Abstract
This work examines the role the lysine methyltransferase KMT1E (Setdb1) in thymocyte development. We have developed and described a T cell-specific conditional knockout of Setdb1. A partial block was seen at the double-positive to single-positive transition, causing reduced numbers of single-positive T cells in the thymus and periphery. Knockout thymocytes had reduced numbers of CD69+ and T-cell receptor TCRβ+ cells and increased numbers of apoptotic cells in the double-positive compartment, suggesting an alteration in the selection process. Transcriptional profiling of thymocytes revealed that Setdb1 deletion derepresses expression of FcγRIIb, the inhibitory Fc receptor. We demonstrate that a KMT1E-containing complex directly interacts with the FcγRIIb promoter and that histone H3 at lysine 9 tri-methylation at this promoter is dependent on Setdb1 expression. Derepression of FcγRIIb causes exacerbated signaling through the TCR complex, with specifically increased phosphorylation of ZAP70, affecting selection. This work identifies KMT1E as a novel repressor of FcγRIIb and identifies an underappreciated role of FcγRIIb in fine tuning thymocyte development.
Similar content being viewed by others
Introduction
Thymocyte development, with its well-defined sequence of lineage fate decisions, has provided an excellent model of epigenetic regulation in the adult animal. Thymocyte development follows a highly defined process wherein early T-cell progenitors enter the thymus from the bone marrow and proceed through the double-negative stage (stages DN1–4 as delineated by their expression of CD44 and CD25) and on to the CD4/CD8 double-positive (DP) and single-positive (SP) stages before exiting to the periphery. Throughout this process thymocytes gradually lose their multipotency in response to cell intrinsic and extrinsic signals, such as Notch, interleukin-7, stem cell factor (SCF) and pre-T-cell receptor (TCR) engagement, as they determine their differentiated cell state.
In addition to the transcription factors identified as controllers of thymocyte lineage choice,1 epigenetic regulators of T-cell differentiation have been described. For example, loss of the DNA methyltransferase Dnmt1 in early DN thymocytes causes significant and global changes to the numbers and function of TCRαβ cells at the DP, SP and mature T-cell stages.2 Conditional ablation of Mi-2β, a member of the Mi-2 chromatin remodeling complex, elicits a more acute defect in the DN to DP transition because of loss of activation at the CD4 locus.3 Histone modifications can fine tune transcriptional activity by marking a gene as active, inactive or silent.4 One of the most abundant and stable of these modifications is methylation of histone H3 at lysine 9 (H3K9). This residue can be mono-, di- or tri-methylated with each methylation event being associated with different biological processes.5 Whereas mono-methylation of H3K9 is associated with transcriptional activity, di- and tri-methylation of H3K9 allows for the recruitment of heterochromatin protein 1 proteins, subsequent chromatin condensation and gene silencing.6, 7
KMT1E (gene name Setdb1) is a histone methyltransferase belonging to the SET-domain family of proteins that mediates di- and tri-methylation of H3K9. By interacting with its corepressor KAP1 (Trim28), KMT1E represses gene expression in euchromatin. This is distinct from the heterochromatic silencing caused by H3K9 methylation by KMT1A (Suv39H1). Previous studies have documented a role for KMT1E in early development, propagation of ES cells, neuronal development and melanoma progression.8, 9, 10, 11 In addition to KAP1, KMT1E has been shown to exist in a complex with heterochromatin protein 1, G9A and SUV39H112 and represses gene expression during terminal differentiation.13 Recent studies have delineated distinct roles for each member of this complex except KMT1E in T-cell development. Neither SUV39H1 nor G9A appear to impact early T-cell development, yet interestingly they both modulate the expression of Th2 lineage-associated cytokines.14, 15, 16 Heterochromatin protein 1 has a role in silencing CD4 in the CD8 lineage.17 Of all the members of this complex, the scaffolding molecule KAP1 has been studied the most in thymoctye development and multiple phenotypes have been reported.18, 19, 20
