Abstract
Background/Objective:
To determine gastrointestinal (GI) responses and maximum tolerated dose of erythritol in young children given as a single oral dose in a 250-ml non-carbonated fruit-flavoured beverage in between meals. This is a multicentre double-blind study with sequential design for multiple dose groups and randomised crossover for comparators of placebo vs dose.
Subjects/Methods:
A total of 185 healthy young children aged 4–6 years were recruited at three clinical investigation centres after informed consent of both parents; 184 children completed the study. Children were included in one of the four dose groups (5, 15, 20 or 25 g erythritol) and exposed randomly to only one single dose vs an isosweet sucrose placebo. After consumption in the clinic and an observation period, GI symptoms and stooling patterns were recorded during the next 48 h.
Results:
Statistically significantly more episodes of diarrhoea and/or severe GI symptoms were observed in the 20 and 25 g groups compared with placebo, but not in the 5 and 15 g groups. Stool consistency, as measured by Bristol stool scale, was lower in the 15-, 20- and 25 g groups for the first 24 -h period, but not at later time points. Incidences of nausea, vomiting, borborygmi, excess flatus and abdominal pain were not significantly different from the placebo controls at all doses of erythritol.
Conclusions:
Rapid ingestion of up to and including 15 g (6% w/v) of erythritol in a beverage in between meals by young children aged 4–6 years was well tolerated. The no observed effect level for diarrhoea and/or severe GI symptoms was 15 g (0.73 g/kg body weight (bw)). Children appeared not to be more sensitive to the GI effects of erythritol than published for adults on a g/kg bw basis.
Similar content being viewed by others
Introduction
Erythritol is a four-carbon polyol with a sweetness potency of ~70% compared with sucrose. It is present naturally in various fruits, fermented foods, mushrooms and cheese, and it is produced by using a fermentation process.1 Per capita erythritol intake from natural occurrence in Japan is ~46 mg/day or ~0.7 mg/kg body weight (bw)/day for a 60- kg individual.2 It is non-caloric,3 non-cariogenic,4 non-glycaemic and non-insulinaemic.5, 6, 7 In adults, most of the ingested erythritol is absorbed from the small intestine within 2 h, and ~90% of the ingested dose is excreted unchanged in the urine over a 72 -h period.5, 6, 8, 9, 10, 11, 12, 13, 14 The remainder is not fermented in the colon in adults.15
Polyols can cause laxative effects, flatulence and abdominal pain when excessive amounts are consumed acutely, especially in a solution.16, 17, 18 These effects occur when too high an amount of the malabsorbed carbohydrate enters the large intestine where it acts osmotically producing a laxative effect.19 Sensitivity depends on absorption rate, which is different for all polyols, the prior and concomitant consumption of other macronutrients, physical characteristics of the food and acute vs staggered consumption throughout the day.
Erythritol absorption in adults is substantially higher than other polyols,7 and its greater tolerance20 is because of the ~90% complete absorption from the small intestine and absence of fermentation in the large intestine.9, 15 Its safety for use in foods and beverages has been confirmed by the Joint WHO/FAO Expert Committee on Food Additives in 1999,21 the EU Scientific Committee on Food in 200320 and by many other Regulatory Authorities around the world. In the EU, erythritol is authorised for the same uses as other polyols.22
Historically, polyols are not authorised for sweetening or non-sweetening purposes in beverages in the EU, because of their potential laxation effect. The key benefit from erythritol use in lower-calorie beverages is as a flavour enhancer, its capability to improve the flavour profile and mouthfeel to mimic full-sugar beverages.1 These flavour-enhancing effects are achieved at levels of 0.5–3.5 g of erythritol per 100 ml.
Although data support high digestive tolerance to erythritol during acute and repeated intake studies in adults,13, 18 little information exists concerning tolerance in children. The present digestive tolerance study was undertaken to assess the maximum dose of erythritol, consumed in a maximum of 15 min, that does not cause diarrhoea and/or severe gastrointestinal (GI) symptoms in young children aged 4–6 years when delivered as a single dose in a beverage compared with placebo (sucrose). On the basis of an independent assessment conducted using the UK National Diet and Nutrition Survey (2008–2009), of all age groups, subjects most exposed to beverages on a g/kg bw basis are children aged 4–6 years.
