Abstract
Background/objectives:
We aimed to quantify the association of sarcopenia with length of hospital stay (LOS) and to identify factors associated with sarcopenia among hospitalized patients.
Subjects/methods:
A total of 655 patients composed the study sample. A longitudinal study was conducted in a University Hospital. Sarcopenia was defined, according to European Consensus criteria, as low muscle mass (bioelectrical impedance analysis) and low muscle function (handgrip strength). Logistic regression, Kaplan–Meier and Cox adjusted proportional hazards methods were used. LOS was determined from the date of hospital admission and discharge home (event of interest).
Results:
Participants were aged 18 to 90 years (24.3% sarcopenic). Factors associated with sarcopenia were male gender, age ⩾65 years, moderate or severe dependence, undernutrition and being admitted to a medical ward. Sarcopenic patients presented a lower probability of being discharged home (hazard ratio (HR), 95% confidence interval (CI)=0.71, 0.58–0.86). However, after stratifying for age groups, this effect was visible only in patients aged <65 years (HR, 95% CI= 0.66, 0.51–0.86). Moreover, sarcopenic overweight or obese patients presented a higher probability of being discharged home (HR, 95% CI=0.78, 0.61–0.99) than nonoverweight sarcopenic patients (HR, 95% CI=0.63, 0.48–0.83).
Conclusions:
Being male, age ⩾65 years, presenting dependence, being undernourished and admitted to a medical ward were factors associated with sarcopenia among hospitalized adult patients. Sarcopenia is independently associated with longer LOS, although this association is stronger for patients aged <65 years. Moreover, sarcopenic overweight was associated with a higher probability of discharge home than nonoverweight sarcopenia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
According to the European Working Group on Sarcopenia in Older People (EWGSOP), sarcopenia is defined as a combination of both low muscle mass and low muscle function.1 This condition has been associated with physical disability, low quality of life and higher mortality.1, 2
Sarcopenia is estimated to occur between 5 and 45% of community-dwelling older adults.3, 4 Although this condition is mainly observed in older adults, it can also be present in younger individuals. A study from 2013 by Cherin et al.5 showed that 9% of the individuals aged between 45 and 54 years and 13.5% of those aged from 55 to 64 years were sarcopenic. Although data concerning sarcopenia in hospitalized patients are scarce, previous studies have described this condition as frequent among hospitalized older patients,6, 7, 8, 9, 10 ranging from 10 to 37.3%. Moreover, it has been recently shown that sarcopenia is present in hospitalized patients aged under 65 years, with a frequency equal to 19.8%.9
It has been previously reported that sarcopenia is related with poor clinical outcome in hospitalized older patients, namely higher mortality 6, 8, 11 and higher risk of non-elective readmission in a 6-month period.6
In a study conducted among hospitalized patients aged ⩾65 years,6 sarcopenic patients presenting a mean age of 79 years were reported to have a higher length of hospital stay (LOS) than nonsarcopenic patients. In contrast, Cerri et al.8 found no differences in LOS between sarcopenic and nonsarcopenic patients, among hospitalized older patients, with a mean age of 84.2 years, ranging from 66 to 100 years. Nevertheless, as far as we are concerned, there are no available data on the impact of sarcopenia on LOS among hospitalized patients aged <65 years. LOS is an indicator of the changes that occur during a hospitalization process and can be used as a surrogate marker of health status.12 Moreover, predicting LOS may lead to a maximization of resources.13
According to our knowledge, data on factors associated with sarcopenia in hospitalized patients are scarce, whereas they are particularly limited among hospitalized younger patients. Moreover, the potential effect of confounding factors on the association between sarcopenia and LOS remains to be described. Identification of sarcopenia and the establishment of an association between this condition and LOS are of utmost importance in order to provide a more effective healthcare plan, thus reducing the adverse consequences this condition entails.
This study aims to quantify the association of sarcopenia with LOS, after adjustment for potential confounders, and to identify factors associated with sarcopenia among a wide-ranging sample of hospitalized adult patients.
Materials and methods
Study sample and design
A longitudinal study was conducted in a general, university and 600-bed hospital between July 2011 and December 2014. A consecutive sampling method was applied in medical and surgical wards. Patients were eligible to participate in the study if they were aged 18 years and over, Caucasian, with an expected hospital stay longer than 24 h, conscious, cooperative and capable of providing written informed consent.
