Abstract
Background/Objectives:
Isolated phytochemicals have been shown to reduce blood pressure; however, combinations of phytochemicals have rarely been tested in humans. We hypothesized that a combination of extracts from grape seed and skin (330 mg), green tea (100 mg), resveratrol (60 mg) and a blend of quercetin, ginkgo biloba and bilberry (60 mg) would reduce blood pressure (BP) in hypertensive subjects.
Subjects/Methods:
Eighteen individuals meeting BP requirements (⩾130 mm Hg systolic or ⩾85 mm Hg diastolic) and criteria for metabolic syndrome were enrolled in a double-blinded, placebo-controlled, crossover trial (ClinicalTrials.gov, NCT01106170). The 28-day placebo and supplement arms were separated by a 2-week washout period, and 14 -h daytime ambulatory BP was assessed at baseline and at the end point of each arm.
Results:
BP was not altered after placebo. After supplement treatment, diastolic pressure was reduced by 4.4 mm Hg (P=0.024, 95% CI, 0.6–8.1), systolic pressure was unchanged and mean arterial pressure trended (P=0.052) toward reduction. Serum angiotensin-converting enzyme activity was similar between placebo and supplement arms, but urinary nitrate and nitrite concentrations were significantly increased (P=0.022) after supplementation. Human aortic endothelial cells treated with metabolites of the polyphenols used in the human supplement trial had a significant increase (P=0.005) in insulin-stimulated eNOS phosphorylation and greater (P<0.001) accumulation of nitrates/nitrites.
Conclusions:
Our clinical and in vitro data support the theory that this combination of polyphenols reduced diastolic pressure by potentiating eNOS activation and nitric oxide production. Such supplements may have clinical relevance as stand-alone or adjunct therapy to help reduce BP.
Similar content being viewed by others
Introduction
In the United States, hypertension is the most prevalent form of cardiovascular disease (CVD), affecting an estimated 76 400 000, or roughly one in three, Americans.1 Further, the World Health Organization estimates that nearly 1 billion people have hypertension.2 This condition increases the risk of left-ventricular hypertrophy, heart attack, stroke and heart failure.3 In this era of advanced health care it is alarming that the number of deaths caused by hypertension-related heart disease remains high, with a reported ~36 000 American deaths in 2009.4 Hypertension is also a common feature of metabolic syndrome, characterized by the presence of three or more of the following criteria: fasting glucose⩾100 mg/dl; waist circumference⩾102 cm in men or ⩾88 cm in women; blood pressure >130/85 mm Hg (systolic/diastolic); triglyceride levels ⩾150 mg/dl; and high-density lipoprotein ⩽40 mg/dl in men or ⩽50 in women.5 Approximately 34% of all adults in the United States over age 20 meet these criteria and are at higher risk for mortality due to CVD and stroke.6, 7, 8
Given the epidemic numbers of hypertensive individuals there is a great interest in efficacious diet and lifestyle-based interventions to lower blood pressure. Many studies have examined the efficacy of various botanically derived phytochemicals to reduce blood pressure. For example, grape seed extract reduces blood pressure in spontaneously hypertensive rats fed normal and high-salt diets.9 In humans both 150 and 300 mg/day grape seed extract can lower blood pressure,10 and a meta-analysis examining nine randomized controlled human trials reported significant reductions in systolic pressure.11 Supplementary green tea polyphenols lowered blood pressure in stroke-prone hypertensive rats12 and angiotensin II-infused rats.13 Green tea supplements are also reported to reduce blood pressure in healthy humans,14 insulin-resistant adults15 and in subjects with pre-hypertension.16 Resveratrol, a polyphenol found in grape skin, lowers arterial pressure in hypertensive17, 18 and obese rat models.19 Quercetin, a flavonol found in onions and apples, can also alleviate hypertension in pressure-overloaded rats20 and spontaneously hypertensive rats.21 Finally, quercetin supplemented (150–730 mg/d) to humans with metabolic syndrome and/or high blood pressure reduces systolic and diastolic blood pressure by ~3–7 mm Hg.22, 23
As data from both animals and humans indicate that individual polyphenolic compounds reduce blood pressure, we hypothesized that a combination of polyphenolic agents may also be an effective intervention. To test our hypothesis we conducted a double-blinded, placebo-controlled trial, in which a supplement containing isolated polyphenols and botanical extracts was administered to hypertensive subjects. The primary outcome measure assessed in this study was ambulatory blood pressure, and secondary outcome measures included serum angiotensin-converting enzyme (ACE) activity and urinary metabolites of nitric oxide. Parallel studies were conducted in human aortic endothelial cells (HAECs) in culture treated with metabolites of the polyphenolic combination used in our human studies. We report here that a 4-week supplementation period of this combination significantly reduced diastolic blood pressure compared with placebo, and present evidence supporting that the responsible mechanism may be increased activation of endothelial nitric oxide synthase and greater nitric oxide production.
