Abstract
The most effective method to prevent and treat bone loss following hematopoietic stem cell transplantation (HSCT) remains uncertain. We conducted a comprehensive search in four electronic databases until August 2015. We retrieved articles describing patients with bone loss or fractures who received HSCT. Controlled trials, with a follow-up period of at least 12 months, were included. Twelve studies (19 publications) met our inclusion criteria. A total of 643 participants underwent HSCT (85.7% allogeneic HSCT). There was a statistically significant lower mean bone mineral density (g/cm2) percentage change of the lumbar spine (mean difference (MD) 7.8, 95% confidence interval (CI) 5.6–10.0) and femoral neck (MD 6.7, 95% CI 5.6–7.9) in the bisphosphonate therapy group compared with the control group with no bisphosphonate therapy at 12 months. In a subgroup analysis, seven different comparison groups were evaluated. The rate of fractures or X-ray findings of subclinical vertebral fractures was similar between groups. Bisphosphonates are promising in the prevention and treatment of bone loss following HSCT. Additional research is required to determine whether they reduce long-term fracture risk.
Similar content being viewed by others

Introduction
With advances in transplantation techniques and post-transplant care, the number of long-term survivors following hematopoietic stem cell transplantation (HSCT) is growing.1, 2 As patients survive long term, their risk of developing late complications such as bone loss also increase. Bone mineral density (BMD) loss can lead to increased bone fragility and fractures that can cause substantial morbidity and mortality and impair the quality of life during the survivorship period.3
Bone remodeling in the context of HSCT is complex and multiple factors are involved in post-transplantation bone loss. Pretransplantation chemotherapy, conditioning regimens, GvHD prophylaxis, glucocorticoid use and several endocrine factors have been implicated in post HSCT bone disease.3, 4 Other known risk factors for bone loss such as older age, immobilization, low body mass index, genetic factors and improper nutrition may also potentially contribute to bone loss following HSCT.3, 5
A few studies have suggested that most of the bone loss in this setting occurs within the first year following transplantation with variable recovery thereafter.6, 7, 8 Our previous research also shows that the incidence of fractures is significantly higher following HSCT compared with the general US population.9 Most patients do not return to normal BMD levels, possibly because of prolonged risk exposure. General preventive measures with calcium (Ca) and vitamin D (VitD) supplementation have not been shown to prevent bone loss in this patient population.10 In recent times a few studies have also been conducted to assess the efficacy of antiresorptive medications in preventing bone loss following HSCT.
To the best of our knowledge, there have been only two summaries of the literature to assess bone loss management strategies in this patient population.2, 11 These summaries did not make any conclusive recommendations. Controversies as to the best modality of prevention and treatment of bone loss and fractures following HSCT still remain. This is the first systematic review and meta-analysis to comprehensively evaluate the evidence and systematically analyze the treatments currently available to treat or prevent bone loss following HSCT.
Materials and methods
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for reporting methods.12, 13
Search and information sources
We searched on Medline, EMBASE, Cochrane Library and Web of Science from inception to August 2015 without any restrictions. Appendix A shows the search terms used in Medline. In addition, the reference lists from the identified clinical trials and the Clinicaltrials.gov registry were searched for possible references not otherwise found. Retrieved citations were exported into reference manager software and duplicates were removed.
Eligibility criteria and study selection
Our review encompassed a two-step screening process. In the first step, the titles and abstracts of the unique citations were independently screened by two reviewers (HIC and GSP). We included studies evaluating the effects of any antiresorptive medication or supplement to prevent or treat bone loss in patients receiving HSCT. We excluded studies reporting on young children (<15 years old), case reports, reviews or not original research articles. In the second step, full-text articles were acquired for the relevant citations. Studies were included if they were controlled trials (randomized or not), with a follow-up period of at least 12 months, and reported separate data on BMD and bone turnover markers for each intervention. Studies were excluded if the control group did not receive HSCT (that is, healthy individuals). A consensus was established at both steps and, when not reached, a third reviewer (MES-A) solved the disagreement.
Data collection process and data items
Data were independently extracted by two reviewers (HIC and GSP). Collected information included: (1) study design and general information, (2) details of treatment and control groups (age, gender, dosage and route of drug administration, duration of treatment and follow-up) and (3) outcome measures. Data were extracted from text, tables or graphs. In addition, we extracted sources of funding and other support such as intervention supply and the role of the funders.
