Abstract
Background/Objectives:
Different diets are used for weight loss. A Paleolithic-type diet (PD) has beneficial metabolic effects, but two of the largest iodine sources, table salt and dairy products, are excluded. The objectives of this study were to compare 24-h urinary iodine concentration (24-UIC) in subjects on PD with 24-UIC in subjects on a diet according to the Nordic Nutrition Recommendations (NNR) and to study if PD results in a higher risk of developing iodine deficiency (ID), than NNR diet.
Subjects/Methods:
A 2-year prospective randomized trial in a tertiary referral center where healthy postmenopausal overweight or obese women were randomized to either PD (n=35) or NNR diet (n=35). Dietary iodine intake, 24-UIC, 24-h urinary iodine excretion (24-UIE), free thyroxin (FT4), free triiodothyronine (FT3) and thyrotropin (TSH) were measured at baseline, 6 and 24 months. Completeness of urine sampling was monitored by para-aminobenzoic acid and salt intake by urinary sodium.
Results:
At baseline, median 24-UIC (71.0 μg/l) and 24-UIE (134.0 μg/d) were similar in the PD and NNR groups. After 6 months, 24-UIC had decreased to 36.0 μg/l (P=0.001) and 24-UIE to 77.0 μg/d (P=0.001) in the PD group; in the NNR group, levels were unaltered. FT4, TSH and FT3 were similar in both groups, except for FT3 at 6 months being lower in PD than in NNR group.
Conclusions:
A PD results in a higher risk of developing ID, than a diet according to the NNR. Therefore, we suggest iodine supplementation should be considered when on a PD.
Similar content being viewed by others
Introduction
Diets with variable macronutrient composition are used for weight loss in obesity.1 In short-term studies,2, 3 a Paleolithic-type diet (PD) has been beneficial for weight reduction and metabolic balance. Positive long-standing effects on fat mass, abdominal obesity and triglyceride levels are also reported.4 However, there is concern whether iodine level remains adequate after long-term use of a PD.
The PD is composed of ingredients consumed by humans before the establishment of agriculture. Therefore, grains, legumes, dairy products, refined sugar, processed oils and salt are excluded.5 In Sweden, >50% of the iodine intake is supplied by iodinated table salt,6 with dairy products and sea food as additional important iodine sources.6 According to the Nordic Nutrition Recommendations (NNR), 150 μg iodine/day is recommended for adults. However, an intake as low as 70 μg iodine/day is the lowest intake level that the thyroid can compensate for, corresponding to a median urinary iodine concentration (UIC) of 35–40 μg/l.7
Iodine deficiency (ID) is one of the most common nutritional disorders worldwide, and the most common cause of goiter8 linked to thyroid dysfunction. Moderate to severe ID results in hypothyroidism with increased conversion of free thyroxine (FT4) to free triiodothyronine (FT3).9 In mild ID, FT4 and thyreotropin (TSH) are unaffected,10 whereas, multi-nodular goiter is common in all levels of ID.11
In Sweden, ID and goiter were widespread at the beginning of the twentieth century.12, 13 National iodization of table salt with 10 mg potassium iodine/kg salt was introduced in 1936. In 1966, goiter was still evident in some areas; therefore, iodization was increased to 50 mg potassium iodine/kg.12 In 2007, the Swedish population was declared iodine sufficient, with median UIC of 125 μg/l.14, 15
UIC from spot urine is the recommended method for determining the iodine status within a population.8 A median UIC of 100–199 μg/l in school children indicates adequate iodine nutrition: mild ID is defined as UIC 50–99 μg/l, moderate ID as 20–49 μg/l and severe ID as <20 μg/l.16 However, the best way of determining iodine status is by calculating the 24-h urinary iodine excretion (24-UIE)17 over multiple days, as daily iodine intake varies.18 Groups at risk of ID can be found in an otherwise iodine sufficient population: these include, pregnant and lactating women with an increased iodine demand,19, 20 and vegans10 because of low iodine intake.
