Abstract
The discovery of T regulatory cells has been one of the most important advances in basic immunology and has opened the door to the development of innovative therapeutic strategies for improving the outcome of solid organ and hematopoietic stem cell transplantation. Basic immunology is rapidly elucidating the complex biology of these cells even though the difficulties in purifying or even expanding them in vitro represent a major limitation to the development of clinical studies. The clinical benefit potentially associated with this therapeutic approach remains to be demonstrated. Meanwhile, several drugs used for the treatment of hematologic malignancies or for other purposes have been shown to upregulate the number and function of Tregs in vivo. In the near future, both ex vivo or in vivo expanded T cells are likely to enter the therapeutic armamentarium of clinical transplantation.
Similar content being viewed by others
Introduction
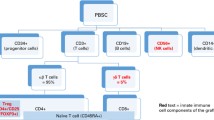
Rejection, graft versus host disease (GvHD) and infections are major complications of allogeneic hematopoietic stem cell transplantation (alloHSCT) and remain the main causes of transplant related morbidity and mortality. These complications still offset a significant part of the transplant benefit and limit the curative use of alloHSCT. Overcoming these complications is the main challenging objective to improve the transplant outcome as well as the patients’ quality of life and to minimize the long-term use of immunosuppressive drugs. The field of immunology was revolutionized when in 1995 Sakaguchi showed that depletion of the small fraction of CD4+ T cells co-expressing CD25 from normal adult T cells left a population of cells that induced a spectrum of autoimmune diseases when transferred to an immunocompromised recipient.1 These T suppressor cells (defined as T regulatory cells, Tregs) suppress the function of other T cells and accordingly, they play a crucial role to limit the immune response, to regulate the immune homeostasis and to maintain self-tolerance.2, 3 Two major classes of Tregs have been identified4 and, according to the Third International Conference on Regulatory T Cells, defined as thymus-derived Treg cell (tTreg cell) and peripherally derived Treg cell (pTreg cell).5 tTreg cells originate from the thymus as CD4+ cells expressing high levels of CD25 and the transcription factor FoxP3, a transcriptional repression factor of the forkhead or winged helix family of transcription factors, also known as Scurfin, IPEX and JM2.6, 7, 8 FoxP3 was initially identified as a Treg marker in mice by Sakaguchi and Rudensky in CD4+ cells.9 tTreg cells represent approximately 1–2% of the total CD4+ T-cell population10 and are positively selected thymocytes with a relatively high avidity for self-Ags.11 The signal to develop into Treg cells comes from the physical interactions between the T-cell receptor/MHC II complex with self-peptides expressed on the thymic stroma.12 pTreg cells are thymic CD4+ cells that differentiate into CD25+ and FoxP3+ expressing Tregs following adequate antigenic stimulation in the presence of cognate Ag and specialized immunoregulatory cytokines such as TGF-β, IL-10 and IL-4 and in the absence of IL-6 and possibly IL-23.13
High levels of Tregs have been found in many malignant disorders including lung, pancreatic and breast cancers suggesting their role in preventing an adequate immune response of the patient against cancer.14 Alterations in the number and function of Tregs have been implicated in several autoimmune diseases including multiple sclerosis, active rheumatoid arthritis and type 1 diabetes.15 Mutations of FOXP3 are associated with the development of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), leading to organ-specific autoimmune diseases, insulin-dependent diabetes and hematological disorders.7
Tregs are ideal candidates for tolerance induction in the clinical context of solid organ and alloHSCT and may represent an ideal tool for an adoptive cellular therapy for inducing tolerance, preventing GvHD and for improving the quality of immune reconstitution without compromising the beneficial graft-versus-leukemia (GvL) effect. Here, we analyze the available data suggesting the role of Tregs in preventing and controlling GvHD. We review experimental data generated in animal models and then the indirect and direct evidence suggesting the clinical role of Tregs in the setting of alloHSCT.