Materials and methods
This was a dose-escalating study that included both a sequential design component (to permit an assessment of tolerability across doses) and a crossover design component (to permit an assessment of tolerability relative to a sucrose placebo). The experimental plan was to first assess the tolerability of 5 g erythritol and to increase the amount of erythritol by 10 g (that is, to 15 g of erythritol and then to 25 g of erythritol) only if the preceding dose was found not to be associated with significant GI effects. Consequently, subjects were not randomly allocated to the various erythritol dose groups; however, within each dose, treatment order was random (that is, the order of erythritol and sucrose testing was random). The blinding of the children, their caregivers and study investigators was maintained throughout the study that was conducted in three sites in France in 4–6-year-old children comparing the incidence of diarrhoea and/or severe GI symptoms following rapid, oral consumption of erythritol or placebo.
All children and their parents/guardians received all information relevant for their consent, including objectives of the trial, study design, number of visits, diet and data collection requirements at home. Parents provided written consent for their child to participate and were free to leave the study at any time for any reason. Inclusion and exclusion criteria are given in Table 1.
After inclusion, parents were given a list of polyol-containing products to be excluded from their child's diet 24 h before and 24 h after the study period. Within each group, all children received erythritol and placebo in random order in accordance with good clinical practice. Each subject was seen in the clinic on two test days (D1 and D2) to consume erythritol or placebo at least five days apart. Each test required one-half day at the clinic. After consumption of the test beverage (about 2 h after breakfast), faecal parameters and GI effects were recorded to determine the threshold dose. In addition, one of the parents (or legal guardian) recorded GI symptoms and stooling patterns in a diary during the following 48 h. After finding a significant difference in tolerance between erythritol and placebo in the 25 -g dose cohort, a protocol amendment was approved allowing the investigators to study the dose of 20 g.
Test materials
Test materials were prepared by Cargill, Vilvoorde, Belgium and supplied in bottles containing 250 ml of a non-carbonated fruit-flavoured (two flavours: strawberry and orange) clear drink sweetened with erythritol at four different dose levels: 5, 15, 20 and 25 g (equivalent to 2, 6, 8 and 10% w/v erythritol, respectively). Placebo was supplied in an identical manner but prepared with common nutritive carbohydrates (sucrose and maltodextrin) and providing an equivalent sweetness to that of the corresponding erythritol beverages (that is, 1.4, 4.2, 5.6 and 7% w/v saccharose).
Randomisation and blinding
For each dose tested, the erythritol and placebo beverages had exactly the same appearance. Independent technicians at Cargill laboratory dispensed either erythritol or placebo beverages in sequentially numbered identical bottles according to a third-party computer-generated randomisation list allocating one of the two possible administration sequences (erythritol first/placebo second or placebo first/erythritol second, first administration coded D1 and second D2). For each dose tested, subjects were identified with a number according to their order of inclusion. The allocation randomisation list was disclosed by third party to the investigators only after all subjects completed that particular dose study and all results were ‘frozen’.
Compliance
The beverage was consumed at the investigation centre under investigator supervision and compliance was assessed by measuring the volume consumed. Each child was expected to drink the entire 250- ml beverage within 15 min. This process was completed twice, that is, for erythritol and placebo (initially and at least 5 days later).
Tolerance and safety variables
The primary outcome variable was an estimate of GI tolerability, as measured by the incidence of diarrhoea and/or severe GI symptoms following consumption of the study beverage. Subjects were categorised according to diarrhoea and/or severe GI symptoms as follows:
Diarrhoea - a single watery stool (Bristol Stool Scale23 score of 7) or >3 bowel movements (regardless of consistency) in a 24 -h period.
Severe GI symptoms – any GI symptoms having an intensity recorded as ‘severe intensity’ (inability to perform everyday activities) in the symptom diary.
Secondary outcomes included bowel movement frequency, stool consistency, GI symptoms intensity score and urinary erythritol excretion. The intake of erythritol by each group is shown in Table 2.