As each member of this complex has subtle or contradictory differences in its role in T-cell development, we sought to investigate the role of KMT1E-mediated H3K9 tri-methylation in this process to identify potentially unique functions of this methyltransferase. By generating a T cell-specific knockout of Setdb1 (referred to as cKO), we identified a novel role for this protein in thymocyte development, as we observed significant reductions in the numbers of SP thymocytes in the thymus and periphery. This was due to a specific defect in normal selection processes as the cKO thymocytes had reduced numbers of TCRβ+/CD69+ cells and increased expression of the apoptotic marker cleaved Caspase 3. Deletion of Setdb1 resulted in a highly restricted alteration to the transcriptional profile of thymocytes. The inhibitory receptor for IgG, FcγRIIb, was revealed to be one of the most highly upregulated genes in the absence of KMT1E. Chromatin immunoprecipitation (ChIP) assays revealed both KMT1E-independent and KMT1E-dependent H3K9me3 marks on the FcγRIIb promoter. The derepression of FcγRIIb that occurs in the absence of KMT1E alters the signal strength at the level of the TCR complex, causing increased phosphorylation of ZAP70 in response to CD3 agonism and resulting in increased cell activation and cell death. This work highlights the selectivity of the H3K9 methyltransferase family and their specificity in regulating unique steps in the thymocyte development process and describes a novel role of KMT1E repression of FcγRIIb in normal generation of the T-cell lineage.
Results
Germline deletion of Setdb1 results in peri-implantation lethality at E3.5–5.5,21 making the study of its role in adult animals difficult. Transcriptional analysis of Setdb1 expression during thymocyte development revealed a peak of expression during the DN2–4 stages that waned with thymocyte maturity (Figure 1a). This data and the multifaceted roles of other H3K9 methyltransferases in thymocyte development mentioned previously prompted us to further investigate the role of KMT1E during this process. To study the potential role of KMT1E in adult tissues and in particular T-cell development, mice carrying a Setdb1 floxed allele (Setdb12lox/+)8 were crossed with mice carrying a Setdb1 null allele (Setdb11lox/+) and the Lck-Cre. Deletion of Setdb1 was mediated by Cre expressed under the control of the Lck promoter, such that deletion specifically occurs during thymocyte development, beginning at the DN stage.22 Setdb12lox/1lox|Lck-Cre (cKO) mice, as well as wild-type (WT) littermates, were identified by PCR genotyping. Setdb1 was efficiently deleted in DN and DP thymocyte populations (Figure 1b). The extent of deletion was lower at the more mature SP stage, suggesting that cells that had escaped Cre-mediated deletion were more likely to mature to this point (Figure 1b). However, western blotting confirmed almost complete loss of protein in total thymocytes from cKO mice (Figure 1c).
Characterization of the KMT1E cKO mouse. (a) RNA from sorted thymocyte populations were subjected to quantitative RT-PCR (qPCR) and Setdb1 expression is expressed as percent of Actin transcript (n=4). (b) RNA from sorted WT or cKO thymocytes (n=6) was subjected to qPCR analysis as in (a). (c) The deletion of KMT1E was confirmed by western blotting of whole-cell lysates from WT or cKO thymocytes. (d) Hematoxylin and eosin and Ki67 staining of thymus sections from WT and cKO mice (C, cortex; M, medulla) (n=3). (e) Total cellularity of thymus, lymph nodes, and spleens from WT (n=6) and cKO (n=7) mice.
The thymi of cKO mice had markedly reduced medullary space as compared with their WT littermates as seen in both hematoxylin and eosin and Ki67 staining (Figure 1d). DP cells are concentrated within the cortex of the thymus and as they mature into SP thymocytes they progress into the medulla. Reduced medullary space suggests a possible reduction in the numbers of SP cells. Deletion of Setdb1 caused reduced total cellularity in the thymus, lymph nodes, and spleen (Figure 1e). To formally address whether these changes in cellularity and morphology were due to deficits in thymocyte development, we utilized flow cytometry to analyze this more closely. Early thymocyte development can be delineated into four well-characterized sequential stages (DN1–4), based on the expression of the hyaluronic acid receptor (CD44) and the alpha chain of the interleukin-2 receptor (CD25) within the lineage-negative (CD11c−, CD11b−, TER119−, CD3−, CD8−, CD4−, B220− and CD19−) subset. Setdb1 deletion caused a modest decrease in the DN1 (LIN−/CD44+/CD25−) and DN4 (LIN−/CD44−/CD25−) stages of thymocyte development and a modest increase in the DN3 (LIN−/CD44−/CD25+) population (Figure 2a). However, these differences were not statistically significant. Significant differences were observed at later developmental stages. cKO mice had significantly reduced numbers of SP CD4 and CD8 cells in both the thymus (Figure 2b) and spleen (Figure 2c).