Patients and methods
Participants and recruitment criteria
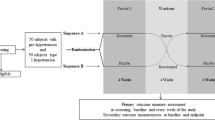
This study was approved by the University of Utah Institutional Review Board and conformed to the Declaration of Helsinki and Title 45, US Code of Federal Regulations, Part 46, Protection of Human Subjects; it has been registered in Clinical Trials.gov (NCT01106170), and adhered to updated 2010 COSORT guidelines.24 Written informed consent was obtained from each participant prior to enrollment in this placebo-controlled, crossover study to determine the efficacy of the phytochemical supplement to reduce blood pressure. Subjects were screened for eligibility at the University of Utah Nutrition Clinic. Blood pressure during screening was measured using an Omron random zero automated blood pressure cuff as previously described.22 All volunteers had to meet 3 of 5 metabolic syndrome inclusion criteria, and exclusion criteria summarized in Table 1. Participants were recruited from the greater Salt Lake City area via flyers, public health screenings, advertisements (radio, the Internet, public transportation) and by word of mouth. Twenty-nine participants were originally recruited; 18 completed all experimental protocols and were included in the final analysis (Figure 1, Table 2). The most common reasons cited for volunteers leaving the study during or after the first intervention arm were health problems, moving out of state, inability to get to the clinic due to transportation problems and loss of interest in continuing participation.
Supplement and placebo
We tested a proprietary product, hereafter referred to as the 'supplement', containing (per capsule) grape seed and skin extract (165 mg), green tea extract (50 mg, decaffeinated, 90% phenols), quercetin dehydrate (25 mg), resveratrol (25 mg, Polygonum Cuspidatum—50%) and a 5 mg combination of gingko biloba, bilberry extract, bromelain and fungal protease (Melaleuca Inc, Idaho Falls, ID, USA). The placebo consisted of cornstarch packaged in identical capsules to that of the supplement. At the end of each arm, bottles of supplement and placebo were collected to determine compliance via pill count.
Overview of study design and blood pressure measurement
Eligible subjects were recruited between March 2010 and June 2011 and were entered into this double-blinded, placebo-controlled, crossover study. The primary outcome measure was blood pressure, and secondary outcome measures were ACE activity and urinary nitrate/nitrite concentrations as potential regulators of blood pressure. The study was conducted at the University of Utah Nutrition Clinic on a final sample of 18 subjects who had resting blood pressure of ⩾130 mm Hg systolic or ⩾85 mm Hg diastolic, and who completed all study protocols. A prospective power calculation using a difference of 6 mm Hg in mean arterial pressure between placebo and supplement, a s.d. of 9 mm Hg and a P<0.05 determined that n=20 would be required to produce a power of β=0.80. This analysis provided us a recruitment target goal when we began the study. The study was halted after a significant difference in blood pressure was achieved. After a 1-week 'wash-in phase' where subjects discontinued any existing supplements, they were assigned (random draw) to a 28-day supplement or a 28-day placebo arm. Identically sealed bottles containing either placebo or the supplement were produced by Melaleuca, Inc. and given identical labels that contained a number identifier, along with the name and contact information of the study Principle Investigator. Subjects and research assistants were blinded to the nature of the bottles of supplement/placebo. Only the Principle Investigator, who did not have a direct patient contact, was aware of the interventions. Subjects were instructed to take two capsules with the meal of their choice. Research assistants randomized subjects to begin either placebo or supplement arm first by blindly selecting a bottle of capsules out of a box. After a 2-week washout period, subjects crossed over to the other arm. The 2-week washout period was selected on the basis of our previous human studies using quercetin, which have indicated no trace of quercetin metabolites in plasma beyond 1 week after supplementation.22, 25 Compliance was monitored by pill count; each bottle was sealed with 58 capsules, two more than required for full compliance. During the course of the study subjects were counseled to maintain their regular diet and physical activity but not take any additional supplements. For each study arm, baseline and end-point blood pressure was measured using an ambulatory blood pressure cuff (Meditech ABPM-05, Budapest, Hungary) fitted on the left arm that recorded heart rate and systolic and diastolic blood pressure every 30 min from ~0800 to 2200 h. This technique has clinical advantages over traditional acute clinic-based blood pressure measurement because it is free from the 'white coat effect' and similar confounding variables, and is a better predictor of cardiovascular events.26
Dietary records
To verify diet consistency, subjects logged a 3-day food record during the second week of both the placebo and the supplement phase. Data were entered and analyzed using the Food Processor dietary analysis program (ESHA Research, Salem, OR, USA).
