proven effective in pre-clinical or clinical studies.30, 31, 32–33 Enhancing surgical repair strength remains the major approach in decreasing the repair rupture of the repaired tendons.34, 35, 36, 37–38 More efforts in optimizing the dosage and method of delivery of the genes to the tendons would be necessary before we proceed to clinical trails.
In summary, flexor tendon repairs treated with AAV2-VEGF exhibited an increased healing response, with greater healing strength and smoother healing surface of tendon. The cellular and molecular events behind may include: (1) inhibition of cellular apoptosis, (2) improvement of ECM component expression, such as type III collagen, (3) increased activity of protease responsible for ECM degradation and remodeling such as MMP2 and (4) formation of new vessels. Currently, the AAV2-VEGF treatment has no significant bearing on gliding function of tendon and adhesion formation during the observed time points. Nevertheless, the long-term effects of VEGF gene transfer remain to be assessed. Our study highlights the potential mechanisms of VEGF gene transfection and provides insight into the molecular modulation in tendon healing.
Materials and methods
This study was approved by the experimental animal care committee of our university. White Leghorn chickens, weighing ∼1.5 kg, were used because of the similarity of flexor mechanism between chicken toe and human finger.39, 40
AAV2-VEGF construction and production
Single-stranded and dsAAV2 vectors were used. AAV2-VEGF vector plasmid was constructed using a method similar to that used for production of pAAV2-bFGF reported in previous study.5, 13 Human VEGF gene (Gene bank accession no. AF486837) that encodes human VEGF 165 isoform was inserted into pAAV-MCS (Stratagene, La Jolla, CA, USA). The final production of both AAV2-VEGF vectors was finished by Vector BioLabs (Philadelphia, PA, USA).
Operative procedure
We anesthetized the chickens by intramuscular injection with pentobarbital (12 mg kg−1 of body weight) and applied Elastic bandages as tourniquets on chicken legs. The operation was performed in a sterile surgical condition. The area between the proximal and distal interphalangeal joint of the long toes was our ideal operation region that corresponds to zone 2 flexor tendons of the human digits. A zig-zag incision was made in the plantar skin and the sheath was opened longitudinally for 1.5 cm in length to expose the flexor tendon system. We resected the flexor digitorum superficialis tendon for ∼1.5 cm and transected the FDP tendon completely. The FDP tendons were injected with 2 × 109 particles of AAV2-VEGF or dsAAV2-VEGF (Vector BioLabs) diluted in 20 μl sterile physiological saline and the control tendons with saline. After injection, each tendon was surgically repaired with modified Kessler method with 5-0 sutures (ETHILON; Ethicon, Somerville, NJ, USA). Peripheral sutures were added with 6-0 sutures and the sheath was not repaired. The skin was closed surgically and the toes immobilized in semi-flexed position with sterile gauze and adhesive tape for 3 weeks. They were released to allow free motion thereafter. An animal care technician administered postoperative pain and care.
Gliding excursion
The toes were disarticulated at the knee joint and mounted to a measurement board with K-wires. The proximal phalanx of the long toe, the metatarsal and the bones of the distal part of the leg were fixed to the board, whereas the distal three phalanges of the long toe were left unrestricted. The proximal end of the FDP tendon of the long toe was connected to the upper clamp of the testing machine (Model 4411; Instron Corp., Canton, MA, USA) and pulled proximally at a constant speed of 25 mm min−1 until the pulling force reach 15 N. The testing software (Series IX software; Instron Corp.) measured the load and displacement of tendon simultaneously and drew the load–displacement curve automatically. The displacement recorded represents the gliding excursion of the FDP tendons.
Tendon morphology and adhesion scores
We took down the chicken toes from the measurement board and exposed the FDP tendon through a volar longitudinal incision in the entire length of the long toe. The tendon morphology and adhesion formation over the surface of the repaired tendon were observed. Adhesions were recorded according to established grading criteria.5
Ultimate strength
We carefully separated the FDP tendon from the circumjacent tissues and adhesions. The FDP tendon connected with the toe tip was fixed in the testing machine. The proximal end of the FDP tendon was pulled upward until the tendon ruptured. A sharp decline in the load–displacement curve represents the ultimate rupture.
