Abstract
Background:
Resistance training (RT) has selective effects on body composition that may counteract the deleterious effects of aging. Changes in phase angle (PhA) may serve to monitor the influence of RT in older people.
Objectives:
To describe the effect of RT in training, detraining and retraining on body composition, including PhA in older women.
Subjects/methods:
Thirty-three older women (⩾60 years old) participated. The RT program was carried out over 12 weeks for each stage (training, detraining and retraining). Whole-body fat-free mass and fat mass (FM) and appendicular lean soft tissue (ALST) measurements were carried out using a dual energy X-ray absorptiometry. Bioimpedance spectroscopy was used to estimate total body water (TBW), intra (ICF) and extracellular (ECF) fluids, whole-body resistance (R) and reactance (Xc) and PhA. Upper and lower body muscle strength were also assessed.
Results:
From baseline to after training muscle strength, ALST and PhA significantly (P<0.05) increased. In detraining, significant (P<0.05) reductions in muscle strength, TBW, ECF, ICF and PhA along with significant (P<0.05) increases in R were observed, with the greatest magnitude observed for PhA (Δ=−7.6%). From detraining to retraining a significant reduction in FM along with increases in Xc, PhA and muscle strength were observed. Although an increase was observed from detraining to retraining in PhA, the values were still lower than baseline PhA.
Conclusions:
In untrained older women, a RT is associated with increases in PhA, whereas detraining results in a marked decrease in PhA, and more time may be required in retraining to counteract the negative influence of absence of exercise stimulus.
Similar content being viewed by others
Introduction
Resistance training (RT) is considered a non-pharmacological intervention with important implications for the health of older adults.1, 2 Among the main benefits of RT are included maintenance and/or increased muscle strength and skeletal muscle mass.3, 4, 5 Additionally, reduction of subcutaneous and ectopic fat, and improvements in physical independence have also been associated with RT.2, 3 Monitoring of changes induced by RT is a key factor for the control and maintenance of the effectiveness of interventions.
The bioelectrical impedance analysis is commonly used to monitor changes in hydration and body composition induced by training due to its speed of use, safety and non-invasiveness.6, 7 Phase angle (PhA) has been suggested as a variable of interest from bioelectrical impedance analysis given that it is independent of body height and weight, it is easily calculated from the directly measured resistance (R) and reactance (Xc), and it is associated with the membrane structure and function and it is an indicator of tissue hydration and nutritional status.8
In older adults, PhA has been set as a predictive indicator for sarcopenia,9, 10 muscular function,11 fragility and risk of mortality,12, 13, 14 and it has also been suggested to monitor changes related to comorbidities.12, 15, 16 The PhA is normally higher in males than females due to the amount of muscle mass, and its value tends to be reduced in each decade of life, decreasing from 6.7 to 4.9 in males and from 6.0 to 5.2 in females from 65 to >90 years old. Such changes reflect alterations in hydration, body composition and cellular health15 that occur with aging.
Despite the growing number of studies that use PhA as an indicator of body composition, the cross-sectional design has prevailed and only one study to date found the influence of exercise on the R and Xc in a very specific population. Additionally, to our knowledge, no research has been conducted to understand the PhA responses after intervention with RT combining multiple time points with periods of training, detraining and retraining in older ages.
Considering the potential role of PhA and the possible effects of exercise on body composition in older adults, the present study aimed to describe the effect of RT in training, detraining and retraining on PhA and body composition in older women.