Blood lipids and glucose
A fasting venous sample of blood (12 ml) was drawn into two serum separator tubes. The blood was allowed to sit for 1 h before being centrifuged at 1000 RCF for 15 min at 4 ºC. Serum was placed into aliquots and stored at −80 ºC. Fasting total cholesterol, low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, triglycerides and glucose in whole blood were determined using a Cholestech (Orlando, FL, USA) blood lipid analyzer as previously described.22
Serum cytokines
Interleukin-8 (IL-8), C-reactive protein (CRP), monocyte chemoattractant protein-1 and interferon gamma-induced protein 10 (IP-10) were measured in fasting serum using multiplex kits from Quansys Biosciences (Logan, UT, USA).
Serum ACE activity
ACE activity was measured using the Buhlmann Assay Kit (Basel, Switzerland) as previously described.25 This method is based on the ability of ACE to cleave the synthetic substrate N-[3-(2–furly)acryloyl]-l-phenylalanine-l-glycyl-l-glycine) into an amino acid derivative and a di-peptide. The kinetics of this cleavage is measured by reading the decrease in absorbance at 340 nm at time 0 and 10 min after the addition of the synthetic substrate to the serum sample.
Nitrates and nitrites measurement
NO2− and NO3−, surrogates for NO bioavailability, were quantified in urine collected from fasting human subjects and in HAECs grown in culture using a colorimetric kit (Cayman Chemical, Ann Arbor, MI, USA) as previously described.25 Total NO2− and NO3− concentrations were normalized to protein concentration.
Urinary F2-α-isoprostane
F2-α-isoprostane, an indicator of oxidative stress, was measured in fasting urine samples as previously described.22
Cell culture and treatment with polyphenols
HAECs (Lonza, Carlsbad, CA, USA) were cultured as previously described27 in media containing 2% FBS and endothelial growth supplements EGM-2 (Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere (5% CO2 / 95% O2, 37 °C). Cells were passaged at 80% confluence, and all experiments were conducted on cells between passages 3 and 6. Reported data are the average of 4–6 independent experiments. Prior to polyphenol treatment, HAECs were incubated overnight in serum-free medium. Next, HAECs were treated with metabolites of polyphenols for 24 h in media containing 2% FBS and 5 mm glucose. After 24 h, HAECs were treated with or without insulin (100 nm) for 10 min. Following insulin treatment, cells were lysed and prepared for detection of eNOS and measurement of nitrate/nitrite concentrations. HAECs were grown in glucose concentration (5 mm) that mimicked the average fasting blood glucose levels of subjects in the clinical trial. Insulin was used to activate eNOS in our cell-based model as it is known to stimulate eNOS activation and NO production in human endothelial cells, rodents and humans.28, 29
HAECs were treated with metabolites of polyphenols found in the supplement used in the human trial. Metabolites, rather than the parent polyphenols, were selected because human studies have demonstrated that polyphenols are rapidly metabolized after ingestion and very little parent compound remains in circulation,30 thus indicating that metabolites may be more biologically relevant than the originating compounds. As multiple metabolites are produced for each compound in vivo, we attempted to use metabolites for our in vitro study that were reported to be prevalent in circulation. Accordingly, metabolites of proanthocyanins, epicatechin and epigallocatechin (major components of grape seed and green tea extract), as well as quercetin and resveratrol, were employed. We acknowledge the use of a single metabolite of each ingredient as a limitation of these in vitro studies. However, we opted for this approach because of the difficulty inherent in employing multiple metabolites for each individual ingredient in combination in cell culture media.