Animal models
Infusion of Tregs reduces the incidence of GvHD, favor post-transplant immune reconstitution and promote GvL
In murine models, experimental data demonstrated that infusion of Tregs reduces the incidence of GvHD and promotes immune reconstitution. Freshly isolated or ex vivo expanded polyclonal Tregs, co-infused with conventional T cells (Tcons) at 1:1 ratio, prevented lethal GvHD.16, 17, 18, 19 The proinflammatory environment induced by pre-transplant irradiation was found to represent a crucial stimulus for early Tregs activation, expansion and migration to GvHD target tissues (skin, gut, liver and lung). Suppression was optimal during the initial phase of inflammation, when Tregs specifically suppressed early alloreactive T-cell proliferation in lymph nodes and in non-lymphoid tissues.17 Conversely, Tregs did not cross-inhibit pathogen-specific Tcons expansion and response, thus allowing functional immune system reconstitution. Indeed, Tregs prevented GvHD-induced damage to the thymus and peripheral lymph node architecture and accelerated donor lymphoid reconstitution with a polyclonal TCR-Vβ repertoire. The enhanced immune reconstitution in Tregs recipient mice protected them from lethal CMV infection.16 The preclinical studies also demonstrated that Tregs could proliferate in the post-conditioning proinflammatory environment of the T-cell depleted host in the 4 to 7 day interval before the Tcons infusion. This in vivo Tregs expansion allowed the subsequent infusion of significant numbers of Tcons without triggering GvHD. More recently, Bolton and colleagues showed that selective Tregs reconstitution before T-cell transfer normalized dendritic cell co-stimulation and provided complete protection against GvHD. Tregs-mediated CTLA-4-dependent downregulation of CD80 and CD86 on dendritic cells was critical to inhibit the rapid proliferation of alloreactive T cells.20
The mechanisms by which Tregs suppress GvHD with no loss of the transplant GvL activity have been described in several studies. In a murine model, Edinger et al. observed that Tregs can inhibit the early expansion of alloreactive donor T cells and their capacity to induce GvHD but do not inhibit co-transplanted Tcons activation and cytotoxicity against leukemia and lymphoma cell lines in vitro and in vivo.21 GvL activity relies mainly on Tcons activation and it is mediated by the perforin lysis pathway so that an efficient GvL effect requires transplantation of a sufficient number of Tcons. In this context, Ruggeri and colleagues, in murine models engrafted with human primary acute myeloid and lymphoid leukemia (AML and ALL) cells, showed that the sequential adoptive transfer of human Tregs and Tcons was able to eradicate leukemia without GvHD. In contrast, mice that received only Tregs died of leukemia, while animals that received only Tcons developed severe GvHD and died within 60 days. These data suggest Tcons can be blocked by Tregs in the periphery and exert unopposed allo-antigen recognition in the bone marrow where Tregs are unable to home because of different migratory properties.22
Recent experimental results indicate that NOTCH should be considered as a major regulator of alloreactivity and tolerance, since its signaling inactivation in donor T cells markedly reduces GvHD severity and mortality.23, 24, 25 Interestingly, a very recent study showed that Tregs trigger NOTCH1 downregulation directly on Tcons through the CD39/adenosine axis.26 NOTCH1 downregulation along with the different Tregs migratory properties might elucidate the observations obtained in clinical trials performed with Treg/Tcons adoptive immunotherapy resulting in potent GvL effects with markedly reduced risk of GvHD.27, 28
Clinical studies
Indirect evidence for a role of Tregs on GvHD
Small retrospective studies have examined the relationship between the content of Tregs in allogeneic hematopoietic stem cell grafts and the transplant outcomes. While some studies suggested a protective effect on GvHD played by a high number of Tregs in the graft29, 30, 31, 32, 33 other studies showed no relationship34, 35 and some even showed the opposite effect.36 More recently, Danby et al.,37 in a cohort of 94 adult patients, reported that patients receiving higher numbers of Tregs had an improved overall survival and a reduced non-relapse mortality with a trend toward less acute GvHD (aGvHD).