Loss of SP cells in cKO mice. For WT and cKO mice, flow cytometric analysis was performed to quantify the CD44 and CD25 expressing populations within the lineage-negative thymus population (DN1–4; parent refers to the lineage-negative population) (a) and CD4 and CD8 expressing thymocyte populations in the thymus (b) and spleen (c) within the live cell gate (parent; WT n=11; cKO n=13).
The reduced medullary space and SP CD4 and CD8 cell numbers prompted us to investigate possible defects in selection. CD69 is upregulated during thymocyte-positive selection in response to TCR engagement by major histocompatibility complex (MHC) molecules.23 Staining for TCRβ and CD69 on thymic CD4+/CD8+ (DP) cells indicated a significant defect in selection, as the cKO mice had reduced percentages of TCRβhigh/CD69+ cells, which represent post-selection thymocytes (Figure 3a). This decrease was also seen in TCRβhigh/CD69− and total TCRβ+ populations. We then compared the abilities of DP thymocytes with response to TCR stimulation. DP thymocytes from the cKO mice responded more strongly to CD3/CD28 signals; cKO mice had increased expression of the activation marker CD69 and increased cell death in response to CD3/CD28 agonism, suggesting a shift toward negative selection (Figure 3b). As CD69 upregulation or cell death in response to TCR agonism is a very distal readout, we sought to investigate proximal TCR signaling. TCR ligation causes the immediate phosphorylation of the mouse zeta chain-associated protein of 70 kD, ZAP70. cKO DP thymocytes were found to respond more strongly to CD3 ligation as seen by increased ZAP70 phosphorylation (Figure 3c). Not only do the cKO thymocytes respond more strongly to CD3 ligation, they also exhibit prolonged kinetics of ZAP70 phosphorylation. No differences were seen in response to H2O2 stimulation or in CD4 SP thymocytes (Supplementary Figure 2). To see if increased cell death was actually occurring in cKO thymoctyes in vivo, we assayed for the presence of the apoptosis marker cleaved caspase 3 (17/19 kDa fragment) on DP thymocytes. Indeed, this was represented in vivo, as naive DP thymocytes from cKO mice had increased numbers of cells positive for the activated caspase 3. (Figure 3d).
KMT1E cKO mice exhibit a defect in selection. (a) Flow cytometric analysis for CD69 and TCRβ shows reduced numbers of post-selection thymocytes in cKO mice (WT n=11; cKO n=13). (b) DP thymocytes from WT and cKO mice were stimulated with different doses of anti-CD3 and anti-CD28 and assayed by flow cytometry for CD69 expression and cell viability (n=6 for each group). (c) Thymocytes from WT and cKO mice were stimulated for various times with plate-bound hamster anti-mouse CD3 antibody. The left panel shows a representative image of ZAP70 phosphorylation after stimulation with 50 μg ml−1 CD3 antibody for 5 min and the right image shows the kinetics of ZAP70 phosphorylation after CD3 ligation (n=3 for each group) (*P<0.05). (d) Flow cytometric analysis for cleaved Caspase 3 within the DP thymocyte population (WT n=3; cKO n=2).
Taken together, these results demonstrate that cKO thymocytes respond more vigorously to TCR agonism than their WT counterparts. This hyper-responsiveness can be seen in both in vitro assays of proximal and distal TCR signaling, and also observed in vivo as reduced numbers of post-selection thymocytes and increased apoptotic DP cells in cKO animals.