Proanthocyanins are found in grape seed extracts, whereas catechin and epicatechin are abundant in green tea extract. Proanthocyanins are poorly absorbed and converted by colonic microflora to the phenolic acid 3-hydroxyphenylpropionic acid,31 which has been detected in human plasma.32 Similarly, catechin and epicatechin can undergo similar conversion.31, 33, 34, 35, 36 On the basis of these data, we used 3-hydroxyphenylpropionic acid (purchased from Sigma Chemical, St Louis, MO, USA) in our in vitro studies to represent a common metabolite for compounds found in both grape seed and green tea extract. Piceatannol is an analog of resveratrol found in grapes and is a product of reservatrol metabolism by cytochrome P450.37, 38, 39 Therefore, we used piceatannol (purchased from A. G. Scientific Inc., San Diego, CA, USA) as a resveratrol metabolite. Finally, for quercetin, quercetin-3-O-glucoronide (purchased from TRC Chemicals, Toronto, ON, Canada) was used as it represents a major metabolite previously reported in human plasma.33, 40, 41, 42 All three metabolites were combined in cell culture media for in vitro experiments. Concentrations were selected by performing a preliminary dose–response experiment using 1–50 μm of the various metabolites, individually. The lowest concentrations that produced significant insulin-stimulated eNOS phosphorylation were selected. These levels were 1 μm for 3-hydroxyphenylpropionic acid, 5 μm for Piceatannol and 2 μm for quercetin-3-O-glucoronide. Regarding these concentrations, the amount of quercetin used in our in vitro experiments was similar to the human plasma levels (1.42 μm) we have measured in our supplement trials,22 albeit following a larger quercetin dose of 730 mg. Other studies have reported ~0.3–0.4 μm quercetin in plasma following ingestion of 50–200 mg quercetin.23, 41, 43 For 3-hydroxyphenylpropionic acid and piceatannol there is a paucity of data on their plasma levels following ingestion of food or supplements. This lack of data prompted our rationale for employing a dose–response approach to first identify the lowest effective dose able to augment NO production.
Our experiments evaluated both basal and stimulated eNOS activation and nitric oxide production in order to better mimic the in vivo condition in which eNOS activation fluctuates between these two states. To stimulate eNOS we used insulin (100 nm, 10 min), whereas control cells were treated with saline. Vehicle control was dimethyl sulfoxide.
Western blotting
Western blots to detect eNOS and p-eNOSSer1177 (Cell Signaling Technology, Beverly, MA, USA) were performed as previously described.44
Statistical analysis
Blood pressure, ACE activity, lipids, inflammatory activity and nitrates were analyzed using paired t-tests (SPSS, Armonk, NY, USA) to compare the difference achieved between placebo and supplement arms. Cell culture data were analyzed using analysis of variance (eNOS and NOx data) and Tukey’s post-hoc test when main effects were detected. Variance was similar between groups in both human and cell studies. Data are reported as mean±standard error and significance was accepted at P<0.05.
Results
Blood pressure and heart rate
Baseline blood pressure levels in placebo and supplement arms were similar to each other. A treatment effect was present as there was a significant decrease in diastolic pressure during the 4-week supplement arm from 88.8±2.1 to 84.2±1.9 mm Hg (P=0.024, mean difference between placebo and supplement=4.4, 95% CI, 0.6–8.1, Figure 2a). However, systolic pressure remained unchanged after supplement treatment (baseline 145.1±3.3 vs end point 141.3±2.2 mm Hg). Mean arterial pressure showed a trend (P=0.052) toward reduction from 107.7±2.3 to 102.8±2.1 mm Hg after the supplement (mean difference between placebo vs supplement=4.0, 95% CI, −0.03 to 8.0). There was no order effect (that is, randomized to placebo or supplement first) on blood pressure reduction. During the placebo arm there was no change in systolic pressure (baseline 145.6±3.5 vs end point 142.8±3.0 mm Hg), diastolic pressure (baseline 85.8±2.5 vs end point 85.4±2.1 mm Hg) or mean arterial pressure (baseline 106.2±2.9 vs end point 105.2±2.6 mm Hg), Figure 2a). Placebo and supplement treatments did not alter the heart rate (Table 3).