Outcome measures
Our primary end points were bone loss measured by BMD (g/cm2) at either the lumbar spine or femoral neck. We also evaluated the following secondary outcomes: fractures, bone turnover markers (for example, osteocalcin, carboxy-terminal collagen crosslinks, bone-specific alkaline phosphatase and so on), hormonal changes (for example, estradiol, parathyroid hormone, testosterone and so on) and adverse events (for example, avascular necrosis, osteonecrosis of the jaw, toxicity, infections, fever, flu-like symptoms, myalgia and so on).
Risk of bias across studies
The quality of the studies was evaluated independently by two reviewers (HIC and GSP). Details on methods used to assess the risk of bias can be found in the Supplementary Information section. Evaluation of publication bias through funnel plot asymmetry was planned if >10 studies were included to assess the primary outcome (power for tests is too low when <10 studies are included).
Synthesis of results and additional analysis
We performed a direct comparison meta-analysis using RevMan v5.3.15 Dichotomous data were analyzed as relative risk (RR) and use 95% confidence intervals (CIs). Continuous data were analyzed as mean difference (MD) with corresponding 95% CI. Missing data was handled in two ways: (1) computing from other statistics (for example, s.e. to s.d.), extrapolating from graphs or other similar studies in the meta-analysis; and (2) exploring the impact of excluding such studies by a sensitivity analysis (dropping studies one by one from each comparison group for those that had missing data and evaluating the effect on the mean change). Fixed-effect model was used, but when heterogeneity was present, we used random-effects model. Heterogeneity was assessed by observing study characteristics, visually inspecting the forest plots to assess for obvious differences in result between the studies, and using the I2 test. An I2 value of >40% was considered substantial heterogeneity. We compared any bisphosphonate with no bisphosphonates for our primary outcome change in BMD at either the lumbar spine or femoral neck. A subgroup analysis was performed to compare therapies with different bisphosphonates separately to those not receiving bisphosphonates.
Results
Study selection
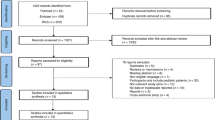
Our search resulted in 3393 citations. We included 12 studies14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 in the qualitative synthesis and, of these, 10 studies were included in the quantitative synthesis.14, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 Results from our stepwise selection process are shown in Figure 1. Eight studies were randomized open-label trials,14, 16, 17, 19, 20, 21, 22, 23, 24, 27, 28, 32, 33 one was a randomized, double-blind trial25, 26 and three studies were nonrandomized and nonblinded trials.18, 29, 30, 31
Study characteristics
Study characteristics are shown in Table 1. Two studies were multicenter.19, 20, 21, 22, 23, 24 One study did not specify the number of centers.27, 28 The rest of the studies were conducted in one center. The sample sizes ranged from 8 persons17 to 116 persons.19, 20, 21 The major outcome measured in 11 of the 12 studies was percent change in BMD (expressed in g/cm2, T- and/or Z-score) of the lumbar spine (L1–L4) and femoral neck measured by dual-energy X-ray absorptiometry before HSCT, and 3, 6, 12, 24 or 36 months after HSCT.14, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Some studies analyzed BMD in other anatomical sites such as total hip14, 17, 19, 20, 21, 25, 26, 27, 28, 33 and trochanter.17, 27, 28 One study evaluated changes in BMD under influences of glucocorticoid and cyclosporin therapy 12 months after HSCT.19, 20, 21 Details on common secondary outcomes and bone turnover markers are listed in Supplementary Table 1. Bone turnover markers were reported in most studies except in three studies.17, 25, 26, 33 Six studies were funded by only pharmaceutical companies or pharmaceutical companies and private organizations.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 32 Three studies did not disclose the source of funding.14, 31, 33 Two studies were supported only by private foundations or organizations.16, 29, 30
Participant characteristics
A total of 643 participants were included. Of these, 562 (87.4%) underwent allogeneic HSCT,14, 16, 18, 19, 20, 21, 22, 23, 24, 27, 28, 29, 30, 31, 32, 33 8 (1.2%) underwent autologous HSCT17 and 73 (11.4%)25, 26 did not report the type of transplant. Table 2 shows participants’ age and percent of females, eligibility criteria and interventions. Information on type of donor, source of stem cells, race, primary disease for undergoing a transplant and smoking status can be found in the Supplementary Information section.