TUNEL assay and immunofluorescence staining
Tendon samples were fixed in 4% paraformaldehyde in phosphate-buffered saline for 24 h at 4 °C followed by gradient alcohol dehydration. The samples were embedded within paraffin and cut longitudinally to 4 μm. TUNEL assay (11684817910; Roche, Mannheim, Germany) was performed according to the manufacturer’s protocol to detect cellular apoptosis. Tendon sections were incubated with TUNEL reaction mixture for 1 h at 37 °C in a humidified chamber followed by converter-peroxidase solution for 30 min. The slides were incubated with the chromogenic substrate 3,3-diaminobenzidine (D-5905; Sigma Aldrich, St Louis, MO, USA) for 5 min at ambient temperature and counterstained with Mayer’s hematoxylin. Positive nuclei stained brown and negative nuclei stained blue. The apoptosis index in tendon surface and core areas was separately calculated (a percentage of the number of positive tenocytes divided by the total number of tenocytes). For immunofluorescence staining, the sections were incubated with the rabbit anti-Collagen type III (1:40, BP8013, Acris Antibodies GmbH, Herford, Germany) and MMP2 antibody (1:100, ab37150, Abcam, Cambridge, MA, USA) overnight at 4 °C and then with the fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (GGHL-90F; Immunology Consultants Laboratory, Newberg, OR, USA) in a 1:200 dilution for 60 min at 37 °C. Subsequently, sections were stained with 50 μl 4′-6-diamidino-2-phenylindole solution (1 μg ml−1) at room temperature for 20 min. In negative controls, the primary antibody was replaced by nonimmune serum.
Statistical analysis
Results are expressed as mean±s.d. The differences between experiment and control groups were assessed by two-tailed Student’s t-test. We estimated the variation within each group and the variance was similar between the groups. The level of significance was set at P<0.05. Statistical analyses were conducted with SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA).
References
Beredjiklian PK . Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am 2003; 85-A: 539–550.
James R, Kesturu G, Balian G, Chhabra AB . Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am 2008; 33: 102–112.
Tang JB . Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg Eur 2007; 32: 118–129.
Wu YF, Tang JB . Recent developments in flexor tendon repair techniques and factors influencing strength of the tendon repair. J Hand Surg Eur Vol 2014; 39: 6–19.
Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY . Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study. J Bone Joint Surg Am 2008; 90: 1078–1089.
Suwalski A, Dabboue H, Delalande A, Bensamoun SF, Canon F, Midoux P et al. Accelerated Achilles tendon healing by PDGF gene delivery with mesoporous silica nanoparticles. Biomaterials 2010; 31: 5237–5245.
Tang Y, Leng Q, Xiang X, Zhang L, Yang Y, Qiu L . Use of ultrasound-targeted microbubble destruction to transfect IGF-1 cDNA to enhance the regeneration of rat wounded Achilles tendon in vivo. Gene Therapy 2015; 22: 610–618.
Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM et al. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg 2003; 112: 1613–1619.
Kaux JF, Janssen L, Drion P, Nusgens B, Libertiaux V, Pascon F et al. Vascular endothelial growth factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons J 2014; 4: 24–28.
Chen CH, Zhou YL, Wu YF, Cao Y, Gao JS, Tang JB . Effectiveness of microRNA substantially down-regulates TGF-beta gene expression of digital flexor tendons: an in vitro andin vivo study. J Hand Surg Am 2009; 34: 1777–1784.
Wang XT, Liu PY, Tang JB . Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surg Am 2004; 29: 884–890.
Wang XT, Liu PY, Tang JB . Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J Hand Surg Am 2005; 30: 222–229.
Wang XT, Liu PY, Xin KQ, Tang JB . Tendon healing in vitro: bFGF gene transfer to tenocytes by adeno-associated viral vectors promotes expression of collagen genes. J Hand Surg Am 2005; 30: 1255–1261.
Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB . Tendon healingin vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am 2008; 33: 1834–1842.
Schulze-Tanzil G, Al-Sadi O, Wiegand E, Ertel W, Busch C, Kohl B et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports 2011; 21: 337–351.
Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni J et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials 2016; 81: 14–26.
Durgam S, Stewart M . Cellular and molecular factors influencing tendon repair. Tissue Eng Part B Rev 2017, [Epub ahead of print].
Wu YF, Chen CH, Cao Y, Avanessian B, Wang XT, Tang JB . Molecular events of cellular apoptosis and proliferation in the early tendon healing period. J Hand Surg Am 2010; 35: 2–10.
Wu YF, Zhou YL, Mao WF, Avanessian B, Liu PY, Tang JB . Cellular apoptosis and proliferation in the middle and late intrasynovial tendon healing periods. J Hand Surg Am 2012; 37: 209–216.
Wu YF, Tang JB . Apoptosis in adhesions and the adhesion-tendon gliding interface: relationship to adhesion-tendon gliding mechanics. J Hand Surg Am 2013; 38: 1071–1078.
Jones ME, Mudera V, Brown RA, Cambrey AD, Grobbelaar AO, McGrouther DA . The early surface cell response to flexor tendon injury. J Hand Surg Am 2003; 28: 221–230.