The relationship between the number of Tregs detected in vivo post-transplant and the development of GvHD is similarly controversial. In an early study, Clark and colleagues investigated the number of peripheral blood CD4+CD25+high T cells in patients after allogeneic hematopoietic stem cell transplantation. Patients with chronic (cGvHD) had markedly elevated numbers of CD4+CD25+high T cells as compared to patients without GvHD and this observation lead authors to claim that cGvHD does not occur as a result of Treg cell deficiency.38 More recently, Ukena reported that in patients with acute/chronic GvHD the number of Tregs always remained lower than observed in patients who never developed GvHD. In addition, numerically deficient recovery of Tregs following a transplant performed using peripheral blood derived stem cells was significantly associated with the development of acute but not chronic GvHD.39 By contrast, in a study based on the post-transplant immune reconstitution analysis, Zorn et al.40 reported a decreased frequency of CD4+CD25+ T cells in patients with cGvHD compared with patients without cGvHD and healthy individuals. Along the same lines, Fujioka and co-workers reported that at the second week after transplantation, patients with aGvHD had significantly lower Treg/CD4(+) T-cell ratios than those without aGvHD. As these differences were seen before the development of aGvHD, these ratios can predict the incidence of aGVHD.41
Tregs as adoptive cellular therapy
Cord blood derived Tregs
The consistent proof of concept derived from preclinical models and the development of efficacious methods to isolate and in vitro expand Tregs from blood, led to test Tregs in clinical trials, in patients who underwent alloHSCT. In the ‘first in human’ clinical trial performed in 23 patients immediately after double-umbilical cord blood (UCB) transplantation, the infusion of UCB-derived tTregs (as defined by an average expression of FOXP3+ in about 60% of cells infused after in vitro expansion) was associated with a reduced incidence of aGvHD compared to historical control subjects. Moreover, the authors did not observe an increased incidence of opportunistic infections or relapse, suggesting that Tregs could provide an immediate, albeit possibly transient, immune suppression to control GvHD without long-term deleterious effects.42
However, in this clinical trial, investigators from the University of Minnesota were not able to generate ex vivo the planned Treg doses for almost 20% of the patients enrolled in their study. With the aim to maximize the infused Tregs dose, selected CD251 UCB cells were expanded in the presence of K562 cells modified to express the high-affinity Fc receptor (CD64) and CD86, the natural ligand of CD28 (KT64/86). In this new study, 11 patients were treated with Tregs doses from 3 up to 100 × 106/kg. Clinical outcomes were compared with contemporary controls who received the same conditioning regimen with sirolimus and mycophenolate mofetil immune suppression. The incidence of grade II–IV aGvHD at 100 days was 9% vs 45% in controls (P=0.05) and cGvHD at 1 year was 0% in patients treated with Tregs and 14% in controls. Moreover, it is important to note that no increase in relapse, infection, or toxicity was observed between the 2 groups of patients, confirming that expanded UCB Tregs were safe and reduced the risk of aGvHD even at highest Treg doses.43
Normal donor-derived, Tregs
The potential clinical application of human Tregs purified and expanded from normal donors could be potentially limited by the small number detectable in the peripheral blood. During the past ten years several laboratory approaches have been tested to implement this clinical technology. Initial attempts were based on the use of artificial APCs for repeated stimulation via CD3 and CD28 in the presence of high-dose IL-2. After in vitro expansion, CD4+ CD25+ bright T cells were shown to be polyclonal, to maintain their original phenotype including the expression of the lymph node homing receptors L-selectin (CD62L) and CCR744 and to retain their suppressive activity. However, the therapeutic application in humans of large numbers of in vitro expanded Tregs requires the application of clinical grade protocols according to Good Manufacturing Practice (GMP) rules. Therefore, subsequent efforts have been put into developing Tregs from standard leukapheresis products by using a two-step magnetic cell-separation protocol performed under GMP conditions. The generated cell products contained about 50% CD4+ CD25+ bright T cells that phenotypically and functionally were fully comparable to Tregs preparations obtained by a non GMP approved, fluorescence-activated cell sorting-based technology.45
Tregs have been also isolated from leukapheresis products by CliniMACS, with a GMP compliant isolation strategy, using anti-CD25, anti-CD8 and anti-CD19 coated microbeads.46 CliniMACS isolation procedures led to 40–60% pure CD4+ CD25+ bright FoxP3+ Tregs populations with moderate suppressive activity and these cells could be also expanded with maintenance of suppressive function. Depletion of unwanted CD19+ and CD127+ cells improved the purity of Tregs up to ~90%.46 Cryopreservation of CliniMACS isolated Tregs is feasible, but after thawing activation is necessary to restore suppressive potential.46 In addition, it has been shown that CD25+ cells isolated from G-CSF-mobilized apheresis contain a significant increase in the proportion of FoxP3 expression when compared with conventional non-mobilized CD25+ cells and showed a superior suppressive capacity in a T-cell proliferation assay.47 Recent results confirmed that in a clinical scale setting significant amounts of functionally active Tregs can be purified from G-CSF mobilized PBSCs by a CD8+CD19+CD14+ cell depletion followed by CD25+ cell selection (two-step process) or by adding an initial CD14+ cell depletion (three-step process) using a CliniMACS device. The three-step approach resulted in a better purity and yield of functionally suppressive in vitro FoxP3+CD127dim Tregs.48