Treatment was triggered by an abnormal baseline BMD in 3 of the 12 studies included in the analysis.14, 23, 29 In one study, although treatment was not triggered by an abnormal BMD, all groups evaluated had osteopenia,16 and in another study, 39% of patients had osteopenia at the lumbar spine and 25% had osteopenia at the femoral neck.32 In contrast, majority of the patients in other studies had normal BMD levels.18, 19, 27 Four of the studies were abstracts and did not provide sufficient information to evaluate baseline BMD status.17, 25, 31, 33
Risk of bias
A summary of judgments about each risk of bias item is presented in Supplementary Figures 1a and b as percentages across all included studies. Several studies have attempted to provide a numerical score of risk of bias for a given study for simplicity.34, 35, 36 However, use of an overall score for the risk of bias (that is, summarizing risk of bias across several outcomes for a given study) is strongly discouraged by the Cochrane workgroup.37 We used the scoring used by Ferreira et al.34 and a summary overall score of risk of bias is presented in Table 1 for each study.
Efficacy of the interventions
We compared changes in BMD at the lumbar spine and femoral neck between studies comparing bisphosphonate therapy with no bisphosphonate therapy. Seven studies reported change in BMD of the lumbar spine at 12 months. There was a statistically significant lower mean BMD (g/cm2) percentage change of the lumbar spine in the bisphosphonate therapy group compared with the control group with no bisphosphonate therapy (MD 7.8, 95% CI 5.6–10.0; Table 3a). Similarly, six studies reported change in BMD of the femoral neck at 12 months. There was a statistically significant increase in mean BMD (g/cm2) percentage change of the femoral neck in the bisphosphonate therapy group compared with the control group with no bisphosphonate therapy (MD 6.7, 95% CI 5.6–7.9; Table 3b).
One randomized controlled trial evaluating the role of ibandronate did not have sufficient information and was not included in the quantitative analysis.33 Seven comparisons were evaluated in a subgroup analysis. We observed a statistically significant lower mean BMD (g/cm2) percentage change in a majority of the comparisons that included a bisphosphonate in the treatment arm at most locations and time points assessed (Table 4). A detailed description of the subgroup analysis can be found in the Supplementary Information section. Findings of all other outcomes are shown in Supplementary Table 1.
Fractures
No statistically significant differences were observed in spontaneous fractures, X-ray findings of subclinical fractures and osteonecrosis of the jaw between any of the comparison groups (Supplementary Table 1).
Adverse events
In the zoledronic acid combined with Ca/VitD versus Ca/VitD alone subgroup, flu-like symptoms (that is, myalgia, nausea and increase in body temperature) were more common in patients in the zoledronic acid group compared with the control group (RR 25.0, 95% CI 1.6–387.4). Participants with at least one serious adverse event were more common in the zoledronic acid group compared with the control group (RR 2.4, 95% CI 1.3–4.5). Death rates at 12 months and overall mortality at 24 months were also increased in the zoledronic acid group compared with the control group (RR 2.3, 95% CI 1.0–5.0; and RR 2.3, 95% CI 1.1–4.8, respectively) (Supplementary Table 1). No statistically significant differences were observed in any of the reported adverse events in any of the other comparison groups (Supplementary Table 1).
Sensitivity analysis and publication bias
To handle missing data we conducted a sensitivity analysis. We did not observe any differences, and the inclusion of these studies did not impact the overall conclusion. As we had <10 studies, publication bias could not be investigated.
Discussion
Bone loss following HSCT occurs early following transplantation and is a multifactorial process.11, 22 The main goal of our study was to evaluate the available evidence on efficacy and safety of bisphosphonates and/or general preventive strategies in the prevention and treatment of post-transplant bone loss. Results of this systematic review and meta-analysis showed that patients receiving any bisphosphonate therapy showed an increase in BMD of the lumbar spine and femoral neck, or a lesser decrease in BMD, from baseline to 12 months compared with those not receiving any bisphosphonates.
Subgroup analysis of the different bisphosphonates used showed that zoledronic acid in combination with Ca/VitD supplementation prevented bone loss of lumbar spine and femoral neck at 1 year. Furthermore, evaluation of Z-scores of zoledronic acid in combination with Ca alone was effective in preventing lumbar spine and femoral neck bone loss at 6, 12 and 24 months following transplant. Similarly, risedronate in combination with Ca/VitD prevented bone loss of the lumbar spine, femoral neck and the total hip at 6 and 12 months.