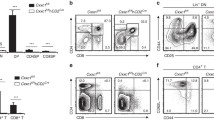
To determine the genes being repressed by KMT1E that may contribute to thymocyte development, we performed transcriptional profiling on lineage-negative cells isolated from WT and cKO mice. Setdb1 deletion resulted in very minor alterations in the transcriptional profile of the thymocytes (Figure 4a and Supplementary Table 1). The most significantly upregulated gene was the ribosomal protein paralog Rpl39L, which has been associated with Setdb1 deletion in another study and has been shown to be upregulated in different tumor subsets.24, 25 Of the genes that were significantly different between the two genotypes, FcγRIIb, the inhibitory receptor for IgG, was the only upregulated gene with known function in T-cell development.26 FcγRIIb is a negative regulatory receptor, as it carries an immunoreceptor tyrosine-based inhibitory motif, allowing it to antagonize BCR-, TCR-, and TLR-mediated signaling by recruitment of phosphatases to the activating receptor.27 Expression of FcγRIIb has been described previously on developing thymocytes28 and mature T cells and antagonism of this receptor was shown to lead to increased numbers of SP thymocytes in vivo and in fetal thymic organ cultures.29 Although the ligand for FcγRIIb during the course of thymocyte development has not been clearly identified, it is hypothesized that a non-IgG ligand is present on medullary thymic epithelial cells.29 FcγRIIb has been shown to antagonize TCR signaling by facilitating dephosphorylation of the linker of activated T cells (LAT).30 Transcriptional upregulation of FcγRIIb was observed in total thymocytes from both Setdb11lox/+ (Heterozygous) and cKO mice (data not shown; Figure 4b). Using an antibody specific to FcγRIIb, we confirmed that a small portion of DN thymocytes express FcγRIIb, and that in the absence of KMT1E this expression is maintained throughout the later stages of T cell development with the highest expression seen at the DP stage (Figure 4c and Supplementary Figure 1). FcγRIIb upregulation was also observed in SP T cells in the periphery albeit at a reduced amount. Upregulation of FcγRIIb was specific to cells of the T-cell lineage, as no differences were seen in B cell or monocyte lineages (data not shown).
KMT1E is a repressor of FcγRIIb. (a) Microarray analyses of thymocytes from WT and cKO mice showed minimal changes to the transcriptional profile; probe sets for FcγRIIb are indicated (WT n=6; cKO n=7). (b) FcγRIIb expression was determined by qRT–PCR in WT (WT n=6; cKO n=11) thymocytes. (c) Flow cytometric analyses were performed for FcγRIIb expression on the indicated thymocyte populations (WT n=11; cKO n=13). (d) ChIPs were performed with the indicated antibodies on WT and cKO thymocytes. Enrichment at the FcγRIIb promoter is represented as percent of total input DNA (representative of at least three independent experiments).
KMT1E exists in a complex that interacts with DNA to mediate transcriptional repression. ChIP experiments showed KMT1E occupancy of the FcγRIIb promoter in murine thymocytes (Figure 4d). KMT1E occupancy correlated with the presence of the repressive H3K9me3 mark at the FcγRIIb promoter, and both were significantly reduced in cKO thymoctyes. Conversely, the active H3K4me3 mark was absent on the FcγRIIb promoter in WT thymocytes, yet enriched in cKO thymocytes correlating with the derepression of FcγRIIb expression seen in various cKO thymocyte populations.