In a phase I study reported by Edinger and Hoffman,49 nine patients at high risk of leukemia relapse after alloHSCT received freshly isolated donor Tregs. In this pre-emptive Tregs infusion strategy, up to 5 × 106/kg cells (>50% FOXP3+) were administered after stopping any pharmacologic GvHD prophylaxis (within one year after alloHSCT). After an observation period of 8 weeks, additional Tcons were administered at the same dose to promote GvL activity. Despite the absence of pharmacologic immunosuppression, no Tregs transfusion-related adverse events were observed. Furthermore, neither GvHD nor opportunistic infections or early disease relapses occurred after Tregs transfusion, suggesting safety and feasibility of this cellular therapy.49
In the most compelling setting of haploidentical transplantation the adoptive infusion of in vitro-selected Tregs might be extremely important since the development of an uncontrolled aGvHD can be rapidly lethal. On the basis of the previously described murine models,22 normal haploidentical donor-derived, in vitro-selected Tregs were infused 4 days before unselected Tcons.27, 28 The primary goal of these studies was to manipulate the graft in order to increase the speed and quality of immune reconstitution for preventing the high risk of infection-related deaths associated with the extensive T-cell depletion used in this type of transplant,50 as well as to reduce the incidence and severity of aGvHD likely expected after the infusion of a minimal number of unmanipulated haploidentical lymphocytes. In a trial of 43 consecutive acute leukemia patients undergoing haploidentical using an ex vivo T-cell depleted graft, authors investigated the infusion of donor Tregs (2 × 106/kg), followed 4 days later by an inoculum of mature donor T cells (Tcons, 1 × 106/kg) and positively immune selected CD34+ cells. Patients did not receive any prophylactic immunosuppression after transplant. The early, post-transplant adoptive transfer of Tregs resulted effective not only in improving the immune reconstitution, but it was also associated with a low incidence of leukemia relapse in a group of patients with very high risk AML.27, 28 However, the non-relapse mortality remained significantly high, possibly related to the inclusion of patients that were heavily pre-treated and/or had a history of significant infectious complications before transplantation.27, 28 The potential future clinical developments of this approach to adoptive immunotherapy with Tregs are summarized in Figure 1.
Very few data are available about the therapeutic use of Tregs when GvHD is already established. In a report of 2 patients the infusion of in vitro expanded donor Tregs (90% FOXP3+, dose of 1 × 105/kg) contributed to improve of cGvHD and allowed the reduction of immunosuppressive drugs in one patient. In contrast, the second patient treated with a higher Tregs dose (3 × 106/kg) for resistant aGvHD did not benefit from this immunotherapy.51 At the time of writing this article, several clinical trials with Tregs-based cellular therapy protocols are ongoing for the treatment of GvHD after alloHSCT. The main aim of all these studies is to assess that these cells do not induce an undesired aggravation of this life-threatening complication as well as their potential efficacy for the treatment of resistant forms of GvHD (see for reference clinicaltrials.gov numbers NCT01903473, NCT02749084, NCT01937468, NCT02385019, NCT01911039 and NCT02519816). Table 1 summarizes the available results from published trials using Tregs as adoptive cellular therapy.
Drug induced, in vivo expansion of Tregs
From what was described in the previous sections it is clear how challenging is the clinical use of in vitro isolated and expanded Tregs. In fact, the need for a cell-separation strategy to purify such a small proportion of peripheral blood T cells10, 52 and the absolute need for appropriate GMP procedures to ensure optimal in vitro expansion to obtain clinically relevant numbers of reasonably pure Tregs prompted several investigators to search for drugs able to expand Tregs in vivo. Studies evaluating drug-induced expansion of Tregs are summarized in Table 2.
Drugs with a direct effect on in vivo expansion of Tregs
Interleukin-2
In a phase I escalation study the administration of low-dose IL-2 to 29 patients with steroid-refractory cGvHD induced a rapid increase of circulating Tregs, associated with a significant clinical response which allowed a clinically relevant reduction of the steroid dose.53 Laboratory investigation performed in the context of this clinical study allowed the demonstration that in patients with cGvHD, IL-2 induces a series of changes in Treg homeostasis, including increased proliferation, increased thymic export and enhanced resistance to apoptosis.54 The same group from Harvard University recently published a phase 2 study, involving 35 adult patients with steroid-refractory cGvHD receiving daily IL-2 at the dosage of 1 × 106 IU/m2 per day for 12 weeks. Twenty of 33 evaluable patients (61%) had clinical responses and only 3 patients (9%) had progressive cGvHD. Compared with pre-treatment levels, Treg and NK-cell counts rose more than fivefold (P<0.001) and fourfold (P<0.001), respectively.55 A similar study performed at Baylor College using ultra low doses of IL-2 for GvHD prophylaxis, confirmed the in vivo expansion of Tregs and suggested a GvHD reduction in patients receiving this experimental treatment.56 Taken together, these results suggest that low-dose IL-2 may be a reasonable candidate as adjuvant for adoptive Tregs cell therapy. Nonetheless, the impact of the therapeutic administration of IL-2 can be controversial as to its role on NK-cell expansion57 that could be regarded as positive for its ability to promote a significant GvL protective effect58 or potentially negative in relation to a possible proinflammatory activity as observed in various autoimmune models and in patients.59