Pamidronate in combination with Ca/VitD and hormone replacement therapy showed varying results; it prevented bone loss in the lumbar spine and trochanter at 6 and 12 months, but failed to do so at 3 and 24 months in the lumbar spine. In addition, this combination also prevented femoral neck bone loss at 3 and 6 months but failed to do so at 12 and 24 months. It also prevented bone loss of the total hip at 3, 6, 12 and 24 months. In contrast, Ca/VitD in combination with hormone replacement therapy did not prevent bone loss following transplantation in comparison with Ca/VitD. Similarly, Ca and calcitonin did not prevent bone loss following transplantation in comparison with no intervention. Ca alone also did not prevent bone loss following transplantation in comparison with no intervention.
Although bisphosphonates are commonly used in the post- transplant setting, they do have some side effects. A previous study found that patients taking zoledronic acid in combination with Ca/VitD had a greater number of deaths at 1 and 2 years. The authors of the primary study concluded that the increased mortality observed in the zoledronic acid group was not related to the adverse events related to bisphosphonates. They speculated that although they had well matched patients in terms of disease- and transplantation-related variables, there was considerable disparity in pretransplantation comorbidity scores and this was likely the explanation for the increased mortality observed. Furthermore, those taking zoledronic acid in combination with Ca/VitD had increased flu-like symptoms and greater number of patients affected with a serious adverse event than those only taking Ca/VitD.23
The risk of fractures or X-ray findings of subclinical vertebral fractures was not increased in any of the comparison groups, but this is likely as the studies were not sufficiently powered or of sufficient duration. Fracture development is an important outcome associated with increased morbidity and financial burden and future studies evaluating the efficacy of bisphosphonates should be designed to evaluate fracture occurrence outcomes. Our study shows that bisphosphonates are the most optimal pharmacological agents currently available to prevent bone loss following HSCT.
Our study is limited in that only different therapeutic groups were compared. The optimal dose, duration of therapy and when to start patients on preventive therapies could not be assessed. Additional questions for future studies include the frequency and timing of screening, the population that would benefit from bisphosphonate and other therapies, effects of treatment on fracture prevention and cost effectiveness of various approaches.
Another limitation of our review is that the included studies started treatment with bisphosphonates before, at the time of or right after the HSCT. Evaluating the role of bisphosphonates in long-term survivors was not comprehensively assessed in this systematic review. However, one randomized study evaluating the role of bisphosphonates in long-term survivors conducted by Tauchmanova et al.14 was included in which risedronate treatment was started 17 to 24 months following grafting and they observed that lumbar spine BMD significantly improved within 12 months; it also prevented further femoral neck bone loss.
Conclusion
Bisphosphonates are promising in the prevention and treatment of bone loss following HSCT. In particular, zoledronic acid was effective in preventing bone loss of the lumbar spine and femoral neck; risedronate was effective in preventing bone loss of the lumbar spine and femoral neck and total hip; and pamidronate was variably effective in preventing lumbar spine, femoral neck, trochanter and total hip bone loss following HSCT.
Before more information is available, we propose that every patient undergoing HSCT should have their bone status evaluated. A dual-energy X-ray absorptiometry scan should be obtained and secondary causes of bone loss should be looked for. Bisphosphonates are the most commonly evaluated pharmacotherapy in this population and have shown to prevent bone loss in the early post-transplant period. However, because of the lack of information on fracture risk and the potential adverse effects of bisphosphonates, all patients on bisphosphonates should be monitored closely in order to assess improvement in bone strength and identify adverse events early on and manage them effectively. We believe that it is reasonable to start bisphosphonates and continue them at least for the first year post HSCT. Future studies directly comparing different bisphosphonate agents, dosing regimens and duration of treatment are imperative.
References
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 348–371.
McClune BL, Polgreen LE, Burmeister LA, Blaes AH, Mulrooney DA, Burns LJ et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant 2011; 46: 1–9.
Weilbaecher KN . Mechanisms of osteoporosis after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2000; 6: 165–174.
Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP . Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res 1999; 14: 342–350.
Stern JM, Sullivan KM, Ott SM, Seidel K, Fink JC, Longton G et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant 2001; 7: 257–264.