Patients who were unable to perform the handgrip strength (HGS) technique were excluded from the study. This impossibility in carrying out HGS measurement was defined as an inability to understand verbal instructions or having a condition limiting HGS measurement (namely pain). Critically ill patients, that is, with a life-threatening medical or surgical condition requiring intensive care unit level care, presenting severe organ system dysfunction and needing for active therapeutic support, were excluded.14 Pregnancy and patient ward isolation were also defined as exclusion criteria. According to these criteria, patients admitted to neurology, clinical hematology and intensive care unit wards were not recruited, whereas participants from the following departments were selected: angiology and vascular surgery, cardiology, digestive surgery, endocrinology, gastroenterology, hepatobiliary surgery, internal medicine, nephrology, nondigestive surgery, orthopedics, otorhinolaryngology and urology. Therefore, from the daily list of inpatients admitted to each of these wards, those who fulfilled the inclusion criteria were invited to participate in the study, until the number of patients had attained the total number of beds of the ward.
From 992 patients who fulfilled the inclusion criteria and were invited to participate, 337 (34%) were not included. The reasons were refusals (n=198), cognitive impairment (n=13) and missing data (n=126).
All patients were followed up from the time of admission until death, hospital discharge or 30 days after admission.
Ethics
This research was carried out according to the recommendations established by the Declaration of Helsinki and approved by the Institutional ethics and review boards of Centro Hospitalar do Porto. All study participants provided a written informed consent.
Data collection
Demographical, clinical data, medical diagnoses and data of hospital admission were retrieved from patient's clinical file at the time of evaluation. Date of hospital discharge, discharge destination (home, another ward, another hospital, continuing care unit and discharge against medical advice or death) and discharge diagnosis were retrieved from hospital records after patient discharge. All other information was obtained by two trained registered nutritionists through a structured questionnaire within 72 h of admission to hospital.
Education was evaluated by the number of completed school years, and the following categories were created: 0–4, 5–12 and >12 years. Marital status was categorized as single, married or in a civil partnership, divorced and widowed. Cognitive impairment was evaluated with the Abbreviated Mental Test (AMT).15 Independence in activities of daily living was assessed with the Katz index.16 Charlson disease severity index17 was obtained by two previously trained interviewers using medical discharge diagnoses in the patient's clinical record.
Patient nutritional status was evaluated with patient-generated subjective global assessment (PG-SGA).18 Standing height (cm) was measured with a metal tape (Rosscraft, Innovations Incorporated, Surrey, Canada) with a 0.1-cm resolution and a headboard. Body weight (kg) was assessed with a calibrated portable beam scale with a 0.5 -kg resolution. All anthropometric measurements were performed by two previously trained registered nutritionists using standard methods.19 The intra- and inter-observer technical error of measurement was calculated for all measurements, respectively, in 17 and 18 individuals. Intra-observer error ranged from 0.2 to 0.6%, and inter-observer error ranged from 0 to 1.4%. These values are considered acceptable for trained anthropometrists.20
Body mass index (BMI) was determined through the standard formula (weight (kg)/height2 (m)), and BMI categories were created according to the World Health Organization cutoffs.21
Sarcopenia was defined according to the EWGSOP as the presence of both low muscle mass and low muscle function.1
Whole-body resistance (ohms) and reactance (ohms) were assessed through tetrapolar bioelectrical impedance analysis (BIA) using a Biodynamics Model 450 (Seattle, WA, USA) with 0.1 ohm resolution, operating at a single frequency of 50 kHz.
Muscle mass was evaluated using the equation of Janssen et al.22 ((height2/resistance × 0.401)+(gender × 3.825)+(age × –0.071))+5.102, with height measured in cm, resistance measured in ohms, for gender, men=1 and women=0, age measured in years. Muscle mass was adjusted for height. Gender-specific cutoff points indicated in the EWGSOP consensus were used.1
Muscle function was evaluated as HGS, using a calibrated Jamar Hydraulic Hand dynamometer (Sammons Preston, Bolingbrook, IL, USA), with a 0.1- kgf resolution. The Jamar dynamometer is proposed by the American Society of Hand Therapists as the gold standard for measurements of HGS.23 Each subject undertook three measurements using the nondominant hand with a 1-min interval between measurements, and the maximum value was selected.24 Low HGS was classified using the cutoffs proposed in the EWGSOP Consensus:1 <30 kgf for men and 20 kgf for women.
Statistics
According to the normality of variables distribution, evaluated through Kolmogorov–Smirnov test, results were described as mean and standard deviation or as median and interquartile range (IQR) if the distribution was non-normal. Categorical variables were reported as frequencies.