Liu X, Wu H, Byrne M, Krane S, Jaenisch R . Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA 1997; 94: 1852–1856.
Page-McCaw A, Ewald AJ, Werb Z . Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007; 8: 221–233.
Loiselle AE, Bragdon GA, Jacobson JA, Hasslund S, Cortes ZE, Schwarz EM et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res 2009; 27: 833–840.
Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X . Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro andin vivo. Gene Therapy 2003; 10: 2105–2111.
Choi SH, Lee HC . Long-term, antidiabetogenic effects of GLP-1 gene therapy using a double-stranded, adeno-associated viral vector. Gene Therapy 2011; 18: 155–163.
Elliot D, Barbieri CH, Evans RB, Mass D, Tang JB . IFSSH Flexor Tendon Committee Report 2007. J Hand Surg Eur Vol 2007; 32: 346–356.
Moriya K, Yoshizu T, Maki Y, Tsubokawa N, Narisawa H, Endo N . Clinical outcomes of early active mobilization following flexor tendon repair using the six-strand technique: short- and long-term evaluations. J Hand Surg Eur Vol 2015; 40: 250–258.
Moriya K, Yoshizu T, Tsubokawa N, Narisawa H, Hara K, Maki Y . Clinical results of releasing the entire A2 pulley after flexor tendon repair in zone 2C. J Hand Surg Eur Vol 2016; 41: 822–828.
Jordan MC, Schmitt V, Dannigkeit S, Schmidt K, Meffert RH, Hoelscher-Doht S . Surgical adhesive BioGlue does not benefit tendon repair strength: an ex vivo study. J Hand Surg Eur Vol 2015; 40: 700–704.
Lees VC, Warwick D, Gillespie P, Brown A, Akhavani M, Dewer D et al. A multicentre, randomized, double-blind trial of the safety and efficacy of mannose-6-phosphate in patients having Zone II flexor tendon repairs. J Hand Surg Eur Vol 2015; 40: 682–694.
Loiselle AE, Kelly M, Hammert WC . Biological augmentation of flexor tendon repair: a challenging cellular landscape. J Hand Surg Am 2016; 41: 144–149.
Wong R, Alam N, McGrouther AD, Wong JK . Tendon grafts: their natural history, biology and future development. J Hand Surg Eur Vol 2015; 40: 669–681.
Khor WS, Langer MF, Wong R, Zhou R, Peck F, Wong JK et al. Improving outcomes in tendon repair: a critical look at the evidence for flexor tendon repair and rehabilitation. Plast Reconstr Surg 2016; 138: 1045e–1058e.
Moriya K, Yoshizu T, Tsubokawa N, Narisawa H, Hara K, Maki Y . Outcomes of release of the entire A4 pulley after flexor tendon repairs in zone 2A followed by early active mobilization. J Hand Surg Eur Vol 2016; 41: 400–405.
Kozono N, Okada T, Takeuchi N, Hanada M, Shimoto T, Iwamoto Y . Asymmetric six-strand core sutures enhance tendon fatigue strength and the optimal asymmetry. J Hand Surg Eur Vol 2016; 41: 802–808.
Leppänen OV, Linnanmäki L, Havulinna J, Göransson H . Suture configurations and biomechanical properties of flexor tendon repairs by 16 hand surgeons in Finland. J Hand Surg Eur Vol 2016; 41: 831–837.
Gibson PD, Sobol GL, Ahmed IH . Zone II flexor tendon repairs in the United States: trends in current management. J Hand Surg Am 2017; 42: e99–e108.
Wu YF, Zhou YL, Tang JB . Relative contribution of tissue oedema and the presence of an A2 pulley to resistance to flexor tendon movement: an in vitro andin vivo study. J Hand Surg Eur Vol 2012; 37: 310–315.
Wu YF, Mao WF, Zhou YL, Wang XT, Liu PY, Tang JB . Adeno-associated virus-2-mediated TGF-β1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury. Gene Therapy 2016; 23: 167–175.
Acknowledgements
YF Wu was supported by grants from the Natural Science Foundation of China (No. 81401797). WF Mao was supported by China Postdoctoral Science Foundation Grant (No. 2016M591895). JB Tang was supported by grants from the Natural Science Foundation of China (No. 81271985).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mao, W., Wu, Y., Yang, Q. et al. Modulation of digital flexor tendon healing by vascular endothelial growth factor gene transfection in a chicken model. Gene Ther 24, 234–240 (2017). https://doi.org/10.1038/gt.2017.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.12
- Springer Nature Limited
This article is cited by
-
MOFs-Based Nitric Oxide Therapy for Tendon Regeneration
Nano-Micro Letters (2021)