Materials and methods
Design and participants
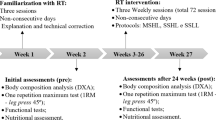
Thirty-three older women (⩾60 years old) volunteered to participate in this study. Recruitment was carried out through newspaper and radio advertisings and home delivery of leaflets in the central area and residential neighborhoods. All participants completed health history and physical activity questionnaires and met the following inclusion criteria: Non-hypertensive (systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg), non-diabetic, free from cardiac or renal dysfunction, nonsmokers, not receiving hormonal replacement therapy, and were not performing any regular physical exercise for more than once a week over the 6 months preceding the beginning of the study. Participants performed a diagnostic graded exercise stress test with 12-lead electrocardiogram reviewed under the supervision of a cardiologist and were released with no restrictions for participation in this study. The study was carried out over a total period of 44 weeks: 12 weeks of a RT program (weeks 3–14), 12 weeks of detraining (weeks 17–28), in detraining volunteers have been instructed to keep the usual physical activity levels and avoid performing systematic exercise, 12 weeks of retraining (weeks 31–42) a RT program and 8 weeks for measurements (weeks 1–2, 15–16, 28–29 and 43–44), muscular strength, dietary intake, anthropometry, muscle strength and body composition measurements were performed. Adherence to the program was high, with all the subjects participating in >85% of the total sessions. Written informed consent was obtained from all the participants after a detailed description of the study procedures was provided. This investigation was conducted according to the Declaration of Helsinki and was approved by the local University Ethics Committee.
Dietary intake
Participants were instructed by a dietitian to complete a food record on three nonconsecutive days (two weekdays and one weekend day). Participants were given specific instructions regarding the recording of portion sizes and quantities to identify all food and fluid intake, in addition to viewing food models in order to enhance precision. Total energy intake, proteins, carbohydrates and lipids were calculated using nutrition analysis software (Avanutri Processor Nutrition Software, Rio de Janeiro, Brazil; Version 3.1.4). All participants were asked to maintain their normal diet during the entire study period. Water ingestion was ad libitum.
Anthropometry
Body mass was measured to the nearest 0.1 kg using a calibrated electronic scale (Filizola, model ID 110, São Paulo, Brazil), with the participants wearing light workout clothing and no shoes. Height was measured with a wooden stadiometer to the nearest 0.1 cm with subjects standing without shoes. Body mass index (BMI) was calculated and classified according to the World Health Organization criteria.17
Body composition
Whole-body fat-free mass and fat mass (FM) and appendicular lean soft tissue (ALST) measurements were carried out using a dual energy X-ray absorptiometry scan (Lunar Prodigy, model NRL 41990, GE Lunar, Madison, WI, USA). Before being scanned, participants were instructed to remove all objects containing metal. Scans were performed with the subjects lying in the supine position along the table’s longitudinal centerline axis. Feet were taped secured together at the toes in order to immobilize the legs while the hands were maintained in a pronated position within the scanning region. Participants remained motionless during the entire scanning procedure. Both calibration and analysis were carried out by a skilled laboratory technician. The equipment calibration followed the manufacturer’s recommendations. The software (Lunar Prodigy DXA System software v.9.3, Madison, WI, USA) generated standard lines that set apart the limbs from the trunk and head. These lines were adjusted by the same technician using specific anatomical points determined by the manufacturer. Analyzes during the intervention were performed by the same technician, who was blinded to intervention time point. Previous test-retest scans of eight older women resulted in an standard error of measurement (SEM) of 0.90 kg and intraclass correlation coefficient (ICC) >0.98 for % FM, and an SEM of 0.60 kg and ICC >0.97 for fat-free mass and SEM of 0.40 kg and ICC= 0.99 for ALST.
Bioimpedance spectroscopy
A phase-sensitive bioimpedance spectroscopy (Xitron Hydra, model 4200, Xitron Technologies, San Diego, CA, USA) was used to obtain whole-body resistance (R) and reactance (Xc) at a single frequency (50 kHz). PhA was calculated taking the arc tangent value of the ratio of Xc versus R. Bioelectrical impedance vector analysis was performed against the Italian reference population,18 total body water (TBW) and intracellular (ICF) and extracellular (ECF) fluids were obtained by the bioimpedance spectroscopy commercial software.