Discussion
The epigenetic control of cell fate decisions has been well established in the regulation of pluripotency seen during embryonic development. Recent studies have also emphasized the importance of epigenetic mechanisms that occur during commitment to the T cell lineage during which multipotency is gradually lost by alterations to the chromatin landscape. In agreement with these studies, this work identifies KMT1E to be an additional epigenetic regulator of thymocyte development. During the DP to SP transition, thymocytes are testing the avidity of their TCR for MHC/peptide ligands on the surface of medullary thymic epithelial cells. TCR/MHC interactions occurring with a weak affinity lead to receipt of a survival signal and subsequent positive selection and progression to CD4 or CD8 SP cells whereas nonproductive interactions lead to death by neglect. This process generates T cells with the ability to appropriately recognize MHC presented antigen in the periphery. In order to generate self-tolerant thymocytes and protect against autoimmunity, thymocytes carrying TCRs that interact with MHC/peptide ligands with too high affinity are deleted by apoptosis in the process of negative selection. Decreasing the amount of KMT1E dramatically affected the DP to SP transition, resulting in reduced numbers of SP thymocytes (Figure 2). Our data suggests that during normal thymocyte development, the inhibitory Fc receptor, FcγRIIb, is repressed by KMT1E at the DN to DP transition (Figure 1), allowing for normal selection to occur. KMT1E was shown to add the repressive H3K9me3 mark on the FcγRIIb promoter (Figure 4), demonstrating for the first time epigenetic regulation at this promoter. In the absence of KMT1E repression, we observe that the developing thymocytes have a bias towards negative selection resulting in reduced mature T cell numbers (Figure 2). Ex vivo studies showed that cKO DP thymocytes were hyper-responsive to TCR agonism, resulting in increased activation as seen by increased ZAP70 phosphorylation, CD69 upregulation, and increased cell death (Figure 3). While the presence of the cleaved forms of caspase 3 is a well-accepted marker of apoptosis, it should be noted that these forms also can occur as a result of TCR signaling in multiple T cell subsets in the absence of apoptosis, although this has not been demonstrated in DP cells.31 Based on studies describing the inhibition of TCR signaling by FcγRIIb which is dependent on its inhibition of LAT,30 we expected that we would see reduced TCR signaling. However our in vitro activation studies suggest quite the opposite. This, however, is consistent with recent work showing that LAT is a modulator of both CD3ζ and ZAP70 phosphorylation. In the absence of LAT, increased and persistent ZAP70 phosphorylation is observed.32 This suggests that in KMT1E cKO thymocytes, LAT is a likely target of FcγRIIb inhibition which results in hyperactive TCR signaling, increased apoptosis of DP T cells, and a subsequent reduction in SP T cell numbers. As mentioned previously, a question remains as to what is the relevant ligand for FcγRIIb during thymocyte development. As these studies were performed in adult animals, circulating IgG is a possible candidate, as are IgG-like molecules expressed on medullary thymic epithelial cells. Future studies will aim to further characterize the identity of FcγRIIb ligands in thymocyte development and investigate the mechanism and targets of inhibition by FcγRIIb.
Materials and methods
T-cell deletion of Setdb1
The generation of the Setdb1 conditional (Setdb12lox) and null (Setdb11lox) alleles has been previously described.8 To delete Setdb1 in the T-cell lineage, the Lck-Cre transgenic mouse (Jackson Laboratories, Bar Harbor, ME, USA) was used. Specifically, Setdb11lox/+ mice were first crossed with Lck-Cre mice and Setdb11lox/+, Cre+ mice were then crossed with Setdb12lox/+ mice to obtain Setdb12lox/1lox, Cre+ (cKO) mice. Littermates without the Lck-Cre transgene were used as WT as controls. Deletion efficiency in thymoctyes was assessed by quantitative real-time PCR. Mice were maintained under pathogen-free environment and according to the guidelines of the Novartis Institutes of Biomedical Research. All experiments in this study were approved by the Novartis IACUC.
Hematoxylin and eosin and Ki67 Staining
Paraffin tissue sections (5 μm) were deparaffinized and processed using the Ventana Discovery XT system (Ventana Medical Systems, Inc., Tuscon, AZ, USA). Briefly, sections were submitted to antigen retrieval and subsequently incubated in with the appropriately diluted primary antibody. The rabbit anti-Ki67 polyclonal antibody was applied for 1 h. After incubation, OmniMap Rabbit HRP was applied for 30 min (Ventana Medical System) followed by detection using 3,3′-diaminobenzidine as the chromogen and hematoxylin as the nuclear counterstain. Paraffin sections (5 μm) were also stained using standard hematoxylin and eosin staining procedures.
