Abstract
We compared the outcomes of immunosuppressive treatment (IST) with those of alternative donor hematopoietic stem cell transplantation (HSCT) in children and adolescents with severe aplastic anemia (SAA). The medical records of 42 patients with SAA who received frontline IST (N=19) or frontline HSCT with an alternative donor (N=23) between 1998 and 2012 were analyzed retrospectively. Six patients responded in the frontline IST group, whereas 11 underwent salvage HSCT after IST failure. Twenty-one of 23 patients who underwent frontline HSCT survived without treatment failure. The estimated failure-free survival rate of the frontline HSCT group was higher than that of the frontline IST group (91.3% vs 30.7% respectively, P<0.001). Six of 11 patients who underwent salvage HSCT experienced event-free survival (EFS). The estimated EFS of the frontline HSCT group was higher than that of the salvage HSCT group (91.3% vs 50.9% respectively, P=0.015). The outcome of alternative donor HSCT was better than commonly reported rates, especially in patients who underwent frontline HSCT. These results suggest that frontline alternative donor HSCT may be a better treatment option than IST for children and adolescents with SAA who lack a human leukocyte Ag-matched familial donor.
Similar content being viewed by others
Introduction
Severe aplastic anemia (SAA) is a life-threatening disorder characterized by pancytopenia of the peripheral blood and hypocellular marrow. Although both allogeneic hematopoietic stem cell transplantation (HSCT) and immunosuppressive treatment (IST) are available treatments, allogeneic HSCT from an HLA-matched familial donor is preferred by SAA patients.1, 2 However, approximately two-thirds of patients do not have a suitable HLA-matched sibling donor; therefore, either IST or alternative donor HSCT is employed in such cases.3, 4
The response rate of IST consisting of antithymocyte globulin (ATG) and cyclosporine (CsA) with or without G-CSF has been reported to be 60–80% in SAA patients.4, 5 However, previous reports have shown that IST is related with a substantial risk of relapse and clonal evolution.6, 7 Furthermore, IST often leads to the amelioration of cytopenia rather than cures.5, 8, 9 A recent study found that the estimated event-free survival (EFS) of IST was 33%, and 91 of 167 patients (55%) ultimately underwent rescue HSCT.10 Notably, the outcomes of patients undergoing alternative donor HSCT have been significantly improving recently.2 Advances in HSCT, such as modification of the conditioning regimen, a better selection of donors by high degree HLA allele level matching and the introduction of low-dose TBI, have improved the outcomes of HSCT in patients with SAA.11, 12, 13, 14 A recent study showed an estimated 5-year failure-free survival (FFS) of 95.01% in children who received 10 Ag-matched unrelated donor HSCTs.15
Although several reports have compared the outcome of frontline IST to that of frontline HSCT from an HLA-matched familial donor,5, 10 studies comparing frontline IST to frontline alternative donor HSCT have been limited. For this reason, this study aimed to investigate the efficacy of alternative donor HSCT as a frontline treatment modality compared with that of IST in children and adolescents with SAA who lack an HLA-matched familial donor.
Materials and methods
Patients
We retrospectively reviewed the medical records of 42 consecutive patients with acquired SAA who lacked an HLA-matched familial donor and received IST or alternative donor HSCT at Samsung Medical Center between January 1998 and December 2012. All patients were younger than 18 years, were newly diagnosed with SAA, and had not undergone any specific prior treatments, except transfusion support. SAA was defined according to the standard criteria16; if patients met these criteria and had a neutrophil count <0.2 × 109/L, their diseases were considered to be very severe aplastic anemia. Patients who had inherited bone marrow failure syndrome or received a matched-related donor HSCT were excluded from this study. The choice of frontline treatment with alternative donor HSCT or IST was made by the patient or his/her guardians. This study was approved by the Institutional Review Board of Samsung Medical Center. The need for informed consent was waived by the board.
Immunosuppressive therapy
IST consisted of horse or rabbit ATG, methylprednisolone, and CsA. Horse ATG (ATGAM; Pharmacia & Upjohn Company) 20 mg/kg per day for 8 days was available between January 1998 and December 2003, but was replaced with rabbit ATG (Thymoglobulin; Genzyme) 3.5 mg/kg per day for 5 days starting in January 2004. Methylprednisolone (1 mg/kg per day) was administered before commencing ATG infusion to prevent allergic reaction, and then was maintained for 2 weeks before being abruptly tapering. CsA (5 mg/kg per day) orally was started on day 13 and was continued for at least 6 months, with dosing adjusted to achieve drug trough levels between 150 and 200 ng/mL.