Drugs with an indirect effect on in vivo expansion of Tregs
Post-transplant cyclophosphamide and rapamycin
Several experimental models suggest the in vivo expansion of Tregs when Cyclophosphamide is given post transplant (PTCy) for GvHD prophylaxis and tolerance induction.60, 61 Despite the potential dominant effect of a deep depletion of alloreactive cytotoxic T cells, these studies suggest that expansion of Tregs is also contributing to the therapeutic effect of this drug. Two studies with mice models confirmed Tregs as necessary for PTCy mediated GvHD prevention. In these studies Tregs were present at higher levels in patients with GvHD and the protective effects against GvHD of PTCy were lost when Tregs were depleted from grafts prior to transplantation.62, 63 Of note, in patients receiving a standard, calcineurin inhibitors based GvHD prophylaxis, low levels of circulating Tregs have been found at onset of aGvHD.29 Interestingly, a quick Tregs reconstitution after alloHSCT using PTCy was observed, despite continued post-transplant CD4+ lymphopenia. Tregs resistance to PTCy appears to be related to induction of aldehyde dehydrogenase (ALDH) expression upon allogeneic stimulation, in contrast to a condition of equilibrium in which ALDH is minimally expressed by human Tregs.63 Preservation of regulatory T cells by PTCy has been postulated even when this GvHD prophylaxis has been used after myeloablative, HLA-matched transplant in acute leukemia and MDS patients.64
Another interesting drug to expand Tregs in vivo is represented by rapamycin, an inhibitor of the mTOR-AKT pathway that promotes growth and expansion of Tregs.65, 66 In a murine model, this drug selectively expands and preferentially preserves Tregs over T-conventional.65 In the human haploidentical setting, Peccatori and colleagues examined the role of rapamycin-based GvHD prophylaxis to allow the infusion of unmanipulated peripheral blood stem cell grafts. In a first study with GvHD prophylaxis based on highly purified rabbit polyclonal antihuman T-lymphocyte Ig (ATG) (Grafalon, Neovii Biotech GmbH, Gräfelfing, Germany), rituximab and oral administration of rapamycin and mycophenolate, T-cell reconstitution was rapid and skewed toward Tregs, as documented by the high frequencies of circulating Tregs. Interestingly, the occurrence and severity of GvHD was negatively correlated with Tregs frequency.67, 68 The role of ATG is noteworthy since data are available suggesting that this drug acts by modulating the circulating T-cell pool through a selective negative impact on the recovery of naive CD4+, central memory CD4+ and naive CD8+ cells, while effector memory T and regulatory T cells are spared.69
Azacytidine and histone deacetylase inhibitors
Promising results indicate that azacytidine can have an immunomodulatory effect by increasing functional Treg numbers. In particular, azacytidine, in addition to the direct cytotoxic effect, seems to accelerate the reconstitution of Tregs and induce CD8+ T-cell response to candidate tumor antigens, enhancing the GvL effect and reducing the risk of GvHD.70, 71, 72 In this context, the potential mechanism of action of azacytidine seems to be related to a delayed effect of the drug leading to demethylation of the FOXP3 promoter and overexpression of FOXP3, driving T-cell differentiation toward a regulatory phenotype.72 Since azacytidine may represent a simple and relatively non-toxic approach to limit GvHD and preserving the GvL effect, future studies combining azacytidine and donor lymphocyte infusion (DLI) or NK cells deserve to be investigated in the prevention of relapse in patients transplanted for high risk AML and myelodysplastic syndrome.73
In experimental models of alloHSCT, a histone deacetylase inhibitor (vorinostat) reduced proinflammatory cytokines, increased Tregs and reduced GvHD.74, 75 On the basis of these observations, recently, Choi and colleagues report a phase 1/2 study testing vorinostat combined with standard immune prophylaxis in the setting of alloHSCT. These investigators showed that vorinostat is a safe and feasible drug and has the potential to reduce aGvHD.76 Interestingly, vorinostat was associated with augmented FOXP3 expression and increased numbers of Treg cells in peripheral blood, suggesting that the beneficial effects of histone deacetylase inhibition may be due to its ability to expand in vivo and activate the function of circulating Tregs.77
Ruxolitinib