Before each test, the analyzer was calibrated by measuring, modeling and computing volume on a provided verification module. The calibration test result is based on the default ECF and ICF resistivity coefficients. The calibration is deemed successful if, when introducing data for a male with height and weight equal to 175 cm, and 70 kg, respectively, the final result obtained in the verification module is between 15.81 and 16.18 liters for ECF, and between 29.39 and 30.74 for ICF.
Participants were instructed to lie in a supine position for about 10 min (serving as an equilibration period). After cleaning the skin with alcohol, four electrodes were positioned on the surface of the right hand and right foot, according to conventional procedures established in the literature. SEM of 0.32 l and ICC=0.98 for ECF, SEM of 0.19 l and ICC=0.99 for ICF and SEM of 0.38 l and ICC=0.98 for TBW, SEM of 15.6 ohms and ICC=0.95 for R, SEM of 3.5 ohms and ICC=0.96 for Xc, SEM of 0.21 degrees and ICC= 0.96 for PhA.
Muscle strength
Maximal dynamic strength was evaluated using the 1 repetition maximum (RM) test assessed on chest press and leg extension performed in this exact order. Testing for each exercise was preceded by a warm-up set (6–10 repetitions), with ~50% of the estimated load used in the first attempt of the 1RM test. This warm-up was also used to familiarize the participants with the testing equipment and lifting technique. The testing procedure was initiated 2 min after the warm-up. Participants were oriented to try to accomplish two repetitions with the imposed load in three attempts in both exercises. The rest period was 3–5 min between each attempt, and 5 min between exercises. The 1RM was recorded as the last resistance lifted in which the subject was able to complete only one single maximal execution.19 Execution technique for each exercise was standardized and continuously monitored to ensure reliability. All 1RM testing sessions were supervised by two experienced researchers for greater safety and integrity of the participants. Verbal encouragement was given throughout each test. Three 1RM sessions were performed separated by 48 h (ICC 0.96). The highest load achieved among the three sessions was used for analysis in each exercise.
RT program
The supervised RT was carried out over 12 weeks in the training period, and again over 12 weeks in the retraining stage. Training took place in the morning and was based on recommendations for RT in elderly population to improve muscular endurance and strength.20, 21 Exercise professionals personally supervised all participants with substantial experience in RT prescription throughout each training session in order to reduce deviations from the study protocol and to ensure participant’s safety. Participants performed RT using a combination of free weights and machines.
The RT protocol consisted of a whole-body program with three sets of eight exercises that were performed in the following order: chest press, horizontal leg press, seated row, knee extension, preacher curl, leg curl, triceps pushdown and seated calf raise. Participants performed between 10–15 RM for each set. The participants were instructed to inspire during the eccentric phase and exhale during the concentric phase of the exercise and to maintain the speed of movements at a ratio of 1:2 (concentric and eccentric phases, respectively). The rest interval ranged between 60–120 s for sets and exercises. The instructor adjusted loads of each exercise according to the participant’s ability and improvements in exercise capacity throughout the study in order ensure that participants were using as much resistance as possible while maintaining proper technique. Progression was planned so that when 15 repetitions were completed for two consecutive sessions, the load was increased 2–5% for the upper limb exercises and 5–10% for lower limb exercises.20 At the end of each session, ~5 min were provided for stretching the exercised muscles. Each training session lasted about 40 min.
Statistical analysis
Data is presented as mean±s.d. for all outcome variables. Normality was verified with the Shapiro–Wilk’s test, if normality was not achieved a logarithmic function was used (log10) was used. Repeated measures analysis of variance were used to determine the overall difference (main-effects) in the dependent variables across the intervention (baseline-training-detraining-retraining) and the Fisher's least significant difference post-hoc was used to test for differences between the two time points (simple effects). Analyzes were adjusted for age and number of sessions attended. All analyzes were computed using the IBM-SPSS Statistics version 22.0 (IBM, Chicago, IL, USA). Statistical significance was set at P<0.05 for all analysis.