Stool patterns
The parents/legal guardians recorded bowel movement frequency and consistency in a defecation diary for 48 h after consumption of the test beverage. Stools were assigned a consistency score using the Bristol stool scale.23
Gastrointestinal symptoms
The parents/legal guardians were trained on how to record the GI symptoms after consumption of the test beverage during the first 6 h and during the 6- to 24 -h time period on special designed forms including the occurrence, intensity and frequency of borborygmi, excess flatus, abdominal pain, distended stomach (bloating) and nausea. For vomiting, information collected included occurrence and frequency. Symptom intensity was graded as 0 (none), 1 (mild, no restriction of everyday activities), 2 (average, partial limitation of everyday activities) and 3 (severe, inability to perform everyday activities) except for vomiting. Forms were checked by the investigator during an end-of-study interview with the child and accompanying adult to ensure that all symptoms experienced by the child after consuming the test drink were properly recorded.
Total symptom scores were the sum of the intensities for each of the five GI symptoms. A score of zero meant no symptoms, and a score of 15 indicated that the intensity of all five symptoms was rated as severe. For each symptom and composite score at each time point, the maximum intensity was added for a maximum symptom intensity score.
Urinary erythritol excretion
Urine was collected for 24 h after consumption of the test drink and analysed for erythritol by high-performance liquid chromatography to estimate the proportion excreted during the 0- to 24- h period.
Adverse events
Any adverse change in health or side effect during the study was recorded as an adverse event. Each adverse event was classified by the investigator who further defined the severity and potential causality.
Ethics
This study was conducted according to the standards of good clinical practice for the evaluation of medical devices and medicinal products (ICH topic 6), the declaration of Helsinki (1975 and 1983) and French legislation. Three clinical investigation centres were involved, all based in France: Robert Debré Hospital in Paris, Louis Pradel Hospital in Bron and Biofortis in Nantes. Study protocol and all amendments were approved by the ethics committee of Saint-Germain-en-Laye and by relevant French Health Authorities.
Statistics
Statistical analyses included descriptive statistics (for example, number of subjects, mean, s.d., median and minimum-maximum) for quantitative variables, and frequencies and percentages of the number of individuals examined for qualitative variables. The number of children required for the 5 g group was estimated assuming that the incidence of diarrhoea and/or severe GI symptoms between erythritol and placebo would be≥30% with a statistical power of 80% and 10% significance level (one sided). Upon completion of the 5 g group, the sample size was re-estimated assuming that the upper limit of the 90% confidence interval for the difference in the incidence of diarrhoea and/or severe GI symptoms would be≤39%. Statistical analyses were conducted using SAS 9.1.3 Service Pack 4 statistical software (SAS Institute, Cary, NC, USA). Statistical analyses for the assessment of the threshold level were with the risk of type 1 error (alpha) set at 0.10, one sided. All other statistical tests were with the risk of type 1 error (alpha) set at 0.05, two-sided. The analysis was performed on the intention to treat (ITT) population. The modified intention to treat population included all randomised children who received at least one dose of one test preparation, regardless of the quantity consumed. Qualitative variables were analysed after each sub-study using Mainland Gart’s test24 as it takes into account that the product groups were not independent. To ensure that erythritol was comparable to placebo when no statistically significant difference was found, a confidence interval at 90% was computed by applying the May and Johnson method.25
For the secondary analysis, Mainland Gart’s test and the bilateral Kappa test26 were used for the binary variable and nominal or ordinal variable.
Quantitative variables were analysed using repeated measures analysis of variance modelling with product group, sequence and product group by sequence interaction as effects. Models were reduced in a stepwise manner until only significant (P⩽0.05) terms or product group remained. Within each group, the effect of treatment was analysed for effects on diarrhoea, bowel movement frequency and BSS ratings, GI symptoms and urinary erythritol concentration.
Results
A total of 185 children were included in one of the 4 dose groups and exposed to only one single dose vs placebo to minimise study burden. The CONSORT flow diagram is shown in Figure 1.
The mean age of the children in the intention to treat population varied between the 4 dose groups from 4.4 to 5.0 years, and no significant differences were found for gender distribution, age, weight and height. The baseline demographic characteristics are summarised in Table 3.