The hypothesis was that PD results in a higher risk of developing ID, than a diet according to the NNR. As there is a lack of data on PD and iodine nutrition, the aim of this trial was to compare 24-UIC in subjects on PD with 24-UIC in subjects on a diet according to the NNR, through data from a 2-year randomized controlled trial.4
Subjects and methods
Study design
A randomized controlled trial was performed August 2007–March 2010 in a tertiary referral center, as described previously.4 At inclusion, participants underwent a clinical investigation and baseline tests, and were randomized to either a PD or a NNR diet. All study employees (except the dieticians) were blinded to the randomization.
The PD aimed at providing 30 energy percent (E%) protein, 40 E% fat and 30 E% carbohydrates. Lean meat, fish and sea food, fruit, vegetables, root vegetables, eggs and nuts were included in the PD, whereas, dairy products, cereals, beans, refined fats and sugar, added salt, bakery products and soft drinks were excluded. Processed, canned and preserved food, in addition to manufactured and semi-manufactured goods were avoided. The NNR diet aimed for an intake of 15 E% protein, 25–30 E% fat and 55–60 E% carbohydrates; that is, lower protein and fat content and higher carbohydrate content than in the PD. The diets were consumed ad libitum during the 2-year study. Each group participated in 12 group sessions with information on the intervention diets and cooking practices. Dietary intake was assessed by self-reported food records, and urine- and blood samples were collected at baseline, and after 6 and 24 months. In this study, data from dietary records, 24-UIC, 24-UIE and thyroid hormones at baseline and after 6 and 24 months were used.
Study participants
Postmenopausal women with a body mass index ⩾27 kg/m2 were recruited through advertisements in local newspapers in Umeå, Sweden. Informed consent was obtained from all subjects. The subjects had no history of heart or kidney disease, osteoporosis, diabetes mellitus, hyper- or hypothyroidism, and had normal plasma glucose levels, and blood pressure ⩽150/90 mmHg. They were not taking estrogens, statins, beta-blockers or psychiatric medications. Subjects who consumed a restricted or a vegetarian diet or who reported allergy to key components in the intervention diets were excluded. Of the 210 subjects assessed for eligibility, 140 were unsuitable, leaving 70 women available for inclusion.
The PD (n=35) and NNR (n=35) groups did not differ in baseline characteristics (Table 1). The women had an average age of 60 years, an average body mass index of 33 kg/m2, and 74% were obese. The dietary interventions resulted in a reduction in body weight, waist circumference and fat mass in both groups.4 The PD-group decreased more in weight (mean 8.6 kg (9.9% from baseline)) during the first 6 months than the NNR-group (4.7 kg (5.4% from baseline; P=0.001)). After 24 months, the total weight loss from baseline was 10.7% in the PD-group and 7.7% in the NNR-group (P=0.08).
As reported previously,4 the PD-group had higher protein and fat intake and lower carbohydrate intake than the NNR-group (P<0.001) (Table 1). The reported energy intake decreased similarly in both groups from baseline to 6 and 24 months.
Ethics
The protocol was in accordance with the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Umeå University, Sweden.
Methods
24-h urinary collections
Urine was collected on 3 separate days for each occasion: at baseline, after 6- and 24-months. The subjects took 3 × 80 mg tablets of para-aminobenzoic acid before every 24-h collection and returned a questionnaire about the sampling.
Urinary iodine measurements
The Sandell–Kolthoff reaction was used for the analyzes of 24-UIC.21 Samples were measured in duplicate and were reanalyzed if the difference was >2% in absorbance. For calculating 24-UIE, the 24-UIC value was multiplied by the urine volume.
Thyroid hormone measurements
Morning serum samples were drawn in fasting state and stored at −80 °C until analyses. Serum levels of TSH (normal range 0.27–4.2 mIE/l coefficient of variation (CV) 5%), FT4 (normal range 12–22 pmol/l, CV 4%) and FT3 (normal range <6.8 pmol/l, CV 7%) were evaluated by immunometric methods with Roche Modular/Cobas (Rotkreuz, Switzerland).
24-h urinary sodium measurements (24U-Na)
24U-Na was analyzed by an indirect method (Roche/Cobas, Rotkreuz, Switzerland) with an ion selective membrane electrode and an internal control of sodium.