Results
The sample consisted of 33 elderly women with a mean±s.d. age of 68.7±5.7 years old, baseline weight of 67.3±13.8 kg and height corresponding to 155.8±6.9 cm. The majority of the participants (63.7%) were overweight (25 kg/m2<BMI<30 kg/m2; 36.4%) or obese (BMI ⩾30 kg/m2; 27.3%). All other participants (36.3%) were normal weighted (18.5 kg/m2<BMI⩽24.9 kg/m2).
Participants’ body composition and dietary intake variables for the four assessments (baseline; training: 12 weeks; detraining: 12 weeks; and retraining: 12 weeks) are presented in Table 1.
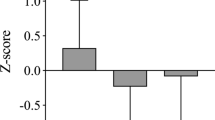
From baseline to after training assessments increases in ALST (Δ=5.3%), PhA (Δ=3.5%), and strength (bench press and leg extension) occurred, along with an increase in the ECF/ICF ratio (Δ=−3.5%). In detraining, reductions were observed in TBW (Δ=−4.2%) and its compartments (ICF (Δ=−3.4%) and ECF (Δ=−4.8%)), PhA (Δ=−7.6%) and strength (bench press and leg extension) along with increases R (Δ=6.9%) and ECF/ICF ratio (Δ=5.1%). As illustrated in Figure 1, the greatest relative change in detraining was observed for PhA (Δ=7.6%). In the retraining, a reduction was observed for FM while Xc (Δ=3.3%), PhA (2.9%) and muscle strength increased. No changes were observed on macronutrients and energy intake over time (P>0.05).
Mean percent changes in body composition after 44 weeks of intervention (Graph A—12-weeks training; Graph B—12-weeks detraining and Graph C–12-weeks retraining). *significant change (P<0.05). Results are presented as means and standard error. FM, fat mass; FFM, fat-free mass; ALST, apendicular lean soft tissue; TBW, total body water; ECF, extracellular fluids; ICF, intracellular fluids; E/I; ECF/ICF ratio; R, resistance; Xc, reactance; PhA, phase angle.
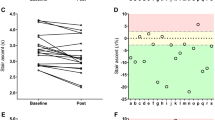
Figure 2 illustrates the individual change (%) in PhA for training, detraining and retraining.
In order to understand how PhA components (R and Xc) changed during the intervention, the sample was split according to PhA changes in participants that decreased PhA and in participants that increased PhA. In Figure 3 are plotted R and Xc by PhA changes.
In Figure 4 is illustrated the bioelectrical impedance vector analysis using the mean R/height and the mean Xc/height against the reference population.18
In all assessments, the mean values were within the 50th percentile. From baseline to training the vector slightly moved upper to the second quadrant (less fluids), in detraining the mean vector moved slightly to the right (less soft tissues) and slightly down (more fluids) and from detraining to retraining the mean vector slightly moved upper (less fluids).
Discussion
This is the first longitudinal study to assess the effect of RT in training, detraining and retraining on PhA and body composition in older adults. It was observed that a RT regimen has a positive impact on body composition, including PhA, but an interruption in the training routine may lead to severe decreases in PhA that may only be partially recovered in a retraining process.
In healthy adults, PhA is influenced by age, sex and BMI. Decreases with age as a result of the reduction in reactance due to loss of muscle mass and an increased resistance due to reduction of body water and increased FM22, 23 Previous studies have shown that the PhA can be modulated by exercise and that these changes can be modulated by the type and intensity of training.24
The feasibility and efficacy of a RT regimen has been previously demonstrated in older adults, with a selective effect of detraining on reduced muscle quality, with retraining counteracting this negative inactivity-related effect.11 Conversely, Rondanelli et al.25 have recently verified that, in sarcopenic older adults, a 12-week comprehensive physical fitness and muscle mass enhancement training program of moderate intensity lead to increases in fat-free mass and ALST only when combined with whey protein, essential amino acids, and vitamin D whey protein, essential amino acids, and vitamin D supplementation. In our investigation, it was observed that both ALST, indicative of muscle mass, and PhA were positively influenced by a 12-week RT regimen. The differences observed when comparing to Rondanelli et al.25 investigation may be related to the fact that the low intensity of the intervention in that study. In our study, we additionally verified that after a detraining period, PhA decreased to values that were lower than baseline assessments, emphasizing that although RT may result in an enhanced body composition profile, it is critical that older adults maintain the training regimens. Interestingly, the same pattern was not observed for ALST, as increases were observed when untrained older women engaged in a RT regimen but no changes were observed in detraining or retraining.