Similar results were observed in the modified intention to treat and the per protocol population. Regardless of the population analysed, compliance was ⩾97% for any of the groups, and there were no significant differences in compliance between or within the four groups. Gastrointestinal tolerability was assessed by the occurrence of diarrhoea and/or severe GI symptoms. The data on GI tolerability of the four doses of erythritol and placebo are presented in Table 4.
A total of 33 children experienced diarrhoea and/or severe GI symptoms after erythritol consumption. In the 5 and 15 g groups, no significant differences were found vs placebo in the incidence of diarrhoea and/or severe GI symptoms. The erythritol group had significantly more events than the corresponding placebo group for 20 g (7 vs 0, P =0.0286) or 25 g (19 vs 1, P<0.0001).
Data for bowel movement frequency and consistency are presented in Table 5. The average number of bowel movements during the study period was not different between the 5, 15 and 20 -g erythritol groups and placebo, whereas an increase was noted in the 25 g group during the first 24- h period (1.49 vs 1.02 bowel movements per day for erythritol beverage and placebo, respectively, P=0.0188). There was a statistically significant difference for the 5- g erythritol group vs placebo during the 24- to 48 -h time period that was not clinically significant.
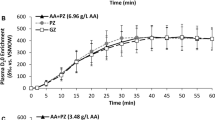
Differences in stool consistency were noted during the first 24- h period, but there were no differences in any of the groups during the later time point of 24–48 h. Stool consistency analysis indicated a significant and clinically meaningful difference in the stools for the 20- and 25- g erythritol groups vs placebo. Stool consistency (BSS) during the first 24- hour period was 4.22 vs 2.91 and 4.94 vs 3.34, respectively, in the 20 and 25 -g erythritol vs placebo (P<0.0001 in both cases), whereas there was no difference for the 5 g group and only a small but statistically significant difference in the 15 g group.
Terms for period, sequence, treatment by period and treatment by sequence were not found to be statistically significant (P>0.05) and were not included in the final statistical model for bowel movements per day and stool consistency.
The incidences of nausea, vomiting, borborygmi, excess flatus, bloating and abdominal pain were not different between erythritol and placebo for any of the dose groups.
The mean total symptom intensity score from GI symptoms questionnaire was only significantly different between 25- g erythritol dose and placebo (0.82 for erythritol and 0.44 for placebo, P=0.0281).
Urinary erythritol excretion over a 24- h period following consumption of erythritol-containing beverage was 66–85% of the ingested dose.
Discussion
Erythritol is a low-molecular-weight non-caloric bulk sweetener that is readily absorbed from the small intestine. About 90% of the ingested dose is excreted unchanged in the urine in 72 hours in adults.16 Consequently, very little moves on to the colon, resulting in a high no observed effect level for laxation, as has been demonstrated in adults13, 18, 27 but not yet in young children. In the present study, young children aged 4–6 y (subjects in this age group are most exposed to beverages on a g/kg bw basis) were included in one of the 4 dose groups (5, 15, 20 and 25 g erythritol) and exposed in random order to a beverage containing erythritol vs an isosweet sucrose placebo. Beverage was chosen as delivery form in order to run the study under most stringent use conditions (fast transit through the small intestine). However, there are limits to the volume that a young child can drink within 15 min. Therefore, a fixed volume of 250 ml was chosen for all doses. This is a weakness of this study, as using concentrations above 3.5% erythritol (isotonic) is likely causing a delayed absorption owing to local hyperosmolar conditions when the test drink reaches the small intestine leading to an influx of water into the gut until iso-osmolarity is achieved.
Results demonstrated that ingestion of up to and including 15 g (0.73 g/kg bw for both genders, 0.72 g/kg bw for boys and 0.76 g/kg bw for girls) of erythritol in 250 ml of beverage (6% w/v) consumed in a single drinking occasion within 15 min by young children is well tolerated.