Statistical measurements
All data are presented as medians and interquartile range (IQR). Data following the Gaussian distribution were evaluated with parametric tests, and non-normally distributed data were analyzed with non-parametric tests. One-way repeated measurements ANOVA and the non-parametric Friedman Test were used to analyze differences over time for related data (that is, longitudinal data). Unpaired two-sample Student’s t-test and independent samples Mann–Whitney U test were used to analyze cross-sectional differences between PD and NNR. The percentage of individuals with 24-UIC <50, <100 or >200 μg/l were tested with the χ2 test. In normally distributed data, associations were tested with Pearson’s correlation test: non-normal distribution was tested with Spearman’s correlation test. IBM SPSS Statistics 22 was used for statistical analyzes (SPSS Institute, Chicago, IL, USA). Variance between groups of data were analyzed with Levene’s Test of Equality of Variances. In cases where variances were unequal the SPSS incorporated function of adjusting the t-statistic to the degrees of freedom using the Welch–Satterthwaite method were employed. Statistical significance was set as P<0.05.
Results
Forty-nine subjects fulfilled the protocol (NNR n=22, PD n=27). The drop-out rate was 30% (n=21): 9 subjects (NNR n=8, PD n=1) discontinued intervention at 6 months, and 12 subjects (NNR n=5, PD n=7) at 24 months. The reasons for discontinuing were lack of time, family reasons, disease, disappointment with allocation and loss of motivation.4
Intake of iodine containing foods
After 6 months, subjects on PD had doubled reported intake of foods containing fish, sea food, spawn and caviar from 46.1 to 97.1 g/day. Simultaneously, the PD group reduced intake of dairy products from 269 to 7.4 g/day and cheese from 28.9 to 1.6 g/day. Other foods were also reduced in the PD group after 6 months: bread from 99.2 to 5.8 g/day, cereals from 68.8 to 0.64 g/day and ice cream from 12.5 to 0.68 g/day. The changed dietary pattern was sustained at 24 months (data not shown). In the NNR-group, the intake of iodine containing foods remained constant (data not shown).
Urinary collection
P ara-aminobenzoic acid was available for 94% of the urinary collections (n=501). Of those, 58% were considered complete and 28% were adjusted with the linear regression equation method.
24-h urinary iodine concentration and excretion
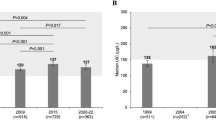
At baseline, overall median 24-UIC was 71.0 (IQR: 46.0) μg/l: 14.9% of subjects had 24-UIC <50 μg/l, 61.2% had 50–100 μg/l, 22.4% had 100–200 μg/l and 1.5% had >200 μgl, with no difference between groups. In the PD-group, median 24-UIC was 36.0 (IQR: 36.0) μg/l at 6 months and 57.0 (IQR: 32.0) μg/l at 24 months, which was lower than at baseline (68.0 (IQR:37.0) μg/l) and lower than the NNR-group. In the NNR-group 24-UIC remained unchanged throughout the study (Figure 1a).
Box-plots of (a) 24-h urinary iodine concentration (24-UIC) and (b) 24-h urinary iodine excretion (24-UIE) for all participants at baseline, 6- and 24-months with a Paleolithic diet (PD) or a diet according to the Nordic Nutrition Recommendations (NNR). P-values from longitudinal and cross-sectional analyses presented. o: mild outlier; *: extreme outlier.
At baseline, the median 24-UIE was 136.0 (IQR: 93.0) μg/day in all study participants. In the PD-group, median 24-UIE was 77.0 (IQR: 65.0) μg/l at 6 months and 113.5 (IQR: 79.0) μg/l at 24 months: 24-UIE in the PD-group was lower at 6 months than at baseline (124.5 (IQR: 88.0) μg/l), and was lower at both 6- group and 24-months group than in the NNR-group group. In the NNR-group, 24-UIE remained unchanged throughout the study (Figure 1b).
At 6 months, more participants on PD had 24-UIC <50 μg/l and fewer had 100–200 μg/l than participants on the NNR diet (Figure 2).