RNA isolation, reverse transcription and analysis of mRNA levels by quntitative real-time PCR
Total RNA was purified using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). For quantitative real-time PCR analysis, cDNA was reverse-transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies, Grand Island, NY, USA) as per manufacturer’s instructions. Quantitative real-time PCR reactions were performed using the Setdb1 (mm_01255836), FcγRIIb (mm_00438878) and β-Actin (4352341E) probe sets on an ABI7900 Real-Time PCR System (Applied Biosystems).
Microarray processing and data analysis
Total RNA was isolated from sorted lineage (CD3, CD4, CD8, CD11b, CD11c, Ter119, B220 and CD19) negative thymocytes using the RNeasy Mini Kit. RNA concentrations were determined using a Nanodrop ND-1000 spectrophotometer (Nanodrop Products, Wilmington, DE, USA) and RNA degradation was ruled out by quality control (on an RNA Nano Chip) using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The GeneChip IVT Express kit (Affymetrix, Santa Clara, CA, USA) was used for cDNA synthesis and in vitro transcription before hybridization to Affymetrix GeneChip Mouse Genome 430 2.0 arrays. R/Bioconductor was used to analyze microarray data (http://www.bioconductor.org/) arrayQualityMetrics was used for quality control and data were normalized using gcrma. Differentially expressed genes were calculated using moderated t-test functions in limma applying a Benjamini–Hochberg multiple hypothesis correction. Probe sets were considered significantly changed if they had an adjusted P-value <0.01 and a fold change >2 (|log2 FC|>1). Genes with at least one probeset passing these criteria were considered significantly changed between groups.
ChIP, primers and antibodies
ChIP assays were performed using 2 × 106 thymocytes per ChIP as previously described.33 Briefly, thymocytes were fixed in 1% paraformaldehyde and quenched in 0.125 m Glycine before sonication in a Bioruptor Plus (Diagenode, Denville, NJ, USA) for 30 cycles. Immunopreciptiations were performed using antibodies to KMT1E (Proteintech, Chicago, IL, USA), H3K9me3 (Active Motif, Carlsbad, CA, USA), H3K4me3 (Diagenode) or rabbit IgG (Cell Signaling Technologies, Danvers, MA, USA). Purified DNA was amplified with primers specific to the FcγRIIb promoter region (Forward: 5′-GTGGAAGGGGAGGAGCCCAGGATTA-3′; Reverse: 5′-TAGGAACGGCAGGATTCCCATCAGC-3′) using SYBR Green PCR Master Mix (Applied Biosystems) on the ViiA 7 Real-Time PCR System according to manufacturer's instructions. Primers were designed using NCBI's Primer-BLAST tool. KMT1E (Proteintech) and β-Actin (Sigma-Aldrich, St Louis, MO, USA) antibodies were used for immunoblotting.
Flow cytometry
Single-cell suspensions of thymus and spleen were subjected to red cell lysis, enumerated on a Beckman Coulter VI-Cell Viability Analyzer (Beckman Coulter, Inc., Brea, CA, USA) and then stained with combinations of fluorophore conjugated antibodies to CD3, CD4, CD8, CD11b, CD11c, Ter119, B220, CD19, CD44, CD25, FcγRIIb, CD69, TCRβ, Cleaved Caspase 3 (Asp175) and phospho-ZAP70/SYK (Y319/Y352). With the exception of Cleaved Caspase 3 (Cell Signaling Technologies) and FcγRIIb, all antibodies were from eBioscience (San Diego, CA, USA). The FcγRIIb-specific antibody has been previously described.34 The Zombie Aqua Fixable Viability dye was used to exclude dead cells from analyses (BioLegend, San Diego, CA, USA). Populations were gated based on forward and side scatter characteristics and viability. DP populations were defined by their expression of CD44 and CD25 and negative for lineage markers (CD3, CD4, CD8, CD11b, CD11c, Ter119, B220 and CD19). Data were acquired on a LSRFortessa cell analyzer (BD Bioscience, San Jose, CA, USA) and analyzed using the FlowJo X data analysis suite (FlowJO, LLC., Ashland, OR, USA). Cell sorting was performed on a FACSAria II (BD Bioscience).