In this study, the primary outcome variable 'GI tolerability' was measured by the incidence of diarrhoea and/or severe GI symptoms where diarrhoea was defined as the passing ⩾1 watery stool (bowel movements with a BSS score of 7) or >3 bowel movements (regardless of consistency) in a 24 -h period. This definition is not perfect and rather conservative by including severe GI symptoms events into it. Additional statistical analysis on the 15 g group solely based on diarrhoea events and excluding severe GI symptoms events also demonstrated no statistical significant difference between 15 g of erythritol and placebo. WHO defines diarrhoea as the passage of three or more loose or liquid stools (bowel movements with a BSS score of 6 or 7) per day or more frequent passage than is normal for the individual (passing of formed stools is not diarrhoea). Applying the WHO definition, there were no incidents at all in the 5- g dose group, one incident with erythritol and 1 incident with the placebo in the 15- g dose group, four incidents with erythritol and none with the placebo in the 20 g group and 14 incidents with erythritol and none with the placebo in the 25 g group.
Differences in stool consistency in the 20 and 25 g group were noted only during the first 24- h period; there were no differences during the 24- to 48 h period, which demonstrates the transient nature of these effects. In addition, there were no differences in the incidence of nausea, vomiting, borborygmi, excess flatus, bloating and abdominal pain between erythritol and placebo for the 5-, 15-, 20- and 25- g dose groups. The small difference in stool consistency in the 15 g group was of minimal clinical significance, as a BSS rating of 3 to 4 is ideal and mean stool consistency was 3.76 with 15 g vs 2.98 during 24 h following placebo consumption. Urinary erythritol excretion as a percentage of ingested dose was consistent with prior studies in adults and not yet completed after 24 h.16
Previous work27 in adults indicated that the maximum bolus of erythritol not causing laxation (no observed effect level) was 0.80 g/kg bw for women and 0.66 g/kg bw for men, equivalent to an approximate 42 -g bolus dose of erythritol for both genders. In the present study, the no observed effect level for young children is very similar on bw basis compared with adults and not significantly different between boys and girls.
Laxation is viewed by the SCF20 and other regulatory authorities as being due to the high local osmotic activity of unabsorbed erythritol in the gut owing to excessive bolus dosing. These intestinal effects are common to all polyols and other low digestible low-molecular-weight carbohydrates such as lactose and tagatose. Rapid consumption of a bolus dose of erythritol in a beverage outside of a normal meal occasion represents a worst case scenario. Dietary consumption of erythritol in foods is more likely to occur spread over the day and in combination with other foods, resulting in a higher threshold dose before GI effects emerge. Tetzloff et al.13 demonstrated in adults that repeated ingestion of erythritol incorporated into various food and beverages spread over the day at a daily dose of 1 g/kg bw (mean 79 g/day) during 5 days was tolerated equally well as sucrose. In comparison, the highest no observed effect level for laxation in adults established after rapid ingestion of a liquid bolus dose of erythritol on an empty stomach is 50 g or 0.78±0.19 g/kg bw (mean±s.d.).18
In conclusion, this study demonstrated that the maximum tolerated bolus dose without causing diarrhoea and/or severe GI symptoms in 4–6-year-old children when consumed in a clear beverage containing 6% w/v erythritol (250 ml total volume) is 15 g of erythritol or 0.73 g/kg bw. This is similar to adults on a g/kg bw basis.18, 27
References
de Cock P . Erythritol. In: O'Donnell K, Kearsley M (eds) Sweeteners and Sugar Alternatives in Food Technology. Wiley-Blackwell, 2012, pp 213–241.
Bernt WO, Borzelleca JF, Flamm G, Munro IC . Erythritol: a review of biological and toxicological studies. Regul Toxicol Pharmacol 1996; 24: S191–S197.
EU. European Union Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. Official J Eur Union 2008; 51 (L 285), 9–12.
Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T . Noncariogenicity of erythritol as a substrate. Caries Res 1992; 26: 358–362.
Bornet FR, Blayo A, Dauchy F, Slama G . Plasma and urine kinetics of erythritol after oral ingestion by healthy humans. Regul Toxicol Pharmacol 1996a; 24: S280–S285.
Ishikawa M, Miyashita M, Kawashima Y, Nakamura T, Saitou N, Modderman J . Effects of oral administration of erythritol on patients with diabetes. Regul Toxicol Pharmacol 1996; 24: S303–S308.
Livesey G . Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev 2003; 16: 163–191.
Bornet FR, Blayo A, Dauchy F, Slama G . Gastrointestinal response and plasma and urine determinations in human subjects given erythritol. Regul Toxicol Pharmacol 1996b; 24: S296–S302.
Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G . Metabolism of erythritol in humans: comparison with glucose and lactitol. Br J Nutr 1993; 69: 169–176.
Noda K, Nakayama K, Oku T . Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects. Eur J Clin Nutr 1994; 48: 286–292.
Noda K, Nakayama K, Modderman J . Fate of erythritol after single oral administration to rats and dogs. Regul Toxicol Pharmacol 1996; 24: S206–S213.
Oku T, Noda K . Erythritol balance study and estimation of metabolisable energy of erythritol. In: Hosoya N (ed) Proceedings of the International Symposium on Caloric Evaluation of Carbohydrates. Japan Association of Dietetic and Enriched Foods: Tokyo, Japan, 1990, pp 65–75.
Tetzloff W, Dauchy F, Medimagh S, Carr D, Bar A . Tolerance to subchronic, high-dose ingestion of erythritol in human volunteers. Regul Toxicol Pharmacol 1996; 24: S286–S295.
Schiweck H, Ziesenitz SC . Physiological properties of polyols in comparison with easily metabolisable saccharides. In: Grenby TH (ed) Advances in Sweeteners. Springer US: Glasgow, UK, 1996, pp 56–84.
Arrigoni E, Brouns F, Amado R . Human gut microbiota does not ferment erythritol. Br J Nutr 2005; 94: 643–646.
Munro IC, Berndt WO, Borzelleca JF, Flamm G, Lynch BS, Kennepohl E et al. Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem Toxicol 1998; 36: 1139–1174.
Lee A, Wils D, Zumbe A, Storey DM . The comparative gastrointestinal responses of children and adults following consumption of sweets formulated with sucrose, isomalt and lycasin HBC. Eur J Clin Nutr 2002; 56: 755–764.
Storey D, Lee A, Bornet F, Brouns F . Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur J Clin Nutr 2007; 61: 349–354.
Lifshitz F, Ament ME, Kleinman RE, Klish W, Lebenthal E, Perman J et al. Role of juice carbohydrate malabsorption in chronic nonspecific diarrhea in children. J Pediatr 1992; 120: 825–829.
SCF. Opinion of the Scientific Committee on Food on Erythritol. European Commission, Health and Consumer Protection Directorate-General. SCF/CS/ADD/EDUL/215 Final. Opinion expressed on 5 March 2003 http://ec.europa.eu/food/fs/sc/scf/out175_en.pdf.
WHO. Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser 2000; 896: 1–128.
EU. European Union Commission Directive 2006/52/EC of 26 July 2006 amending Directive 95/2/EC on food additives other than colours and sweeteners and Directive 94/35/EC on sweeteners for use in foodstuffs. Official J Eur Union 2006; 49 (L 204), 10–22.
Lewis SJ, Heaton KW . Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924.
GART JJ . An exact test for comparing matched proportions in crossover designs. Biometrika 1969; 56: 75–80.
May WL, Johnson WD . Confidence intervals for differences in correlated binary proportions. Stat Med 1997; 16: 2127–2136.
Agresti A . Categorical Data Analysis. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002.
Oku T, Okazaki M . Laxative threshold of sugar alcohol erythritol in human subjects. Nutr Res 1996; 16: 577–589.
Acknowledgements
This research was funded by Cargill. Erythritol-containing beverages were provided by Cargill R&D Centre Europe. The funder had no role in study design, data collection and statistical analysis. A special thanks goes to all children who participated in this research. Roles of the authors: EJA was responsible for study design, execution and reporting; EJA, BK and JMC designed and performed the study; CC contributed to study execution; BH, MC, PDC and AB contributed to study planning; JMP and MB performed statistical analysis; AB and PDC wrote the paper with contributions from all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Cargill funded this research. AB and PDC were employed by funder. No further conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Jacqz-Aigrain, E., Kassai, B., Cornu, C. et al. Gastrointestinal tolerance of erythritol-containing beverage in young children: a double-blind, randomised controlled trial. Eur J Clin Nutr 69, 746–751 (2015). https://doi.org/10.1038/ejcn.2015.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.4
- Springer Nature Limited