To identify variables associated with sarcopenia by bivariable analysis, sarcopenic and nonsarcopenic patients were compared for several demographic and clinical characteristics. Bivariable and multivariable logistic regression models were also conducted. Variables were included in the multivariable logistic regression model considering their potential confounding effect. LOS was dichotomized according to a cutoff of 7 days based on the median LOS of the entire sample, and in agreement with the median LOS in Portuguese hospitals.25 Variables associated with longer LOS (⩾7 days) were identified comparing patients with and without a long LOS. All the comparisons for continuous variables were computed using the Mann–Whitney test or Student’s t-test for independent samples. For categorical variables, Pearson χ2 or Fisher’s Exact test were used.
LOS was determined from the date of hospital admission and discharge to usual residence (the main event of interest). Patients who were not discharged from the hospital to usual residence within the study period were censored at the time of other events, namely death, transfer (to another hospital ward, to another hospital or to continuing care units) and discharge against medical advice (n=40). LOS was censored at 30 days, and thus patients who remained hospitalized 30 days after hospital admission were also censored (n=16). The Kaplan–Meier method was used to estimate the cumulative probability of being discharge-free over time (that is, to experience the event of interest, defined as discharge home within the follow-up interval), according to the presence or the absence of sarcopenia.
Multivariable Cox proportional hazards regression models were used to estimate adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The following characteristics were considered in the multivariable procedure: presence of sarcopenia (categorical), age (categorical), Charlson index (continuous), nutritional status categories according to PG-SGA (categorical), education (categorical), Katz index (categorical), gender (categorical), marital status (categorical) and AMT (continuous).
Statistical significance was set at P<0.05. All analyses were conducted with the Software Package for Social Sciences (SPSS) for Windows (version 20.0; SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics of the 655 hospitalized patients enrolled in this study, according to sarcopenia status, are shown in Table 1. Approximately half of the patients were women (46.1%), and their ages ranged between 18 and 90 years (median (IQR)=56 (22) years). Frequency of sarcopenia was 24.3%. Within the period this study was conducted two patients had died. Therefore, the mortality rate was 0.3%.
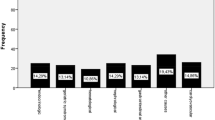
Sarcopenic patients were older and presented longer LOS than nonsarcopenic patients (Table 1). In addition, they were more likely to be male, to be undernourished and to present higher Charlson index score than nonsarcopenic patients (Table 1). There was a higher proportion of sarcopenic patients in medical wards than in surgical wards. The highest proportion of sarcopenic patients (34.3%) was observed in internal medicine wards. Otorhinolaryngology presented the lowest proportion of sarcopenic patients (1.9%).
It is worth noticing that patients aged⩾65 years presented lower muscle mass (median (IQR) 24.8 (11.4) kg) than patients aged <65 years (median (IQR) 26.4 (11.4) kg), P=0.008. Older patients also presented lower HGS than patients aged <65 years (median (IQR) 22.0 (9.8) kgf versus median (IQR) 24.1 (17.5) kgf), P<0.001.
As shown in Table 2, after adjusting for potential confounders, being male, age ⩾65 years, presenting moderate or severe dependence, being undernourished and being admitted to a medical ward were factors associated with sarcopenia.
It is worth mentioning that, compared with patients with a short LOS, patients with longer hospital stay were older, had a lower education level, were less likely to be single, presented a lower AMT score, were more likely to be dependent according to Katz index, were less likely to be overweight or obese, presented reduced HGS, were more likely to be undernourished, sarcopenic and presented a higher Charlson index score.
Figure 1 shows the probability of being discharge-free over time according to the presence of sarcopenia, considering all the participants (Figure 1a), and stratified by age groups, <65 years (Figure 1b) and ⩾65 years (Figure 1c). Sarcopenic patients presented a lower probability of experiencing the event of interest (being discharged home), as displayed in Figures 1a and b. However, for patients aged ⩾65 years, this effect was no longer visible (Figure 1c).
The association of sarcopenia with overweight or obesity (BMI ⩾25 kg/m2) was also evaluated. From all sarcopenic patients (n=159), 44% presented only sarcopenia and 56% of the patients were simultaneously overweight (or obese) and sarcopenic. It is noteworthy that sarcopenic patients who were simultaneously overweight or obese presented higher muscle mass than nonoverweight sarcopenic patients, median 25.5 (12.4) kg vs median 24.1 (9.8) kg, P<0.001, and also higher muscle mass adjusted for height, mean 10.1 (2.0) kg/m2 vs mean 9.1 (1.7) kg/m2, P<0.001. Compared with nonsarcopenic patients, sarcopenic patients present a lower probability of being discharged home. However, patients with nonoverweight sarcopenia presented a lower probability of being discharged home compared with sarcopenic overweight patients (P<0.001; Figure 1d).