(a) Diastolic (diastolic blood pressure) but not systolic (systolic blood pressure) pressure (mm Hg) was reduced (P<0.024) from baseline after 28 days of supplement treatment compared with placebo (n=18/group). (b) Urinary nitrate/nitrite concentration (μm) was lower (P<0.022) after 28 days of supplementation compared with placebo (n=17/group). Values are normalized to baseline for each treatment phase and presented as fold change from baseline. *P<0.05.
Urinary nitrate and nitrite concentrations, serum ACE activity and F2-α-isoprostane.
Reduction in mean arterial pressure during the supplement arm was accompanied by a significant increase in total urinary nitrate and nitrite concentrations (P=0.022, mean difference= 540±214 μm, 95% CI, 87–993, Figure 2b). ACE activity, a known regulator of blood pressure, was unchanged between supplement and placebo phase (Table 3). F2-α-isoprostane concentration was also similar between the supplement and placebo periods (Table 3).
Markers of inflammation, blood lipids, body weight
There was no change in serum concentrations of CRP, IL-8, monocyte chemoattractant protein-1 or IP-10 between baseline and end point in either the placebo or the supplement arm (Table 3). Fasting total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides and glucose also remained unchanged during the course of the study (Table 3). Body weight and body mass index remained unchanged during the study (Table 3).
Diet analysis and supplement compliance
Analysis of 3- day food records indicated that there were no significant differences in the intake of carbohydrates, proteins and fats between the placebo and supplement arms (Table 4). Micronutrient intake, including sodium, potassium and calcium, was similar between supplement and placebo arms (Table 4). Pill count at the end of each study arm indicated that 86±5% of allocated supplements were consumed during the supplement phase and 93±4% during the placebo phase.
Nitric oxide and eNOS in HAECs
We coupled our human study with in vitro experiments to shed light on potential mechanisms regulating the observed increase in the urinary marker of NO bioavailability. HAECs were treated with metabolites of the polyphenols used in the clinical supplement trial. Under basal (no insulin stimulation) conditions NO production and eNOS phosphorylation was similar in polyphenol-treated vs untreated HAECs (Figures 3a and b). Insulin stimulation did not alter NO production in untreated HAECs, but did produce a robust increase of NO (P<0.001), eNOS phosphorylation (P=0.005) and the p-eNOS to total eNOS ratio (P=0.05) in polyphenol-treated HAECs (Figures 3a and b).
Human aortic endothelial cells were incubated for 24 h with polyphenol metabolites, and then insulin was used to activate eNOS to stimulate NO production. (a) Cells that received the mix of polyphenol metabolites prior to insulin stimulation exhibited increased eNOS phosphorylation (P=0.005) and a greater ratio of p-eNOS to total eNOS (P=0.05) vs vehicle-treated insulin-stimulated cells. (b) Accumulation of nitrates and nitrites was also increased (P<0.001) in human aortic endothelial cells incubated with polyphenol metabolites compared with vehicle-treated cells. N=4–6 for all experiments. *P<0.05.
Discussion
The major finding of this study is that diastolic blood pressure was reduced in hypertensive subjects in the supplement arm. There was also a trend (P=0.052) toward reduction of mean arterial pressure. These results were achieved in the absence of adverse events. It is unclear why only diastolic pressure was reduced in this study, but differential blood pressure findings have previously been reported. For example, a study examining grape-derived polyphenols reported a reduction of only systolic pressure, but not diastolic pressure.45 In the present study, reduction of diastolic blood pressure was accompanied by a significant increase in urinary nitrate and nitrite concentrations. This observation suggests that the mechanism responsible for blood pressure reduction could be related to an increase in NO bioavailability. Additional evidence for this was obtained in HAECs, where incubation with polyphenol metabolites markedly increased insulin-stimulated NO production and eNOS phosphorylation. As there were no changes in body weight, body mass index or micronutrient intake, we conclude that the attenuated blood pressure was due to the supplement employed. Although we did not see changes in micronutrients related to blood pressure control (sodium, magnesium, potassium and calcium), our dietary analysis did not evaluate intake of dietary nitrate, which could affect blood pressure levels. For example, beetroot juice is a dietary source of nitrate and is reported to lower blood pressure.46, 47 Therefore, it is possible that acute changes in nitrate consumption could also have influenced blood pressure.