In vitro activation of DP thymocytes
For prolonged stimulation, thymocytes were cultured on hamster anti-mouse CD3 antibody (clone 145-2C11) coated 96-well plates at 5 × 105 cells per well in the presence or absence of soluble hamster anti-mouse CD28 antibody (clone 37.51). Cells were stained (16 h later) for CD4, CD8, CD69 and the viability marker Zombie Aqua. Viability and CD69 expression was determined within the CD4+/CD8+ population by flow cytometry.
For acute stimulation, after resting for 3 h at 37 °C and 5% CO2, 5 × 105 thymocytes were resuspended into ice-cold 1% bovine serum albumin in phosphate-buffered saline and spun (100 g, 1 min) on to anti-CD3 coated 96-well plates containing prewarmed media and maintained at 37 °C for the duration of the stimulation. After stimulation, cells were fixed by direct addition of 4% paraformaldehyde for 10 min at 37 °C. Cells were permeabilized by incubation in ice-cold 90% methanol for 30 min before staining for CD4, CD8 and phospho-ZAP70/SYK (Y319/Y352).
Statistical analyses
All statistical analyses were performed using the GraphPad Prism 6 software suite (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between groups utilized a multiple t-test with a Holm–Sidak correction for multiple comparisons. Dose–response curves compare the LogEC50 of the nonlinear regression of the data. All the data are representative of at least two independent experiments.
References
Naito T, Tanaka H, Naoe Y, Taniuchi I . Transcriptional control of T-cell development. Int Immunol 2011; 23: 661–668.
Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 2001; 15: 763–774.
Williams CJ, Naito T, Arco PG-d, Seavitt JR, Cashman SM, De Souza B et al. The chromatin remodeler Mi-2β is required for CD4 expression and T cell development. Immunity 2004; 20: 719–733.
Wilson CB, Rowell E, Sekimata M . Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 2009; 9: 91–105.
Martin C, Zhang Y . The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 2005; 6: 838–849.
Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T . Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 2001; 410: 116–120.
Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z et al. High-Resolution Profiling of Histone Methylations in the Human Genome. Cell 2007; 129: 823–837.
Lohmann F, Loureiro J, Su H, Fang Q, Lei H, Lewis T et al. KMT1E mediated H3K9 methylation is required for the maintenance of embryonic stem cells by repressing trophectoderm differentiation. Stem Cells 2010; 28: 201–212.
Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA et al. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington's disease. Proc Natl Acad Sci 2006; 103: 19176–19181.
Dodge JE, Kang Y-K, Beppu H, Lei H, Li E . Histone H3-K9 methyltransferase ESET is essential for early development. Mol Cell Biol 2004; 24: 2478–2486.
Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 2011; 471: 513–517.
Fritsch L, Robin P, Mathieu JRR, Souidi M, Hinaux H, Rougeulle C et al. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol Cell 2010; 37: 46–56.
Ait-Si-Ali S, Guasconi V, Fritsch L, Yahi H, Sekhri R, Naguibneva I et al. A Suv39h-dependent mechanism for silencing S-phase genes in differentiating but not in cycling cells. EMBO J 2004; 23: 605–615.
Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med 2010; 207: 915–922.
Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 2012; 487: 249–253.
Thomas LR, Miyashita H, Cobb RM, Pierce S, Tachibana M, Hobeika E et al. Functional analysis of histone methyltransferase G9a in B and T lymphocytes. J Immunol 2008; 181: 485–493.
Taniuchi I, Sunshine MJ, Festenstein R, Littman DR . Evidence for distinct CD4 silencer functions at different stages of thymocyte differentiation. Mol Cell 2002; 10: 1083–1096.
Santoni de Sio FR, Barde I, Offner S, Kapopoulou A, Corsinotti A, Bojkowska K et al. KAP1 regulates gene networks controlling T-cell development and responsiveness. FASEB J 2012; 26: 4561–4575.