Results from multivariable Cox proportional hazards regression models were displayed for the entire sample and according to age groups (Table 3). The model was adjusted for age, gender, marital status, education, nutritional status, Charlson index, AMT score and Katz index, as these variables could be considered as potential confounders in the association between sarcopenia and LOS.
Considering the entire sample and the group of patients aged <65 years, sarcopenia was consistently associated with lower HR (<1) for being discharged home, meaning that sarcopenic patients presented a lower probability of being discharged home. However, for patients aged ⩾65 years, sarcopenia was not independently associated with the probability of being discharged home.
It is worth noticing that sarcopenic overweight or obese patients presented a higher probability of being discharged home (adjusted HR (95% CI)=0.78 (0.61–0.99)) than nonoverweight sarcopenic patients (adjusted HR (95% CI)=0.63 (0.48–0.83)).
In addition, LOS had also been stratified according to hospital ward (medical or surgical) and, as expected, there was a higher proportion of patients with a longer LOS (⩾7 days) admitted to medical wards (53%) than in surgical wards (47%), P=0.019. Thus, the type of hospital ward was included in an additional multivariable Cox proportional hazards regression model. However, the inclusion of this variable did not modify the results concerning the probability of being discharged home.
Discussion
This study results show that sarcopenic patients presented a lower probability of being discharged from the hospital. Cox analysis revealed that sarcopenia is associated with longer LOS after considering the confounding effect of age, gender, marital status, education, nutritional status, disease severity, cognitive impairment and independence in daily living activities. However, after stratifying this analysis by age groups, this association was only observed for patients aged <65 years. This may be explained by a lower proportion of older patients in the study sample (approximately 31%), which leads to a loss of statistical power, increasing the possibility of occurrence of a type two error or, alternatively, by different clinical characteristics; that is, the simultaneous presence of several comorbidities in older patients could have diminished the strength of the association of sarcopenia with LOS. There may be other explanations for this situation, such as an overall lower probability of being discharged home, and a higher prevalence of undernutrition in those patients who were aged ⩾65 years, resulting in a coexistence of undernutrition with sarcopenia. Although our results were adjusted in a multivariable model for age and the presence of undernutrition, the confounding effect of other factors not assessed in the present study cannot be disregarded, while residual confounding may still be present.
The results of this study increased the knowledge and highlighted the impact of sarcopenia on LOS, specifically among hospitalized younger patients (<65 years). Besides, as far as we are concerned, there were no previous data concerning factors associated with sarcopenia among hospitalized younger patients, with the exception for previous results from a recent study undertaken by our research team.9
Gariballa and Alessa,6 in a study conducted among hospitalized older patients, which defined sarcopenia with muscle mass assessed through mid-arm muscle circumference and muscle function evaluated by HGS, concluded that LOS was significantly higher in sarcopenic patients compared with nonsarcopenic patients. Otherwise, in a study conducted by Cerri et al.8 among hospitalized undernourished older patients no differences in LOS were found between sarcopenic and nonsarcopenic patients. Using similar methodology, our results for older patients corroborate the findings of Cerri et al.8 Although the present results are not in accordance with Gariballa and Alessa, the observed differences between studies may be explained by the use of different methodologies, BIA and anthropometry, in the assessment of muscle mass and by different patients’ characteristics. However, our results clearly show a significant association of sarcopenia with prolonged LOS for patients aged under 65 years.
The difference observed for the <65-year and ⩾65-year groups concerning the association of sarcopenia with LOS may be justified by the existence of different characteristics, diagnoses and, even, higher severity of comorbidities between younger and older adult patients, besides the possible occurrence of a type two error, as hypothesized before in this section.
This study results also showed that sarcopenic overweight or obese patients (BMI ⩾25 kg/m2) had a higher probability of being discharged home than sarcopenic nonoverweight patients. This can possibly be explained by the coexistence of undernutrition and sarcopenia, as there was a considerable proportion of sarcopenic patients (approximately 60%) who were simultaneously undernourished. This situation can hypothetically lead to a protective effect of overweight and obesity. Another possible explanation is that overweight (or obese) patients presented significantly higher muscle mass than nonoverweight patients. Thus, characteristics related to overweight and higher muscle mass could have introduced a protective effect for being discharged from the hospital. Notwithstanding this, because of the presence of overweight or obesity, these patients may not present obvious frailty physical features. This may have influenced caregivers and biased the indication for discharge destination.