Ruxolitinib is a potent inhibitor of the JAK1/2 signal transduction pathway and it has been licensed for the treatment of patients with myelofibrosis. Interestingly, in this disease a marked reduction in the total number of circulating Tregs has been reported.78 In a very recent study, the same authors confirmed a reduced frequency of Tregs in 202 consecutive patients with myelofibrosis compared with healthy subjects. Interestingly, in this new study a higher Tregs frequency was reported in subjects with JAK2V617F mutation, in particular in those with an allele burden >50%, suggesting that the degree of activation of the JAK-STAT pathway may influence Tregs levels.79 While a possible relationship between the myelofibrosis related cytokine storm80 and the reduced amount of Tregs found in these patients is conceivable, the therapeutic use of ruxolitinib has been associated to further reduction of Tregs.81 However, in experimental transplant models, higher frequencies of CD4+ FoxP3+ regulatory T cells have been observed in ruxolitinib-treated mice compared to controls.82 The conflicting observation of a reduced number of Tregs in myelofibrosis and the preclinical data showing higher Treg frequencies in ruxolitinib-treated mice must be clarified in future studies, evaluating also the potential role played by different myelofibrosis genotypes79 and the possible ruxolitinib dose effect.82 However, the potent immunosuppressive activity related to the JAK1/2 inhibition mediated by ruxolitinib led to hypothesize the clinical use of this drug for the treatment of steroid resistant GvHD. Interestingly, ruxolitinib has been reported to be clinically effective in a cohort of 95 patients with steroid-refractory acute and chronic GvHD treated in Europe and United States with an overall response rate of 81% with a complete responses of 46% and a 6 months survival of 79%.83 On the basis of these results, an international randomized trial for the use of ruxolitinib in steroid-refractory GvHD is on the way to start and it will offer the unique opportunity to investigate the role of this drug in the biology of Tregs.
Conclusions
The discovery of Tregs and their enormous biologic potential has opened the door to the development of clinically applicable therapeutic strategies to modulate the most critical aspects of GvHD following allogeneic stem cell transplantation. Collectively, the available data suggest that promises generated from basic and preclinical models will translate into innovative and effective clinical therapies. Both adoptive cellular therapy programs as well as drug induced in vivo expansion of Tregs will likely be transferred into clinical practice. However, the challenge of adoptive cellular therapy programs is remarkable. The need of a cell-separation strategy that easily and efficiently allows isolation of this cell population and its subsequent in vitro expansion is still problematic. In fact, selection and in vitro expansion of Tregs remains a difficult laboratory task since the purity of the expanded population is not fully satisfactory and not yet standardized. Most importantly and even more problematic is the issue about the complexity of the laboratory manipulation in compliance with internationally validated GMP rules and the costs of such GMP grade procedures. An additional concern is also related to the fact that the long-term immunologic properties of in vitro expanded Tregs are not yet known. The possible long-term loss of the immune suppression properties of in vitro expanded Tregs following their infusion into patients remains to be carefully investigated. All in all, to date only a few clinical protocols have been designed and clinically tested by academic facilities and this may represent a limit for the wide clinical application of any adoptive cellular therapy approach. However, the possibility of a pharmacologic manipulation of the immune system to favour the in vivo expansion of Tregs is another attractive possibility. Several drugs may offer such a possibility to expand and activate Tregs and are currently under clinical investigation even though the complex interactions between different concomitant drugs for prevention of GvHD and their relationship with different transplant modalities in term of type of grafts, conditioning regimens and underlying disease should be clarified. The understanding of these mechanisms with appropriate investigations will help us to translate into optimal therapeutic decisions the outstanding body of scientific information that basic science has provided about Tregs.
References
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M . Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164.
Shevach EM . Immunology. Regulating suppression. Science 2008; 322: 202–203.
Shevach EM . Special regulatory T cell review: how I became a T suppressor/regulatory cell maven. Immunology 2008; 123: 3–5.
Bluestone JA, Abbas AK . Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003; 3: 253–257.
Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 2013; 14: 307–308.
Fontenot JD, Gavin MA, Rudensky AY . Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336.
Hori S, Nomura T, Sakaguchi S . Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061.
Khattri R, Cox T, Yasayko SA, Ramsdell F . An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4: 337–342.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA . FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500.
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA . CD4+CD25high regulatory cells in human peripheral blood. J Immunol 2001; 167: 1245–1253.
Hsieh C-S, Lee H-M, Lio C-WJ . Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12: 157–167.
Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2: 301–306.
Chen W, Jin W, Hardegen N, Lei K-j Li L, Marinos N et al. Conversion of peripheral CD4+CD25− naive T Cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 2003; 198: 1875–1886.
Liu C, Workman CJ, Vignali DAA . Targeting regulatory T cells in tumors. FEBS J 2016; 283: 2731–2748.
Alice Long S, Buckner JH . CD4(+)FOXP3(+) Treg in human autoimmunity: more than a numbers game. J Immunol 2011; 187: 2061–2066.