A lower PhA may be a consequence of either cell death or an increase in the fragility of the cell membranes, whereas a higher PhA is related to large quantities of intact cells membranes and body cell mass. Basile et al.9 have recently suggested that PhA may be a useful marker of sarcopenia in older ages due to its association with muscle strength and mass, both of which are criteria to diagnose these conditions. It is of importance to counteract the deleterious effects that are associated with the aging process. In a review, Norman et al.23 have previously established an association between PhA and muscle function parameters, including measures of muscle strength and the capability to perform daily activities. The data presented in the present investigation have, therefore, practical application as it was demonstrated that, more than muscle mass (ALST), PhA is sensitive to RT in training, detraining and retraining.
Reactance represents the delay in the conduction or passage of the administered current by cell membranes (structure and function) and tissue interfaces, whereas, resistance is the opposition to the flow of an alternating current through intracellular and extracellular ionic solutions. PhA decreases with age, due to the reduction in reactance, which parallels the loss of muscle mass, and due to the increase in resistance, associated with the declining proportion of body water at the expense of increasing FM in older ages. In this investigation, we observed that PhA decrease was accompanied by a large increase in R while retraining lead to increases in PhA that were parallel to increases in reactance.
Considering the possible effects of RT on changes in body composition, with a more pronounced effect on PhA comparing to other traditionally used body composition parameters in training, detraining and retraining, we suggest this marker to be used to evaluate the efficacy of RT regimens in older ages. The use of this marker is not only sensitive to training, as observed in our study, but it has also been associated with other relevant health markers in older ages.
Conclusion
In untrained older women, a RT program improves body composition and increases PhA. However, the interruption for a prolonged time will result in losses that will overcome the benefits of the training program, and more time may be required in retraining to counteract the negative influence of prolonged periods of absence of stimulus. Accordingly, it is recommended that untrained women engage in a RT regimen program to achieve healthier body composition phenotypes and avoid long periods of inactivity.
References
Hurley BF, Hanson ED, Sheaff AK . Strength training as a countermeasure to aging muscle and chronic disease. Sports Med 2011; 41: 289–306.
Phillips SM . Resistance exercise: good for more than just Grandma and Grandpa's muscles. Appl Physiol Nutr Metab 2007; 32: 1198–1205.
Peterson MD, Gordon PM . Resistance exercise for the aging adult: clinical implications and prescription guidelines. Am J Med 2011; 124: 194–198.
Peterson MD, Rhea MR, Sen A, Gordon PM . Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010; 9: 226–237.
Peterson MD, Sen A, Gordon PM . Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011; 43: 249–258.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 2004; 23: 1226–1243.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr 2004; 23: 1430–1453.
Lukaski HC . Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr 2013; 67 (Suppl 1), S2–S9.
Basile C, Della-Morte D, Cacciatore F, Gargiulo G, Galizia G, Roselli M et al. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp Gerontol 2014; 58: 43–46.
Marini E, Buffa R, Saragat B, Coin A, Toffanello ED, Berton L et al. The potential of classic and specific bioelectrical impedance vector analysis for the assessment of sarcopenia and sarcopenic obesity. Clin Interv Aging 2012; 7: 585–591.
Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB . Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 2009; 55: 217–223.
Norman K, Wirth R, Neubauer M, Eckardt R, Stobaus N . The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc 2015; 16: 173.e117–22.