This study shows a frequency of sarcopenia among hospitalized older adults of 36.4%, being higher than previous reports, 10% from Gariballa and Alessa,6, 7 25.3% from Smoliner et al.,7, 8 26% from Rossi et al.10, 11 and 21.4% from Cerri et al.8, 9 These differences may be because of the use of different methodologies and because of patients’ characteristics. This study also identified sarcopenia in 18.8% of the hospitalized patients aged under 65 years. However, it is noteworthy that cutoff points used were previously defined for use in older adults, as sarcopenia was considered as a geriatric condition. This situation may have biased present results with a possible underdiagnosis of sarcopenia. Moreover, the EWGSOP also indicates physical performance as a possible parameter in sarcopenia diagnosis; that is, sarcopenia can be defined as low muscle mass and low muscle strength or as low muscle mass and low physical performance (for example, gait speed test). In this study, physical performance was not assessed because of the characteristics of our sample, as this test is not applicable to all hospitalized patients.8 Consequently, some sarcopenia diagnoses in patients with low muscle mass and low physical performance but normal HGS could have been missed.
Patients from intensive care units and other critical patients were excluded from this study because of their inability to perform the required functional tests to identify sarcopenia. This situation may constitute a study limitation because critical patients because of their clinical condition would be likely to present muscle mass depletion and reduced function and, therefore, to be sarcopenic. Furthermore, the inclusion of muscle function (physical performance) in the definition and diagnostic criteria of sarcopenia may impair the identification of sarcopenia among critical patients and patients unable to perform functional tests. Moreover, it is important to highlight that HGS of patients unable to stand was measured with individuals on a bed. Although a differential may exist between measurements performed with the individual in a sitting or lying position, care was taken in order to strictly follow the HGS measurement protocol.24 Specifically, HGS was obtained from all participants with the unsupported elbow.26
In this study, muscle mass was estimated through BIA, instead of using computed tomography or magnetic resonance imaging, the golden standards for quantifying muscle mass, or dual energy X-ray absorptiometry, the selected alternative for estimating muscle mass in research and clinical use.1 This could be regarded as a study limitation. However, BIA results are readily reproducible, and this is an economical, practical and portable method, which, used under standard conditions, has been found to be a good alternative to dual energy X-ray absorptiometry.5 Although BIA may not be reliable in conditions such as heart failure, kidney failure and dehydration, after applying inclusion criteria, not all patients with these conditions were excluded. This may have led to a misclassification of muscle mass and subsequently to a misclassification of sarcopenia.
According to hospital discharge records, the proportion of discharged patients aged over 65 years was 38.3% in 2012 and 40% in 2013. Our sample contains less patients aged over 65 years (31%). This may have resulted in a lower representation of an important group of high-risk patients, underestimating sarcopenia burden. Nonetheless, the diagnostic criteria of sarcopenia recommended by the European Consensus necessitate the application of functional tests, thus excluding patients who are unable to carry out these tests.8 The lower representation of older patients in this sample may be explained by the need to fulfill the criteria.
Several strengths of this study could be highlighted. A large number of hospitalized patients composed this study sample, with a wide age range, 18–90 years. The patients enrolled in this study were from a multiplicity of hospital surgical and medical wards, which ensured a large variety of diagnoses and different diseases. These characteristics strengthen the generalizability of our results for other hospitalized patients.
Survival analysis has the ability of handling data that are censored, which in this study were death, transfer, discharge against medical advice and LOS >30 days. This allows for a better hospital representation, because it permits the inclusion of cases that could not be included with other statistical approaches, namely, with follow-up information unavailable after a certain point, which in our study was 30 days after hospital admission. Nonetheless, only 16 participants (2.4% of the study sample) had a LOS longer than 30 days, and thus an extended follow-up period probably would not have changed the results obtained.
Although there are some results available concerning mortality and hospital readmission for older patients,6, 8, 11 further research is required in order to assess short-term and long-term consequences of sarcopenia in hospitalized patients.
Being male, age ⩾65 years, presenting dependence, being undernourished and being admitted to a medical ward are factors associated with sarcopenia among hospitalized adult patients. Sarcopenia is independently associated with longer LOS, although this association is stronger for patients aged <65 years. Moreover, sarcopenic overweight is associated with a higher probability of discharge to usual residence than nonoverweight sarcopenia.