Distribution of 24-h urinary iodine concentration (24-UIC) at baseline and at 6 months for women on Paleolithic diet (PD) or a diet according to the Nordic Nutrition Recommendations (NNR). P-values from the cross-sectional analysis (#P) at 6 months and from the longitudinal analysis (§P) in the PD group are presented, when significant.
In the subgroup of subjects with higher adherence to study protocol, that is, those who had left urine samples at both baseline and 6 months, cross-sectional analyzes of 24-UIC and 24-UIE rendered the same results (data not shown).
Thyroid hormone evaluation
TSH, FT4 and FT3 levels did not differ between groups at any time except for FT3 at 6 months, which was lower in the PD group. In longitudinal analyzes, TSH and FT4 increased in PD group, whereas, NNR group remained unaltered. The FT3/FT4 ratios were lower in the PD than in the NNR group at both 6 months (P=0.008) and 24 months (P=0.010). We have not performed any statistical analysis on thyroid dysfunction (hyper- or hypo-thyroidism) because of the low number of abnormal values, and especially given the absence of group differences in thyroid hormone levels.
Urinary sodium excretion
On all occasions, both groups had similar 24U-Na. In the PD-group, median 24U-Na was 173.0 (IQR: 69.3) mmol/day at baseline, 145.5 (IQR: 68.5) mmol/day at 6 months and 36.5 (IQR: 89.8) mmol/day at 24 months. In the NNR-group, median 24U-Na was 156.0 (IQR: 62.0) mmol/day at baseline, 166.0 (IQR: 70.0) mmol/day at 6 months and 145.0 (IQR: 28.0) mmol/day at 24 months. In the PD-group, 24U-Na decreased from baseline to 6 months (P=0.003), but not from 6 to 24 months and was unaltered in the NNR-group during the whole study. In PD-group, a positive correlation was observed between 24-UIE and 24U-Na on all occasions (Figures 3a–c).
Discussion
In this study it is for the first time demonstrated that long-term use of a PD is associated with the development of mild ID, whereas a normal iodine status is maintained while on the NNR diet. This occurred during ad libitum intake of both diets and concurrent weight reduction.
Before the start of the study, the subjects were considered iodine sufficient, despite a 24-UIC within 50–99 μg/l, the level defined as mild ID in school children.16 The studies confirming the Swedish population as being iodine sufficient, with a spot UIC 125 μg/l, were performed on school children;14, 15 however, reference levels for adults are not well established and 24-UIC is strongly dependent on urinary volume. Therefore, a 24-UIC of 81.7 μg/l, as in this study, predicts a 24-UIE of 151 μg/day, which is within the average Swedish UIE of 150–200 μg/day.22
At 6 months of intervention, both 24-UIC and 24-UIE were consistent with mild ID in subjects eating the PD. A median 24-UIC of 36 μg/l, as in the PD-group, corresponds to a daily iodine intake of about 70 μg, which is considered the lowest recommended level for adults.23 This implied the PD diet could be included in the group of diets that might cause ID. Median UIC for vegans is 20 μg/l in the UK20 and 78.5 μg/l in the US.10 However, in the UK, salt is not iodized and in the US study, 20% of the participants used iodine supplementation and ~75% did not use iodinated salt. This illustrated the importance of compensating for the lack of major iodine sources when such diets are chosen.
At 24 months, 24-UIC and 24-UIE increased in the PD-group, compared with at 6 months, probably reflecting commonly occurring difficulties of maintaining a long-term diet.24
At 6 months, thyroid function hormones in the PD group were unaltered compared to baseline, although iodine was at its lowest level, which was consistent with mild ID. Thyroid function hormones in mild ID are not affected in vegans and vegetarians with a large difference in spot UIC but similar FT4 and TSH.10 Conversely, lower FT4 and higher TSH are expected in moderate ID, which was not observed in this study. In general, ID results in an increased FT3/FT4 ratio, which maintains the level of active hormone. In this study, the FT3/FT4 ratio was lower in the PD group, which is difficult to interpret, as caloric restriction lowers FT3.25 However, there were no changes in FT4 and TSH consistent with moderate ID; so the PD group was defined as mild ID at 6 months.