Kananen K, Volin L, Tahtela R, Laitinen K, Ruutu T, Valimaki MJ . Recovery of bone mass and normalization of bone turnover in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant 2002; 29: 33–39.
Kashyap A, Kandeel F, Yamauchi D, Palmer JM, Niland JC, Molina A et al. Effects of allogeneic bone marrow transplantation on recipient bone mineral density: a prospective study. Biol Blood Marrow Transplant 2000; 6: 344–351.
Schulte C, Beelen DW, Schaefer UW, Mann K . Bone loss in long-term survivors after transplantation of hematopoietic stem cells: a prospective study. Osteoporos Int 2000; 11: 344–353.
Pundole XN, Barbo AG, Lin H, Champlin RE, Lu H . Increased incidence of fractures in recipients of hematopoietic stem-cell transplantation. J Clin Oncol 2015; 33: 1364–1370.
Massenkeil G, Fiene C, Rosen O, Michael R, Reisinger W, Arnold R . Loss of bone mass and vitamin D deficiency after hematopoietic stem cell transplantation: standard prophylactic measures fail to prevent osteoporosis. Leukemia 2001; 15: 1701–1705.
Tauchmanova L, Colao A, Lombardi G, Rotoli B, Selleri C . Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J Clin Endocrinol Metab 2007; 92: 4536–4545.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–34.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151 (264-269): W264.
Tauchmanova L, Selleri C, Esposito M, Di Somma C, Orio F Jr, Bifulco G et al. Beneficial treatment with risedronate in long-term survivors after allogeneic stem cell transplantation for hematological malignancies. Osteoporos Int 2003; 14: 1013–1019.
Review Manager (RevMan) [Computer program]. The Cochrane Collaboration, Version 5.3. The Nordic Cochrane Centre: Copenhagen, Denmark, 2014.
Tauchmanova L, De Simone G, Musella T, Orio F, Ricci P, Nappi C et al. Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant 2006; 37: 81–88.
Arabi A, Bazarbachi A, Dib L, Makarem J, Hatoum H, Kanj AR et al. Prevention of post-bone marrow transplantation bone loss with zoledronate. Bone 2006; 38: S66–S66.
Chae YS, Kim JG, Moon JH, Kim SN, Lee SJ, Kim YJ et al. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplant 2009; 44: 35–41.
Grigg AP, Shuttleworth P, Reynolds J, Schwarer AP, Szer J, Bradstock K et al. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J Clin Endocrinol Metabol 2006; 91: 3835–3843.
Ebeling PR, D'Souza AB, Reynolds J, Shuttleworth P, Szer J, Grigg AP . Pamidronate or zoledronic acid reduce bone loss after allogeneic stem cell transplantation. J Bone Miner Res 2006; 21: S309–S309.
Grigg AC, Shuttleworth P, Reynolds J, Szer J, Schwarer AP, Roberts AW et al. Pamidronate therapy for one year after allogeneic bone marrow transplantation (AlloBMT) reduces bone loss from the lumbar spine, femoral neck and total hip. Blood 2004; 104: 620A–620A.
Hari P, Defor TE, Vesole DH, Bredeson C, Burns LJ . Intermittent zolendronic acid (ZA) for the prevention of osteoporosis after allogeneic hematopoietic cell transplantation (HCT). Blood 2012; 120: 1965.
Hari P, DeFor TE, Vesole DH, Bredeson CN, Burns LJ . Intermittent zoledronic Acid prevents bone loss in adults after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2013; 19: 1361–1367.
Burns L. Phase II Randomized Study of Zoledronate in Preventing Osteoporosis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. National Institutes of Health: Bethesda, MD, USA, 2006. Report no. NCT00321932. https://clinicaltrials.gov/ct2/show/NCT00321932?term=NCT00321932&rank=1.
Jang E, Baek K, Ko S, Kang Ml . The effect of risedronate on serum dickkopf-1, osteoprotegerin, and RANKL in patients with hematologic malignancies following HSCT. J Bone Miner Res 2010; 25 (Suppl 1): S403.
Back K, Kim G, Kim M, Lim D, Lee S, Han J et al. Effect of risedronate on bone density in hematopoietic stem cell transplant patients: a prospective, randomized, double-blind placebo-controlled trial. J Bone Miner Res 2008; 23 (Suppl 1): S346.
Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Valimaki MJ . Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab 2005; 90: 3877–3885.