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 2010; 39: 412–423.
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012; 31: 652–658.
Abellan van Kan G . Epidemiology and consequences of sarcopenia. J Nutr Health Aging 2009; 13: 708–712.
Volpato S, Bianchi L, Cherubini A, Landi F, Maggio M, Savino E et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci 2014; 69: 438–446.
Cherin P, Voronska E, Fraoucene N, de Jaeger C . Prevalence of sarcopenia among healthy ambulatory subjects: the sarcopenia begins from 45 years. Aging Clin Exp Res 2014; 26: 137–146.
Gariballa S, Alessa A . Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 2013; 32: 772–776.
Smoliner C, Sieber CC, Wirth R . Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 2014; 15: 267–272.
Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr 2014; 34: 745–751.
Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF . Sarcopenia among hospitalized patients - a cross-sectional study. Clin Nutr 2015; 34: 1239–1244.
Rossi AP, Fantin F, Micciolo R, Bertocchi M, Bertassello P, Zanandrea V et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc 2014; 15: 303 e7–303 12.
Vetrano DL, Landi F, Volpato S, Corsonello A, Meloni E, Bernabei R et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci 2014; 69: 1154–1161.
Kyle UG, Genton L, Pichard C . Hospital length of stay and nutritional status. Curr Opin Clin Nutr Metab Care 2005; 8: 397–402.
Omachonu VK, Suthummanon S, Akcin M, Asfour S . Predicting length of stay for Medicare patients at a teaching hospital. Health Serv Manage Res 2004; 17: 1–12.
ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr 2002; 26: 1SA–138SA.
Hodkinson HM . Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972; 1: 233–238.
Katz S . Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983; 31: 721–727.
Charlson ME, Pompei P, Ales KL, MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383.
Ottery F . Patient-generated subjective global assessment. In: Polisena PMC (ed). The Clinical Guide to Oncology Nutrition. The American Dietetic Association: Chicago, IL, USA, 2000, pp 11–23.
Marfell-Jones MOT, Stewart A, Carter L . International Standards for Anthropometric Assessement. ISAK: Potchefstroom, South Africa, 2006.
Pederson D, Gore C . Anthropometry Measurement Error. University of New South Wales Press: Sydney, Australia, 1996.
WHO Expert Committee on Physical Status. Physical status: the use and interpretation of anthropometry, Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995; 854: 1–452.
Janssen I, Heymsfield SB, Baumgartner RN, Ross R . Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000; 89: 465–471.
Fess E . Grip Strength, 2nd edn. American Society of Hand Therapists: Chicago, 1992.
Vaz M, Thangam S, Prabhu A, Shetty PS . Maximal voluntary contraction as a functional indicator of adult chronic undernutrition. Br J Nutr 1996; 76: 9–15.
Matos L, Teixeira MA, Henriques A, Tavares MM, Alvares L, Antunes A et al. [Nutritional status recording in hospitalized patient notes]. Acta Med Port 2007; 20: 503–510.
Hillman TE, Nunes QM, Hornby ST, Stanga Z, Neal KR, Rowlands BJ et al. A practical posture for hand grip dynamometry in the clinical setting. Clin Nutr 2005; 24: 224–228.
Acknowledgements
We thank Centro Hospitalar do Porto and all ward directors for facilitating the data collection. RSG received a scholarship from Fundação para a Ciência e a Tecnologia, financing program POPH/FSE, under the project SFRH/BD/61656/2009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sousa, A., Guerra, R., Fonseca, I. et al. Sarcopenia and length of hospital stay. Eur J Clin Nutr 70, 595–601 (2016). https://doi.org/10.1038/ejcn.2015.207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.207
- Springer Nature Limited
This article is cited by
-
A Critical Review of Current Worldwide Definitions of Sarcopenia
Calcified Tissue International (2023)
-
CT-derived measures of muscle quantity and quality predict poorer outcomes from elective colorectal surgery: a UK multicentre retrospective cohort study
Techniques in Coloproctology (2023)
-
Screening, Diagnosis and Management of Sarcopenia and Frailty in Hospitalized Older Adults: Recommendations from the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group
The Journal of nutrition, health and aging (2022)
-
Force-time curve features of handgrip strength in fibromyalgia syndrome
Scientific Reports (2020)
-
Correlation between the Charlson comorbidity index and skeletal muscle mass/physical performance in hospitalized older people potentially suffering from sarcopenia
BMC Geriatrics (2019)