Ruiz M . Capsule commentary on Wilhelm-Leen et al., phase angle, frailty and mortality in older adults. J Gen Intern Med 2014; 29: 183.
Wilhelm-Leen ER, Hall YN, Horwitz RI, Chertow GM . Phase angle, frailty and mortality in older adults. J Gen Intern Med 2014; 29: 147–154.
Beberashvili I, Azar A, Sinuani I, Kadoshi H, Shapiro G, Feldman L et al. Longitudinal changes in bioimpedance phase angle reflect inverse changes in serum IL-6 levels in maintenance hemodialysis patients. Nutrition 2014; 30: 297–304.
Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Stav K et al. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur J Clin Nutr 2014; 68: 683–689.
Walker DG, Obesity: preventing and managing the global epidemic. Report of a WHO consultation. In: World Health Organization technical report series, Geneva. 2001/03/10 ed, 2000, pp i-xii, 1-253.
Piccoli A, Nigrelli S, Caberlotto A, Bottazzo S, Rossi B, Pillon L et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am J Clin Nutr 1995; 61: 269–270.
Amarante do Nascimento M, Januario RS, Gerage AM, Mayhew JL, Cheche Pina FL, Cyrino ES . Familiarization and reliability of one repetition maximum strength testing in older women. J Strength Cond Res 2013; 27: 1636–1642.
American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009; 41: 687–708.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359.
Bosy-Westphal A, Danielzik S, Dorhofer RP, Later W, Wiese S, Muller MJ . Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN. J Parenter Enteral Nutr 2006; 30: 309–316.
Norman K, Stobaus N, Pirlich M, Bosy-Westphal A . Bioelectrical phase angle and impedance vector analysis—clinical relevance and applicability of impedance parameters. Clin Nutr 2012; 31: 854–861.
Torres AG, Oliveira KJF, Oliveira-Junior AV, Gonçalves MC, Koury JC . Biological determinants of phase angle among Brazilian elite athletes. Proceedings of the Nutrition Society 30 June–3 July 2008; 67, (OCE8) Nottingham, UK: E332 doi:10.1017/S0029665108000062.
Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 2016; 103: 830–840.
Acknowledgements
We wish to express our gratitude to all the participants for their cooperation in this study. LS and MA, conducted this work during a visiting scholar period to the Faculty of Human Kinetics, University of Lisbon, Portugal, supported by the Capes Foundation within the Ministry of Education, Brazil (Process: 88887.065407/2014-00 and process: 88887.090477/2014-00, respectively). DAS is supported by a scholarship from the Portuguese Foundation for Science and Technology (SFRH/BPD/92462/2013).
Author contributions
LBS: study concept and design. DS: acquisition of subjects and data analysis. All authors: data analysis, interpretation and preparation of the paper. Sponsor’s Role: No study sponsors had any role in the study design, conduct of the study, data collection, data interpretation or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
dos Santos, L., Cyrino, E., Antunes, M. et al. Changes in phase angle and body composition induced by resistance training in older women. Eur J Clin Nutr 70, 1408–1413 (2016). https://doi.org/10.1038/ejcn.2016.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.124
- Springer Nature Limited
This article is cited by
-
Physiological adaptations of active postmenopausal women and matched men to the multi-day Sardinia Selvaggio Blu wild trek: a gender-comparative pilot study
European Journal of Applied Physiology (2024)
-
Effect of resistance training on bioelectrical phase angle in older adults: a systematic review with Meta-analysis of randomized controlled trials
Reviews in Endocrine and Metabolic Disorders (2023)
-
Change in phase angle is associated with improvement in activities of daily living and muscle function in patients with acute stroke
European Geriatric Medicine (2023)
-
Not only muscle mass but also muscle quality is associated with knee extension muscle strength in patients with hip fractures
Archives of Osteoporosis (2023)
-
Phase angle (PhA) in overweight and obesity: evidence of applicability from diagnosis to weight changes in obesity treatment
Reviews in Endocrine and Metabolic Disorders (2023)