Kananen K, Volin L, Laitinen K, Ruutu T, Valimaki MJ . Serum osteoprotegerin and receptor activator of nuclear factor-kappaB ligand (RANKL) concentrations in allogeneic stem cell transplant-recipients: a role in bone loss? Osteoporos Int 2006; 17: 724–730.
Tauchmanova L, Ricci P, Serio B, Lombardi G, Colao A, Rotoli B et al. Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. J Clin Endocrinol Metab 2005; 90: 627–634.
Seno B, Tauchmanova L, Ricci P, Cerciello G, Imperatore I, Risitano A et al. Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. Bone Marrow Transplant 2005; 35 (Suppl 2): S189–S189.
Wang M, Masuda H, Takemoto Y, Kanamaru A, Kakishita E . Osteoporosis as a late complication of allogeneic bone marrow transplantation. Exp Hematol 1997; 25: 331–331.
Valimaki MJ, Kinnunen K, Volin L, Tahtela R, Loyttyniemi E, Laitinen K et al. A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant 1999; 23: 355–361.
Pundole X, Champlin RE, Popat UR, Escalante CP, Murphy WA, Gagel RF et al. A randomized controlled trial of ibandronate for the prevention of bone loss following allogeneic stem cell transplantation. J Clin Oncol 2015; 33 (suppl): (abstract 7029).
Ferreira CA, Loureiro CAS, Saconato H, Atallah ÁN . Assessing the risk of bias in randomized controlled trials in the field of dentistry indexed in the Lilacs (Literatura Latino-Americana e do Caribe em Ciências da Saúde) database. Sao Paulo Med J 2011; 129: 85–93.
Chen Z, Chen Y, Zeng J, Wang Y, Ye T, Zhou Q et al. Quality of randomized controlled trials reporting in the treatment of melasma conducted in China. Trials 2015; 16: 1.
Lai R, Chu R, Fraumeni M, Thabane L . Quality of randomized controlled trials reporting in the primary treatment of brain tumors. J Clin Oncol 2006; 24: 1136–1144.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J 2011; 343: d5928.
Acknowledgements
We are grateful to Dr Gregory Pratt from the Research Medical Library of the University of Texas MD Anderson Cancer Center for conducting the search in the electronic databases. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author contributions
Dr Suarez-Almazor had full access to all of the data in the study and takes responsibility for the integrity and the accuracy of the data analysis. Study concept and design: Suarez-Almazor and Lu; search strategy: Pratt; selection of the studies: Cheema, Sanchez Petitto, Pundole and Lopez-Olivo; quality appraisal and data extraction: Cheema, Sanchez Petitto, Pundole and Lopez-Olivo; analysis and interpretation of data: Cheema, Sanchez Petitto, Pundole, Lopez-Olivo, Lu and Suarez-Almazor; drafting of the manuscript: Cheema, Pundole, Lu and Lopez-Olivo; critical revision of the manuscript for important intellectual content: Cheema, Sanchez Petitto, Pundole, Lopez-Olivo, Lu and Suarez-Almazor; administrative, technical or material support: Suarez-Almazor; study supervision: Suarez-Almazor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Lopez-Olivo reports grants from Rheumatology Research Foundation, outside the submitted work. Dr Suarez-Almazor reports advisory/consulting role for Ardea Biosciences and a Pfizer Aspire Grant, outside the submitted work.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Pundole, X., Cheema, H., Sanchez-Petitto, G. et al. Prevention and treatment of bone loss and fractures in patients undergoing a hematopoietic stem cell transplant: a systematic review and meta-analysis. Bone Marrow Transplant 52, 663–670 (2017). https://doi.org/10.1038/bmt.2016.312
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.312
- Springer Nature Limited
This article is cited by
-
International recommendations for screening and preventative practices for long-term survivors of transplantation and cellular therapy: a 2023 update
Bone Marrow Transplantation (2024)
-
Assessing quality and quantity of cortical bone in childhood cancer survivors using anthropometric indices
Oral Radiology (2023)
-
Bone turnover markers as an aid to monitor osteoporosis following allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2020)
-
Predictors of impaired bone health in long-term survivors after allogeneic stem cell transplantation
Bone Marrow Transplantation (2019)
-
Fracture risk prediction using FRAX in patients following hematopoietic stem cell transplantation
Archives of Osteoporosis (2018)