Nguyen VH, Shashidhar S, Chang DS, Ho L, Kambham N, Bachmann M et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood 2008; 111: 945–953.
Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood 2007; 109: 2649–2656.
Taylor PA, Lees CJ, Blazar BR . The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002; 99: 3493–3499.
Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest 2003; 112: 1688–1696.
Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY et al. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest 2015; 125: 3627–3641.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150.
Ruggeri L, Di Ianni M, Urbani E, Mancusi A, Falzetti F, Carotti A et al. Tregs suppress GvHD at the periphery and unleash the Gvl effect in the bone marrow. Blood 2014; 124: 842–842.
Sandy AR, Chung J, Toubai T, Shan GT, Tran IT, Friedman A et al. T cell-specific notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol 2013; 190: 5818–5828.
Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest 2013; 123: 1590–1604.
Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood 2011; 117: 299–308.
Del Papa B, Pierini A, Sportoletti P, Baldoni S, Cecchini D, Rosati E et al. The NOTCH1/CD39 axis: a Treg trip-switch for GvHD. Leukemia 2016; 30: 1931–1934.
Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117: 3921–3928.
Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014; 124: 638–644.
Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 2006; 108: 1291–1297.
Pabst C, Schirutschke H, Ehninger G, Bornhauser M, Platzbecker U . The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res 2007; 13: 2916–2922.
Wolf D, Wolf AM, Fong D, Rumpold H, Strasak A, Clausen J et al. Regulatory T-cells in the graft and the risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation 2007; 83: 1107–1113.
Pastore D, Delia M, Mestice A, Carluccio P, Perrone T, Gaudio F et al. CD3+/Tregs ratio in donor grafts is linked to acute graft-versus-host disease and immunologic recovery after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 887–893.
Delia M, Pastore D, Mestice A, Carluccio P, Perrone T, Gaudio F et al. Outcome of allogeneic peripheral blood stem cell transplantation by donor graft CD3+/Tregs ratio: a single-center experience. Biol Blood Marrow Transplant 2013; 19: 495–499.
Noel G, Bruniquel D, Birebent B, DeGuibert S, Grosset JM, Bernard M et al. Patients suffering from acute graft-versus-host disease after bone-marrow transplantation have functional CD4+CD25hiFoxp3+ regulatory T cells. Clin Immunol 2008; 129: 241–248.
Rosenzwajg M, Dhedin N, Maury S, Bensimon G, Landau DA, Norol F et al. Regulatory T cell content in the bone marrow graft does not predict the occurrence of acute GVHD. Biol Blood Marrow Transplant 2011; 17: 265–269.
Stanzani M, Martins SL, Saliba RM, St John LS, Bryan S, Couriel D et al. CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk for graft-versus-host disease after HLA-identical stem cell transplantation in humans. Blood 2004; 103: 1140–1146.
Danby RD, Zhang W, Medd P, Littlewood TJ, Peniket A, Rocha V et al. High proportions of regulatory T cells in PBSC grafts predict improved survival after allogeneic haematopoietic SCT. Bone Marrow Transplant 2016; 51: 110–118.
Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood 2004; 103: 2410–2416.
Ukena SN, Grosse J, Mischak-Weissinger E, Buchholz S, Stadler M, Ganser A et al. Acute but not chronic graft-versus-host disease is associated with a reduction of circulating CD4(+)CD25 (high)CD127 (low/-) regulatory T cells. Ann Hematol 2011; 90: 213–218.
Zorn E, Mohseni M, Kim H, Porcheray F, Lynch A, Bellucci R et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 382–388.
Fujioka T, Tamaki H, Ikegame K, Yoshihara S, Taniguchi K, Kaida K et al. Frequency of CD4(+)FOXP3(+) regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHD. Bone Marrow Transplant 2013; 48: 859–864.
Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011; 117: 1061–1070.
Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 2016; 127: 1044–1051.
Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M . Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 2004; 104: 895–903.
Hoffmann P, Boeld TJ, Eder R, Albrecht J, Doser K, Piseshka B et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol Blood Marrow Transplant 2006; 12: 267–274.
Peters JH, Preijers FW, Woestenenk R, Hilbrands LB, Koenen HJ, Joosten I . Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS ONE 2008; 3: e3161.
Samuel ER, Beloki L, Newton K, Mackinnon S, Lowdell MW . Isolation of highly suppressive CD25+FoxP3+ T regulatory cells from G-CSF-mobilized donors with retention of cytotoxic anti-viral CTLs: application for multi-functional immunotherapy post stem cell transplantation. PLoS ONE 2014; 9: e85911.