The subjects enrolled in our study met at least three out of the five criteria accepted for metabolic syndrome,5 a condition that is associated with elevations in inflammatory cytokines and chemokines.48 It is unclear whether polyphenols can reduce inflammatory markers, such as CRP, a widely used marker of inflammation that is linked to greater CVD risk. For example, resveratrol supplementation does not alter CRP in healthy subjects,49 but reduces CRP in smokers.50 On the other hand, a meta-analysis of human trials using grape seed extract found no change in CRP, despite widely reported reductions in blood pressure.11 In our present study, the observed benefits to blood pressure were not accompanied by reduction in inflammatory markers. Taken together, there is a lack of conclusive evidence supporting the ability of polyphenols to reduce plasma markers of inflammation in humans.
Clinical trails evaluating combinations of compounds used in this supplement are very rare, but the individual polyphenols and extracts used in this supplement have been previously evaluated. For example, acute25 (10 h, 1000 mg) and long-term22, 23, 51 (>4 weeks, 150–750 mg/day) quercetin supplementation reduces blood pressure in subjects with hypertension. Freeze-dried grape extract (46 g/day, ~232 mg of total phenols and 31 mg anthocyanins) reduces systolic blood pressure (−6 mm Hg), but not diastolic blood pressure.45 Purified grape seed extract supplements (150 and 300 mg/day) lower systolic and diastolic blood pressure by 11 and 6 mm Hg, respectively.10 The blood pressure effects of resveratrol in humans are mixed. Some studies demonstrate that chronic (30+ days) supplementation of resveratrol in doses ranging from 150 to 250 mg/d (vs 60 mg/d employed in the present study) reduces only systolic blood pressure in subjects with metabolic syndrome or diabetes.52, 53 However, others report no change.54, 55 Similarly, green tea extract has variable effects on blood pressure, ranging from almost no change (using 379–400 mg catechins/day)56, 57 to nearly 4 mm Hg reduction (using 456-583 mg catechins/day). It is notable that the blend of polyphenols tested in our experiment produced similar reductions in diastolic blood pressure as some of the aforementioned studies, but used smaller amounts of quercetin, resveratrol and green tea extract to do so. On the other hand, there may be no differences in the magnitude of the antihypertensive effect resulting from a combination supplement versus the use of the individual ingredients given at a higher dose.
We evaluated ACE activity and urinary nitrite/nitrate concentrations to gain insight into the mechanism responsible for reduction of blood pressure. No change was observed in ACE activity; however, urinary nitrite/nitrate concentrations were increased during the supplement arm, which could indicate enhanced nitric oxide bioavailability. Our subsequent studies using HAECs support this notion as cells treated with ingredients contained in the polyphenol supplement had greater insulin-stimulated NO production and eNOS phosphorylation compared with untreated cells. Our results are in general agreement with previous work using endothelial cells,58 rodents59 and humans.45 A limitation inherent to our in vitro studies is that many metabolites are produced after ingestion of the polyphenols found in this supplement. Therefore, it is possible that the metabolites selected for the in vitro studies may have lesser or greater impact upon NO bioavailability compared with the in vivo situation. As such, we note that our in vitro experiments may convey proof of principle that metabolites of polyphenols used in this supplement could generally impact NO bioavailability but are not sufficient to identify a specific mechanism that reflects the in vivo state.
Public interest in the use of supplements, including polyphenols, to prevent CVD is high. For example, in the United States the 2007–2010 National Health and Nutrition Examination Survey reported that 49% of all adults use dietary supplements as a tool to 'improve or maintain' health.60 Furthermore, 7.5% of adults reported the use of botanical supplements, which include polyphenol-containing compounds and extracts such as those evaluated in this study, to promote overall health.60 This reality underscores the need for research to evaluate the efficacy and mechanisms regulating the biological effects of polyphenols in humans. To this end, we found that the blend of isolated polyphenols and botanical extracts used in the present study reduced diastolic blood pressure in subjects with metabolic syndrome and hypertension. Data from our human and cell-based experiments support the theory that this supplement reduced diastolic blood pressure through potentiation of eNOS phosphorylation and NO bioavailability, a novel mechanism of action compared with most traditional antihypertensive medications. Although our study attempted a careful investigation of this polyphenol supplement to reduce blood pressure, there are limitations that must be acknowledged. Volunteers were counseled to maintain their normal daily activity to minimize this effect on weight control and blood pressure, but physical activity records were not collected to verify this. Also, the final sample size analyzed was smaller than originally planned because of the greater than expected dropout rate. Therefore, the study was slightly underpowered, as a retrospective power analysis using the actual results of the study indicated that the observed power was β=0.70.