A possible adverse health effect of a PD is valid for all countries, regardless of table salt iodization programs. The PD group was recommended to refrain from manufactured products and restrict table salt consumption. In Sweden, most consumed table salt is iodinated, whereas, the iodization of manufactured and semi-manufactured products can vary, because the iodization of salt is voluntary. As salt intake, estimated by 24U-Na, did not differ between groups, salt ingestion in the PD group must have been higher than recommended in the study, probably due to the salt content not always being obvious to consumers. Despite the positive correlation between 24-UIE and 24U-Na in the PD-group suggesting some ingested salt was iodized, the PD group developed mild ID. In countries without an iodization program, PD may lead to more apparent ID than the mild form detected in this study.
The low iodine content in a PD may have adverse effects, especially if the diet is used by fertile women. In adults, mild and moderate ID may result in toxic multi-nodular goiter.11 Although this was not observed in this study, a longer follow-up is needed. During pregnancy and lactation, the need for iodine is higher,19, 26 and ID may impair cognitive development of the fetus27 and of the growing child.28 We suggest pregnant women avoid PD.
This is the first study investigating the consequences of PD on iodine nutrition. A major strength was increased precision with the use of 24-h collections, the use of p ara-aminobenzoic acid to exclude insufficient samplings, and the calculation of 24-UIE. As the sample size was small, these strengths were particularly important. It is argued that 125 spot UIC samples are needed for proper precision,29 however, the collection method used considerably increased the precision. One weakness may be the 30% drop-out frequency during this 2-year trial. However, drop-out was only 12.9% after 6 months when the most pronounced effect on iodine nutrition was observed. If there is lack of motivation, being on a diet is difficult and it is better to drop-out rather than be incoherent with the diet regime, which may affect the results. Therefore, we consider the differences in iodine levels between the PD and NNR groups were true effects of the dietary changes.
In summary, diets used for reducing obesity may have adverse effects that are not immediately recognized. The iodine issue is an ongoing concern, as is exemplified by the reoccurrence of ID in the UK (31), and awareness of new risk groups is necessary. In this study, the low 24-UIE in subjects on PD confirms for the first time the hypothesis that a PD results in a higher risk of developing ID, than a diet according to the NNR. Therefore, we suggest iodine supplementation should be considered when on a PD.
References
Acheson KJ . Diets for body weight control and health: the potential of changing the macronutrient composition. Eur J Clin Nutr 2013; 67: 462–466.
Lindeberg S . Paleolithic diets as a model for prevention and treatment of Western disease. Am J Hum Biol 2012; 24: 110–115.
Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C et al. A Palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med 2013; 274: 67–76.
Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr 2014; 68: 350–357.
Klonoff DC . The beneficial effects of a Paleolithic diet on type 2 diabetes and other risk factors for cardiovascular disease. J Diabetes Sci Technol 2009; 3: 1229–1232.
National Food AdministrationRiksmaten 1997–1998 Kostvanor och näringsintag i Sverige (The Second National Food Consumption Survey Dietary Habits and Nutrient Intake in Sweden 1997–98). National Food Administration: Uppsala, Sweden, 1998.
Nordic Council of MinistersNordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. Nordic Council of Minsters: Copenhagen, Denmark, 2014..
Vanderpas J . Nutritional epidemiology and thyroid hormone metabolism. Annu Rev Nutr 2006; 26: 293–322.
Leung AM, Lamar A, He X, Braverman LE, Pearce EN . Iodine status and thyroid function of Boston-area vegetarians and vegans. J Clin Endocrinol Metab 2011; 96: E1303–E1307.
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 2010; 24: 13–27.
Nystrom HF, Berg G, Eggertsen R, Hulthen L, Milakovic M . Swedish iodination of salt has decreased the incidence of goiter. But low use of iodinated salt in the schools raises concerns for the future. Lakartidningen 2012; 109: 90–93.
Höjer J . Kropfstudien Die Verbreitung des endemischen Kropfes in Schweden. Svenska Läkarsällskapets Handlingar 1931; 57: 1–104.