Patel P, Mahmud D, Park Y, Yoshinaga K, Mahmud N, Rondelli D . Clinical grade isolation of regulatory T cells from G-CSF mobilized peripheral blood improves with initial depletion of monocytes. Am J Blood Res 2015; 5: 79–85.
Edinger M, Hoffmann P . Regulatory T cells in stem cell transplantation: strategies and first clinical experiences. Curr Opin Immunol 2011; 23: 679–684.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454.
Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 2009; 133: 22–26.
Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A . Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol 2007; 178: 320–329.
Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011; 365: 2055–2066.
Matsuoka K-i, Koreth J, Kim HT, Bascug OG, McDonough S, Kawano Y et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 2013; 5: 179ra143.
Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft vs. host disease. Blood 2016; 128: 130–137.
Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res 2014; 20: 2215–2225.
Fehniger TA, Bluman EM, Porter MM, Mrózek E, Cooper MA, VanDeusen JB et al. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J Clin Invest 2000; 106: 117–124.
Soiffer R, Murray C, Gonin R, Ritz J . Effect of low-dose interleukin-2 on disease relapse after T-cell- depleted allogeneic bone marrow transplantation. Blood 1994; 84: 964–971.
Sharma R, Fu SM, Ju S-T . IL-2: A two-faced master regulator of autoimmunity. J Autoimmun 2011; 36: 91–97.
Kong YY, Eto M, Omoto K, Umesue M, Hashimoto A, Nomoto K . Regulatory T cells in maintenance and reversal of peripheral tolerance in vivo. J Immunol 1996; 157: 5284–5289.
Tomita Y, Mayumi H, Eto M, Nomoto K . Importance of suppressor T cells in cyclophosphamide-induced tolerance to the non-H-2-encoded alloantigens. Is mixed chimerism really required in maintaining a skin allograft tolerance? J Immunol 1990; 144: 463–473.
Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014; 124: 2131–2141.
Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013; 5: 211ra157.
Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014; 124: 3817–3827.
Battaglia M, Stabilini A, Roncarolo MG . Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005; 105: 4743–4748.
Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 2008; 111: 453–462.
Peccatori J, Forcina A, Clerici D, Crocchiolo R, Vago L, Stanghellini MT et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia 2015; 29: 396–405.
Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G et al. Post-transplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan-based myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant 2015; 21: 1506–1514.
Servais S, Menten-Dedoyart C, Beguin Y, Seidel L, Gothot A, Daulne C et al. Impact of pre-transplant anti-T cell globulin (ATG) on immune recovery after myeloablative allogeneic peripheral blood stem cell transplantation. PLoS ONE 2015; 10: e0130026.
Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 2010; 116: 129–139.
Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012; 119: 3361–3369.
Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, Caballero-Velazquez T, Blanco B, Herrero-Sanchez C et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood 2010; 115: 107–121.
Schroeder T, Frobel J, Cadeddu RP, Czibere A, Dienst A, Platzbecker U et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia 2013; 27: 1910–1913.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol 2011; 31: 2066–2078.
Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA 2004; 101: 3921–3926.
Choi SW, Braun T, Chang L, Ferrara JL, Pawarode A, Magenau JM et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol 2014; 15: 87–95.
Choi SW, Gatza E, Hou G, Sun Y, Whitfield J, Song Y et al. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood 2015; 125: 815–819.
Massa M, Rosti V, Campanelli R, Fois G, Barosi G . Rapid and long-lasting decrease of T-regulatory cells in patients with myelofibrosis treated with ruxolitinib. Leukemia 2014; 28: 449–451.
Massa M, Campanelli R, Fois G, Villani L, Bonetti E, Catarsi P et al. Reduced frequency of circulating CD4+CD25brightCD127lowFOXP3+ regulatory T cells in primary myelofibrosis. Blood 2016; 128: 1660–1662.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Parampalli Yajnanarayana S, Stubig T, Cornez I, Alchalby H, Schonberg K, Rudolph J et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol 2015; 169: 824–833.
Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014; 123: 3832–3842.
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015; 29: 2062–2068.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lussana, F., Di Ianni, M. & Rambaldi, A. Tregs: hype or hope for allogeneic hematopoietic stem cell transplantation?. Bone Marrow Transplant 52, 1225–1232 (2017). https://doi.org/10.1038/bmt.2017.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.30
- Springer Nature Limited
This article is cited by
-
Acute invasive fungal sinusitis with orbital tip syndrome in patients on long-term use of ruxolitinib: a case report
Journal of Medical Case Reports (2024)
-
Haplo-identical allografting with post-transplant cyclophosphamide in high-risk patients
Annals of Hematology (2018)