The fact that diastolic but not systolic pressure was reduced has clinical relevance as diastolic pressure is a better predictor of CVD risk in people less than 50 years of age.61, 62, 63 It is possible that this type of polyphenol blend could be useful in those under age 50 who prefer to initially avoid a conventional pharmaceutical and try a naturopathic approach to reduce diastolic blood pressure. Given the general prevalence of supplement use to reduce CVD risk coupled with results of small studies such as ours, we believe pursuit of larger-scale clinical trials is warranted to evaluate the efficacy of polyphenols used alone, or even in coordination with traditional pharmaceutical treatments, to achieve optimal blood pressure control.
References
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012; 125: e2–e220.
World Health Organization Global Status Report On Noncommunicable Diseases 2010. World Health Organization: Geneva, 2011.
Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO . Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis 2009; 52: 153–167.
Vital Statistics Public Use Data Files – 2011 Mortality Multiple Cause Files Centers for Disease Control and Prevention; 2008 [cited 9 July 2012]. Available from: http://www.cdc.gov/nchs/data/dvs/deaths_2009_release.pdf.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645.
Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007; 49: 403–414.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132.
Ervin RB . Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 2009; 1–7.
Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM . Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2005; 289: R771–R775.
Sivaprakasapillai B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T . Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism 2009; 58: 1743–1746.
Feringa HH, Laskey DA, Dickson JE, Coleman CI . The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J Am Diet Assoc 2011; 111: 1173–1181.
Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y . Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr 2004; 134: 38–42.
Antonello M, Montemurro D, Bolognesi M, Di Pascoli M, Piva A, Grego F et al. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens 2007; 20: 1321–1328.
Nantz MP, Rowe CA, Bukowski JF, Percival SS . Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009; 25: 147–154.
Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A . Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 2012; 32: 421–427.
Nagao T, Hase T, Tokimitsu I . A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007; 15: 1473–1483.
Moraloglu O, Engin-Ustun Y, Tonguc E, Var T, Tapisiz OL, Ergun H et al. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J Matern Fetal Neona 2012; 25: 845–848.
Bhatt SR, Lokhandwala MF, Banday AA . Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol 2011; 667: 258–264.
Rivera L, Moron R, Zarzuelo A, Galisteo M . Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009; 77: 1053–1063.
Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC et al. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol 2006; 47: 531–541.
Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A et al. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol 2001; 133: 117–124.
Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T . Quercetin reduces blood pressure in hypertensive subjects. J Nutr 2007; 137: 2405–2411.
Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 2009; 102: 1065–1074.
Schulz KF, Altman DG, Moher D . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010 7: e1000251.
Larson A, Witman MA, Guo Y, Ives S, Richardson RS, Bruno RS et al. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: nitric oxide. Nutr Res 2012; 32: 557–564.
Chavanu K, Merkel J, Quan AM . Role of ambulatory blood pressure monitoring in the management of hypertension. Am J Health Syst Pharm 2008; 65: 209–218.
Babu PVA, Si H, Fu Z, Zhen W, Liu D . Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr 2012; 142: 724–730.
Zeng G, Quon MJ . Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 1996; 98: 894–898.
Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 2000; 101: 676–681.
Manach C, Williamson G, Morand C, Scalbert A, Remesy C . Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81: 230S–242S.
Ward NC, Croft KD, Puddey IB, Hodgson JM . Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic Acid, an important metabolite of proanthocyanidins in humans. J Agric Food Chem 2004; 52: 5545–5549.
Saura-Calixto F, Perez-Jimenez J, Tourino S, Serrano J, Fuguet E, Torres JL et al. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol Nutr Food Res 2010; 54: 939–946.
Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA . The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med 2002; 33: 220–235.
Rios LY, Gonthier MP, Remesy C, Mila I, Lapierre C, Lazarus SA et al. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr 2003; 77: 912–918.
Deprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C et al. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr 2000; 130: 2733–2738.
Gu L, House SE, Rooney L, Prior RL . Sorghum bran in the diet dose dependently increased the excretion of catechins and microbial-derived phenolic acids in female rats. J Agric Food Chem 2007; 55: 5326–5334.