Conditioning regimen and GVHD prophylaxis
The conditioning for alternative donor HSCT consisted of various regimens. Details of the conditioning regimens were as follows: (1) cyclophosphamide (Cy, 60 mg/kg/day on days –8 and –7) +fludarabine (FLU, 40 mg/m2/day on days –6 to –2) +ATG (on days –3 to –1), (2) busulfan (Bu, 0.8–1.1 mg/kg/day on days –7 and –6) +Cy (50 mg/kg/day on days –5 to –2) +ATG (on days –4 to –2), (3) TBI (500 cGy) +Cy (60 mg/kg/day on days –4 and –3) +ATG (on days –4 to –2), (4) Cy (50 mg/kg/day on days –5 to –2) +ATG (on days –4 to –2) and (5) TBI (500 cGy) +FLU (25 mg/m2/day on days –4 to –2) +Cy (60 mg/kg/day on days –3 and –2). Horse ATG (30 mg/kg for 3 days) in the conditioning regimens had been changed to rabbit ATG (2.5 mg/kg for 3 days) since January 2004. Prophylaxis against GVHD consisted of a combination of CsA (3 mg/kg per day) or tacrolimus (0.03 mg/kg per day) plus short-term methotrexate. The doses of CsA and tacrolimus were adjusted to achieve drug trough levels between 200 and 300 ng/mL, and between 15 and 20 ng/mL, respectively.
Comparison of the response and survival outcomes
To assess the effect of treatment modalities on the outcomes, the probable overall survival (OS), FFS or EFS rates were estimated. Treatment failure of IST was defined as death, no response by 6 months and beyond, relapse, disease progression requiring HSCT from an alternative donor or second IST, clonal evolution and evolution to paroxysmal nocturnal hemoglobinuria. Complete response was defined as a normal hemoglobin level for age, neutrophil count >1.5 × 109/L and platelet count >150 × 109/L. Partial response was defined as transfusion independent and no longer meets the criteria for severe disease. The overall response included both complete and partial responses. Relapse was defined as the occurrence of transfusion dependency or a neutrophil count <0.5 × 109/L after attainment of a response. Treatment failure or an event after HSCT was defined as death, primary graft failure, late rejection, relapse and secondary malignancy, whichever occurred first. Primary graft failure was defined as failure to achieve a neutrophil count >0.5 × 109/L on 3 consecutive days after transplantation, and secondary graft failure was defined as graft loss following the initial achievement of graft function.
Statistical analysis
Patient’s characteristics and clinical outcomes were compared using chi-square or Fisher’s exact tests for binary variables, and the Mann-Whitney U-test for continuous variables. FFS and EFS were calculated by using the Kaplan–Meier method, and comparisons between survival curves were performed using the log-rank test. P-values <0.05 were considered to indicate statistical significance.
Results
Patients
Figure 1 shows the overall treatment flow and outcomes of patients in this study. Three of 42 patients had very severe aplastic anemia. Nineteen patients received frontline IST, and the remaining 23 received frontline HSCT. Frontline IST was performed using either horse (n=10) or rabbit ATG (n=9) in combination with CsA and a corticosteroid. Eleven of 19 patients who received frontline IST underwent salvage HSCT after IST failure.
Overall outcomes of 42 patients with SAA who lacked an HLA-matched familial donor. Six of 19 patients who received frontline IST and 2 of 6 patients who received second-line IST had a response. The overall response of IST was 42.1%. In addition, seven non-responders with first-line IST and four non-responders with second-line IST received salvage HSCT. HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; IST, immunosuppressive treatment; SAA, severe aplastic anemia.
Frontline IST vs frontline HSCT
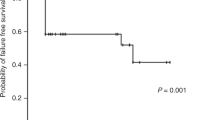
The characteristics of patients in the frontline IST and frontline HSCT groups are summarized in Table 1. Sex, age and the disease status at treatment were comparable between the 2 groups; however, the median time from diagnosis to treatment was longer in the frontline HSCT group than in the frontline IST group (10.1 months vs 4.0 months respectively, P=0.025). Six of 19 patients who received frontline IST showed responses (complete response in 2 patients and partial response in 4), and there was no difference in the response rates between IST with horse ATG and that with rabbit ATG (40.0% vs 22.2% respectively, P=0.628). The remaining 13 patients did not achieve responses with frontline IST, and 6 of them received a second IST; Two of these 6 patients, 1 each achieved complete response and partial response after the second IST. The overall response rate of IST was 42.1%. Eleven patients who had no responses with frontline or second-line IST underwent salvage HSCT (57.9%). Among the 23 patients who underwent frontline HSCT, 21 experienced FFS. Although the 5-year OS was comparable between the frontline HSCT and frontline IST groups (91.3% vs 71.2% respectively, P=0.187), the 5-year FFS was significantly higher in the former than the latter (91.3% vs 30.7% respectively, P<0.001) (Figure 2).