Andersson M, Berg G, Eggertsen R, Filipsson H, Gramatkovski E, Hansson M et al. Adequate iodine nutrition in Sweden: a cross-sectional national study of urinary iodine concentration in school-age children. Eur J Clin Nutr 2009; 63: 828–834.
Filipsson Nyström H, Andersson M, Berg G, Eggertsen R, Gramatkowski E, Hansson M et al. Thyroid volume in Swedish school children: a national, stratified, population-based survey. Eur J Clin Nutr 2010; 64: 1289–1295.
World Health Organization, United Nations Children's Fund and International Council for the Control of Iodine Deficiency DisordersAssessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. 3rd edn. World Health Organization: Geneva, Switzerland, 2007.
Andersson M, Karumbunathan V, Zimmermann MB . Global iodine status in 2011 and trends over the past decade. J Nutr 2012; 142: 744.
Hetzel BS, Dunn JT . The iodine deficiency disorders: their nature and prevention. Annu Rev Nutr 1989; 9: 21–38.
Rasmussen LB, Ovesen L, Christiansen E . Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr 1999; 53: 401–407.
Zimmermann MB . The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid 2007; 17: 829–835.
Lightowler HJ, Jill Davies G . Iodine intake and iodine deficiency in vegans as assessed by the duplicate-portion technique and urinary iodine excretion. Br J Nutr 1998; 80: 529–535.
Pino S, Fang SL, Braverman LE . Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem 1996; 42: 239–243.
Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA . Iodine status, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr 1998; 52: 351–355.
Becker WBK, Ohlander Å, Lyhne EM, Pedersen N, Aro AN, Fogelholm A et al JI Nordic Nutrition Recommendations 2004. 4th ed. Nordic Council of Ministers: Copenhagen, Denmark, 2004..
Dombrowski SU, Avenell A, Sniehott FF . Behavioural interventions for obese adults with additional risk factors for morbidity: Systematic review of effects on behaviour, weight and disease risk factors. Obesity Facts 2010; 3: 377–396.
Yarrington C, Pearce EN . Iodine and pregnancy. J Thyroid Res 2011; 2011: 934104.
Velasco I, Carreira M, Santiago P, Muela JA, Garcia-Fuentes E, Sanchez-Munoz B et al. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab 2009; 94: 3234–3241.
Zimmermann MB, Connolly K, Bozo M, Bridson J, Rohner F, Grimci L . Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: a randomized, controlled, double-blind study. Am J Clin Nutr 2006; 83: 108–114.
Andersen S, Karmisholt J, Pedersen KM, Laurberg P . Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 2008; 99: 813–818.
Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K et al. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet 2011; 377: 2007–2012.
Acknowledgements
Thanks to Elisabeth Gramatkovski for invaluable help with the iodine analyzes and to Michael Hoppe for the statistical analyzes. Inger Arnesjö, Katarina Iselid and Monica Holmgren contributed with important technical assistance. Johanna Larsson helped process food records. This study was supported by grants from The Swedish Council for Working Life and Social Research (2006-0699 and 2010-0398), the Swedish Research Council (K2011-12237-15-6), the Swedish Heart and Lung Foundation, the County Council of Västerbotten and Umeå University, Sweden, and the LUA/Alf Agreement in the County Council of Västra Götaland, Sweden. Registration number at clinicaltrials.gov: NCT00692536.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Manousou, S., Stål, M., Larsson, C. et al. A Paleolithic-type diet results in iodine deficiency: a 2-year randomized trial in postmenopausal obese women. Eur J Clin Nutr 72, 124–129 (2018). https://doi.org/10.1038/ejcn.2017.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.134
- Springer Nature Limited
This article is cited by
-
Thyroid function and iodine intake: global recommendations and relevant dietary trends
Nature Reviews Endocrinology (2024)
-
The effect of sodium restriction on iodine prophylaxis: a review
Journal of Endocrinological Investigation (2022)
-
Carbohydrate knowledge, dietary guideline awareness, motivations and beliefs underlying low-carbohydrate dietary behaviours
Scientific Reports (2020)
-
Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations
European Journal of Nutrition (2020)