Piotrowska H, Kucinska M, Murias M . Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res 2012; 750: 60–82.
Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F et al. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol 2004; 68: 773–782.
Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer 2002; 86: 774–778.
Mullen W, Edwards CA, Crozier A . Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br J Nutr 2006; 96: 107–116.
Perez A, Gonzalez-Manzano S, Jimenez R, Perez-Abud R, Haro JM, Osuna A et al. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: correlation with beta-glucuronidase activity. Pharmacol Res 2014; 89: 11–18.
Terao J, Murota K, Kawai Y . Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct 2011; 2: 11–17.
Erlund I, Kosonen T, Alfthan G, Maenpaa J, Perttunen K, Kenraali J et al. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol 2000; 56: 545–553.
Bosse JD, Lin HY, Sloan C, Zhang QJ, Abel ED, Pereira TJ et al. A low-carbohydrate/high-fat diet reduces blood pressure in spontaneously hypertensive rats without deleterious changes in insulin resistance. Am J Physiol Heart Circ Physiol 2013; 304: H1733–H1742.
Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML . Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J Nutr 2012; 142: 1626–1632.
Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008; 51: 784–790.
Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 2010; 56: 274–281.
Goncalves FM, Jacob-Ferreira AL, Gomes VA, Casella-Filho A, Chagas AC, Marcaccini AM et al. Increased circulating levels of matrix metalloproteinase (MMP)-8, MMP-9, and pro-inflammatory markers in patients with metabolic syndrome. Clin Chim Acta 2009; 403: 173–177.
Heger A, Ferk F, Nersesyan A, Szekeres T, Kundi M, Wagner KH et al. Intake of a resveratrol-containing dietary supplement has no impact on DNA stability in healthy subjects. Mutat Res 2012; 749: 82–86.
Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem 2013; 20: 1323–1331.
Egert S, Boesch-Saadatmandi C, Wolffram S, Rimbach G, Muller MJ . Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr 2010; 140: 278–284.
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011; 14: 612–622.
Bhatt JK, Thomas S, Nanjan MJ . Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 2012; 32: 537–541.
Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 2012; 67: 1307–1312.
Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M et al. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res 2011; 31: 842–847.
Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H . Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr 2008; 62: 953–960.
Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T . Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr 2011; 106: 1880–1889.
Feng Z, Wei RB, Hong Q, Cui SY, Chen XM . Grape seed extract enhances eNOS expression and NO production through regulating calcium-mediated AKT phosphorylation in H2O2-treated endothelium. Cell Biol Int 2010; 34: 1055–1061.
Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y et al. Grape seed proanthocyanidin extract alleviates ouabain-induced vascular remodeling through regulation of endothelial function. Mol Med Report 2012; 6: 949–954.
Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT . Why US adults use dietary supplements. JAMA Intern Med 2013; 173: 355–361.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252.
Greenberg J . Are blood pressure predictors of cardiovascular disease mortality different for prehypertensives than for hypertensives? Am J Hypertens 2006; 19: 454–461.
Taylor BC, Wilt TJ, Welch HG . Impact of diastolic and systolic blood pressure on mortality: implications for the definition of "normal". J Gen Intern Med 2011; 26: 685–690.
Acknowledgements
We acknowledge Maureen Murtaugh, PhD, RD, for providing statistical consultation. This study was funded by a grant from Melaleuca, Inc., and by the University of Utah College of Health Research Incentive grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TJ received research funding from Melaleuca, Inc. for this study. ABR is an employee of Melaleuca, Inc. Other authors do not declare any conflict of interest.
Rights and permissions
About this article
Cite this article
Biesinger, S., Michaels, H., Quadros, A. et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr 70, 10–16 (2016). https://doi.org/10.1038/ejcn.2015.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.88
- Springer Nature Limited
This article is cited by
-
Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus
BMC Cardiovascular Disorders (2021)
-
The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials
Lipids in Health and Disease (2020)
-
The Effects of Resveratrol Supplementation on Endothelial Function and Blood Pressures Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
High Blood Pressure & Cardiovascular Prevention (2019)
-
Metabolites of flavonoid compounds preserve indices of endothelial cell nitric oxide bioavailability under glucotoxic conditions
Nutrition & Diabetes (2017)
-
The therapeutic potential of resveratrol: a review of clinical trials
npj Precision Oncology (2017)