Overall survival (OS) and failure-free survival (FFS) according to the initial treatment modality. (a) No difference in 5-year OS rate was found between frontline HSCT and frontline IST (91.3 vs 71.2% respectively, P=0.187). (b) The 5-year FFS rate was significantly different for frontline HSCT versus frontline IST (91.3% vs 30.7% respectively, P<0.001). HSCT, hematopoietic stem cell transplantation; IST, immunosuppressive treatment.
Frontline vs salvage HSCT
The characteristics and clinical outcomes between the frontline and salvage HSCT groups are summarized in Table 2. Matched unrelated bone marrow (BM)/peripheral blood stem cell (PBSC) grafts were more frequent in the frontline HSCT group compared to those in the salvage HSCT group. Moreover, the median time from diagnosis to HSCT was shorter in the frontline HSCT group than in the salvage HSCT group. There was 1 case of primary graft failure in the salvage HSCT group (9.1%), but all patients in the frontline HSCT group were successfully engrafted. Seven deaths occurred among 34 transplants; 2 patients in the frontline HSCT group died of cardiogenic shock due to coronary disease in one and from Cy-induced cardiomyopathy in the other. However, there were 5 deaths in the salvage HSCT group which were caused by post-transplant lymphoproliferative disease (n=2), graft failure (n=1), fungemia (n=1) and chronic GVHD (n=1). The estimated 5-year EFS rate of the frontline HSCT group was substantially higher than that of the salvage HSCT group (91.3% vs 50.9% respectively, P=0.015) (Figure 3). There were no statistically significant differences in EFS among different graft sources in the frontline HSCT group (BM 90.0% vs. PBSC 88.9%, P=0.907; cord blood [CB] 100% vs. PBSC 88.9%, P=0.505; CB 100% vs. BM 90.0%, P=0.527).
Acute and chronic GVHD
In the frontline HSCT group, grade II–IV acute GVHD developed in 11 patients (47.8%), whereas grade III–IV developed in only 1 patient (4.3%) (Table 2). Fourteen patients developed chronic GVHD (60.8%, 7 limited and 7 extensive). Of 7 extensive chronic GVHD patients, 4 had received BM, 2 PBSC, and 1 CB. Most chronic GVHD patients (n=12, 85.7%) eventually became free of immunosuppressant at a median of 16 months after HSCT, and no patient died of chronic GHVD. The incidence of acute GVHD (P=0.582) and chronic GVHD (P=0.157) between BM transplantations and PBSC transplantations in the frontline HSCT group were not different.
In the salvage HSCT group, the incidence of grade II–IV acute, III–IV acute, and chronic GVHD were 45.5%, 18.2%, and 27.3% (extensive 9.1%), respectively. The only mortality from extensive chronic GVHD in the salvage HSCT group occurred in a patient who received a haploidentical graft. There was no difference in either acute GVHD (P=0.150) or chronic GVHD (P=0.141) between the frontline HSCT and salvage HSCT groups.
Discussion
Both IST and alternative donor HSCT have improved the survival rate of patients with SAA who lack an HLA-matched familial donor. Currently, the SAA treatment guideline in Europe indicates that IST is the first-line treatment of choice for younger patients who lack an HLA-matched familial donor, and matched unrelated donor HSCT is then recommended in the event of failure of first-line IST.17 However, recent advances in the field of HSCT, including better HLA matching by high-resolution allele typing, improved supportive care for the management of transplant-related complications and better availability of new drugs has produced alternative donor HSCT outcomes similar to those of matched sibling donor HSCT in some studies.3, 11 Additionally, previous studies have shown that the FFS rates of alternative donor HSCT were higher than those of IST. One previous study showed that the estimated 5-year FFS following 10 Ag-matched (HLA-A, -B, -C, -DRB1 and -DQB1) unrelated donor HSCTs was 95.01%, and that following IST was 13.3%.15 Another study showed that the estimated FFS rate was 83.9% among patients receiving alternative donor HSCT compared with 9.5% in those receiving second course of IST.8 Most patients in these studies had received IST prior to alternative donor HSCT (90.9% and 100.0%, respectively). However, our study instead focused on the impact of the frontline treatment modality on outcomes, and demonstrated that the 5-year FFS rate of the frontline alternative donor HSCT group was superior to that of frontline IST group (91.3% vs 30.7% respectively, P<0.001). Moreover, the EFS rate after frontline HSCT was higher than that after salvage HSCT in the current study (91.3% vs 50.9% respectively, P=0.015). We believe that treatments aimed at a cure rather than an improvement may be a more desirable goal considering the longer life expectancy of children and adolescents. Because only 4 patients received frontline CB transplantation (CBT) in this study, we cannot draw any conclusion on its benefit over frontline IST. However, we did demonstrate a clear advantage for BM or PBSC transplantations over IST as a frontline treatment modality in pediatric SAA patients without HLA-matched familial donors.