Zhou X-F, Yu J, Chang M, Zhang M, Zhou D, Cammas F et al. TRIM28 mediates chromatin modifications at the TCRα enhancer and regulates the development of T and natural killer T cells. Proc Natl Acad Sci 2012; 109: 20083–20088.
Chikuma S, Suita N, Okazaki I-M, Shibayama S, Honjo T . TRIM28 prevents autoinflammatory T cell development in vivo. Nat Immunol 2012; 13: 596–603.
Dodge JE, Kang YK, Beppu H, Lei H, Li E . Histone H3-K9 methyltransferase ESET is essential for early development. Mol Cell Biol 2004; 24: 2478–2486.
Wang X, Werneck MBF, Wilson BG, Kim H-J, Kluk MJ, Thom CS et al. TCR-dependent transformation of mature memory phenotype T cells in mice. J Clin Invest 2011; 121: 3834–3845.
Yamashita I, Nagata T, Tada T, Nakayama T . CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int Immunol 1993; 5: 1139–1150.
Karimi MM, Goyal P, Maksakova IA, Bilenky M, Leung D, Tang JX et al. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 2011; 8: 676–687.
Wong QW-L, Li J, Ng SR, Lim SG, Yang H, Vardy LA . RPL39L is an example of a recently evolved ribosomal protein paralog that shows highly specific tissue expression patterns and is upregulated in ESCs and HCC tumors. RNA Biol 2014; 11: 33–41.
Sandor M, Galon J, Takacs L, Tatsumi Y, Mueller AL, Sautes C et al. An alternative Fc gamma-receptor ligand: potential role in T-cell development. Proc Natl Acad Sci 1994; 91: 12857–12861.
Daëron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S et al. The same tyrosine-based inhibition motif, in the intra-cytoplasmic domain of FcγRIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity 1995; 3: 635–646.
Rodewald H-R, Moingeon P, Lucich JL, Dosiou C, Lopez P, Reinherz EL . A population of early fetal thymocytes expressing FcγRIIIII contains precursors of T lymphocytes and natural killer cells. Cell 1992; 69: 139–150.
Collazo MM, Wood D, Paraiso KHT, Lund E, Engelman RW, Le C-T et al. SHIP limits immunoregulatory capacity in the T-cell compartment. Blood 2009; 113: 2934–2944.
Jensen WA, Marschner S, Ott VL, Cambier JC . FcgammaRIIB-mediated inhibition of T-cell receptor signal transduction involves the phosphorylation of SH2-containing inositol 5-phosphatase (SHIP), dephosphorylation of the linker of activated T-cells (LAT) and inhibition of calcium mobilization. Biochem Soc Trans 2001; 29: 840–846.
Alam A, Cohen LY, Aouad S, Sékaly R-P . Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med 1999; 190: 1879–1890.
Salek M, McGowan S, Trudgian DC, Dushek O, de Wet B, Efstathiou G et al. Quantitative phosphoproteome analysis unveils LAT as a modulator of CD3ζ and ZAP-70 tyrosine phosphorylation. PLoS ONE 2013; 8: e77423.
Schoppee Bortz PD, Wamhoff BR . Chromatin Immunoprecipitation (ChIP): revisiting the efficacy of sample preparation, sonication, quantification of sheared DNA, and analysis via PCR. PLoS ONE 2011; 6: e26015.
Veri M-C, Burke S, Huang L, Li H, Gorlatov S, Tuaillon N et al. Therapeutic control of B cell activation via recruitment of Fcγ receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum 2010; 62: 1933–1943.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FJM, YX, FL, TBN, JJL and QH are employees of the Novartis Institutes of Biomedical Research.
DNC is an employee of AbbVie Inc. The remaining author declares no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Rights and permissions
About this article
Cite this article
Martin, F., Xu, Y., Lohmann, F. et al. KMT1E-mediated chromatin modifications at the FcγRIIb promoter regulate thymocyte development. Genes Immun 16, 162–169 (2015). https://doi.org/10.1038/gene.2014.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2014.70
- Springer Nature Limited