Previous studies have shown that horse ATG is superior to rabbit ATG when used for IST. A prospective randomized study from the National Institute of Health showed a clear difference in response rates between horse and rabbit ATGs (68% vs 37% respectively, P<0.001).18 Another prospective study from the European Blood and Marrow Transplant Severe Aplastic Anemia Working Party reported that the OS of patients with aplastic anemia treated upfront with horse ATG was superior to that in patients treated with rabbit ATG (86% vs 68% respectively, P=0.009). Moreover, transplant-free survival was better in the former compared to the latter (76% vs 52% respectively, P=0.002).19 In this study, the response rate of horse ATG was higher than that of rabbit ATG; however, the difference was not significant, probably owing to the small number of patients in each group. As stated previously, only rabbit ATG has been available in Korea since 2004. Under these circumstances, alternative donor HSCT, especially using BM or PBSC, may be desirable for the upfront treatment of patients with SAA given the inferior outcome generally observed for IST with rabbit ATG. Furthermore, those who receive IST usually cannot avoid multiple transfusions, which may negatively affect any subsequent HSCT. Regardless, further randomized prospective studies are required to identify the better modality with greater certainty.
CBT has become a treatment option for patients with non-malignant disease with the benefits of rapid accessibility and a lower incidence of GVHD despite HLA discrepancy.20 In general, CBT has not been recommended as frontline treatment for SAA because of the higher probability of graft failure and transplant-related complications.20, 21 A report by the Japan Cord Blood Bank Network showed that the probability of OS at 2 years was 41% in 31 patients who underwent CBT,22 and data from Eurocord showed that the probability of 2-year OS was ~40% among 57 single CB unit recipients.23 In our study, although the number of CB recipients is too small to draw clear conclusions regarding the role of CBT, it is encouraging that all 4 patients in the frontline HSCT group achieved FFS. As the outcome of CBT in various diseases has recently shown improvement,24, 25, 26 we believe that the role of CBT as a frontline treatment for pediatric SAA patients should be re-evaluated using well-designed prospective studies. Although a haploidentical donor may be readily available within a family, the higher risk of GVHD and graft rejection following transplantation are significant limitations associated with it. Our study included 4 patients who underwent haploidentical HSCT; the survival rate among whom was only 50.0%. However, several recent reports have shown relatively acceptable outcomes following haploidentical transplantation. Esteves et al.27 reported a 1-year OS of 67.1% in 16 Brazilian children and young adults with SAA who underwent haploidentical donor HSCT. Xu et al.28 similarly showed an OS rate of 64.6% in 19 Chinese children and young adults with a median follow-up of 746 days. Although number of patients in these previous reports were small, their results suggest that haploidentical HSCT may be a particularly important treatment modality for patients who fail frontline treatment and require urgent transplantation but have no matched familial or unrelated donors.
Although the majority of patients (85.7%) with chronic GVHD were eventually able to discontinue all immunosuppressants, the high incidence of chronic GVHD in our cohort suggests a negative influence of frontline HSCT using an alternative donor on the quality of life of children with SAA. Thus, high quality general supportive measures, as well as a multidisciplinary approach, should be provided during the long-term follow-up of these patients.
There are several limitations in this study. First, this was a retrospective analysis in which the number of patients was small. Second, it was difficult to clarify the main reasons for improved outcomes of alternative donor transplants because of the diversity of conditioning regimens employed. Third, the outcome of IST may be underestimated because of the recent unavailability of horse ATG in our country. Whatever the reason, the outcome of alternative donor HSCT in our cohort was better than expected, especially in those who received HSCT without prior IST, whereas the overall response rate of IST was not satisfactory.
In conclusion, our data suggests that frontline alternative donor HSCT may be a better treatment option than IST for children and adolescents who lack an HLA-matched familial donor, especially in situations where horse ATG is not available. Further study is required to identify the best graft source and the best conditioning regimen that can further improve the outcome of alternative donor HSCT for children with SAA.
References
Kojima S, Horibe K, Inaba J, Yoshimi A, Takahashi Y, Kudo K et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol 2000; 111: 321–328.
Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2007; 92: 11–18.
Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socie G, Maury S et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant 2005; 36: 947–950.
Pongtanakul B, Das PK, Charpentier K, Dror Y . Outcome of children with aplastic anemia treated with immunosuppressive therapy. Pediatr Blood Cancer 2008; 50: 52–57.
Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica 2014; 99: 1784–1791.
Kamio T, Ito E, Ohara A, Kosaka Y, Tsuchida M, Yagasaki H et al. Relapse of aplastic anemia in children after immunosuppressive therapy: a report from the Japan Childhood Aplastic Anemia Study Group. Haematologica 2011; 96: 814–819.
Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood 2002; 100: 786–790.
Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood 2008; 111: 1054–1059.
Scheinberg P, Wu CO, Nunez O, Young NS . Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr 2008; 153: 814–819.
Dufour C, Pillon M, Socie G, Rovo A, Carraro E, Bacigalupo A et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol 2015; 169: 565–573.
Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica 2010; 95: 976–982.
Deeg HJ, Seidel K, Casper J, Anasetti C, Davies S, Gajeweski JL et al. Marrow transplantation from unrelated donors for patients with severe aplastic anemia who have failed immunosuppressive therapy. Biol Blood Marrow Transplant 1999; 5: 243–252.
Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Margolis D, Camitta B, Pietryga D, Keever-Taylor C, Baxter-Lowe LA, Pierce K et al. Unrelated donor bone marrow transplantation to treat severe aplastic anaemia in children and young adults. Br J Haematol 1996; 94: 65–72.
Samarasinghe S, Steward C, Hiwarkar P, Saif MA, Hough R, Webb D et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br J Haematol 2012; 157: 339–346.
Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol 2003; 123: 782–801.
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 2009; 147: 43–70.
Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med 2011; 365: 430–438.
Marsh JC, Bacigalupo A, Schrezenmeier H, Tichelli A, Risitano AM, Passweg JR et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood 2012; 119: 5391–5396.
Liu HL, Sun ZM, Geng LQ, Wang XB, Ding KY, Tang BI et al. Unrelated cord blood transplantation for newly diagnosed patients with severe acquired aplastic anemia using a reduced-intensity conditioning: high graft rejection, but good survival. Bone Marrow Transplant 2012; 47: 1186–1190.
Chan KW, McDonald L, Lim D, Grimley MS, Grayson G, Wall DA . Unrelated cord blood transplantation in children with idiopathic severe aplastic anemia. Bone Marrow Transplant 2008; 42: 589–595.
Yoshimi A, Kojima S, Taniguchi S, Hara J, Matsui T, Takahashi Y et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant 2008; 14: 1057–1063.
MacMillan ML, Walters MC, Gluckman E . Transplant outcomes in bone marrow failure syndromes and hemoglobinopathies. Semin Hematol 2010; 47: 37–45.
Bizzetto R, Bonfim C, Rocha V, Socie G, Locatelli F, Chan K et al. Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica 2011; 96: 134–141.
Locatelli F, Kabbara N, Ruggeri A, Ghavamzadeh A, Roberts I, Li CK et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood 2013; 122: 1072–1078.
Mitchell R, Nivison-Smith I, Anazodo A, Tiedemann K, Shaw P, Teague L et al. Outcomes of hematopoietic stem cell transplantation in primary immunodeficiency: a report from the Australian and New Zealand Children's Haematology Oncology Group and the Australasian Bone Marrow Transplant Recipient Registry. Biol Blood Marrow Transplant 2013; 19: 338–343.
Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V et al. Haploidentical BMT and post-transplant Cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transplant 2015; 50: 685–689.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Acknowledgements
We thank the residents and nurses who cared for the patients, and without whom this study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, Y., Yi, E., Lee, J. et al. Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched familial donor. Bone Marrow Transplant 52, 47–52 (2017). https://doi.org/10.1038/bmt.2016.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.223
- Springer Nature Limited
This article is cited by
-
Allo-HSCT compared with immunosuppressive therapy for acquired aplastic anemia: a system review and meta-analysis
BMC Immunology (2020)
-
Who is the best haploidentical donor for acquired severe aplastic anemia? Experience from a multicenter study
Journal of Hematology & Oncology (2019)
-
Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia
Bone Marrow Transplantation (2019)
-
Unmanipulated haploidentical transplantation conditioning with busulfan, cyclophosphamide and anti-thymoglobulin for adult severe aplastic anaemia
Bone Marrow Transplantation (2018)
-
Cyclophosphamide
Reactions Weekly (2017)