Abstract
Data on 68 146 hematopoietic stem cell transplants (HSCTs) (53% autologous and 47% allogeneic) gathered by 1566 teams from 77 countries and reported through their regional transplant organizations were analyzed by main indication, donor type and stem cell source for the year 2012. With transplant rates ranging from 0.1 to 1001 per 10 million inhabitants, more HSCTs were registered from unrelated 16 433 donors than related 15 493 donors. Grafts were collected from peripheral blood (66%), bone marrow (24%; mainly non-malignant disorders) and cord blood (10%). Compared with 2006, an increase of 46% total (57% allogeneic and 38% autologous) was observed. Growth was due to an increase in reporting teams (18%) and median transplant activity/team (from 38 to 48 HSCTs/team). An increase of 167% was noted in mismatched/haploidentical family HSCT. A Strengths, Weaknesses, Opportunities, Threats (SWOT) analysis revealed the global perspective of WBMT to be its major strength and identified potential to be the key professional body for patients and authorities. The limited data collection remains its major weakness and threat. In conclusion, global HSCT grows over the years without plateauing (allogeneic>autologous) and at different rates in the four World Health Organization regions. Major increases were observed in allogeneic, haploidentical HSCT and, to a lesser extent, in cord blood transplantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The guiding principles of the World Health Organization (WHO; www.who.org) declare the transplantation of organs, cells and tissues to be a global task, with the collection of activity data being one of the prime prerequisites.1, 2 The Worldwide Network for Blood and Marrow Transplantation (WBMT; www.wbmt.org), an umbrella organization in the field of hematopoietic stem cell transplantation (HSCT) and affiliated as a Non-Governmental Organization (NGO) with the WHO, has taken up the challenge of collecting and disseminating worldwide data on a regular basis. Macroeconomic details of the participating countries are considered alongside the transplant activity data. The first report was published in 2010 based on the global transplant activity in 2006,3 and was followed by a report on the data available in 2010 and a retrospective of the first one million HSCT in 2015.4, 5
HSCT presents a valid treatment option for many congenital and acquired disorders of the hematopoietic system, for chemo- and immune-sensitive diseases and for the replacement of deficient cells or cellular components.4, 5, 6, 7 It is used with increasing frequency worldwide.8, 9, 10, 11 This fourth report concentrates on the HSCT activity in 2012 and considers major trends since 2006. The report includes a Strengths, Weaknesses, Opportunities, Threats (SWOT) analysis of the current WBMT policy by key personnel in the field.,12 It illustrates the strengths and weaknesses, the opportunities and threats of the current perspectives in HSCT. The report incorporates both active and inactive countries and HSCT from all sources and donor types. As such, it can serve as a comprehensive guide for competent authorities and transplant organizations as well as patients.
Materials and methods
Study design, data collection and data validation
This retrospective observational survey from the 194 WHO member states on transplants performed in 2012 followed the previously described design. The primary measures of outcome were numbers of HSCT by disease, donor type, stem cell source and WHO region, and secondary outcome were key trends in relation to the first report in 2006.3
Transplant activity data were supplemented by a SWOT analysis via qualitative written interviews as indicated below.12, 13
As no individual patient data were used, no ethics committee approval was asked for this particular study.
Definitions
Transplant rates were computed as the number of patients treated with a first HSCT per 10 million inhabitants. Definition of team density, allocation of individual countries to a WHO region, information on population data and Gross National Income/capita were obtained as described previously.3, 4, 5
SWOT analysis
Six selected representatives of regional transplant organizations were presented with the 2012 survey data and asked in a qualitative written interview to define the strengths, weaknesses, opportunities and threats for WBMT based on the present data and trends. Strengths and weaknesses refer in this context to aspects within WBMT as an organization, opportunities and threats to third parties such as competent authorities, patients or the community at large. The responses were analyzed by two members of the writing committee and experts in the evaluation of qualitative interviews using qualitative research criteria.13 In a Delphi-like process returned to the same representatives for rereview before final presentation in Table 1.
Statistical analysis
The data analysis was performed using ordinary least-squares regressions for trends, χ2 tests for independent proportions of indications and binomial tests for equal shares of donor type. Calculations were performed in Eviews 8 and Excel 2010 (Microsoft, Redmond, WA, USA).
Role of the funding source
The study was funded by the participating organizations. The corresponding author had full access to all of the data and is responsible for the submitted publication.
Results
Global transplant numbers and transplant rates in 2012
A total of 68 146 HSCT (31 926 allogeneic, 47%; 36 220 autologous, 53%) were reported by 1566 teams from 77 of the 79 countries known to have performed HSCT for the year 2012. No transplants were performed in countries with a population of <300 000 inhabitants, with a surface area of <700 km2 or a gross national income/cap of <US$1260.
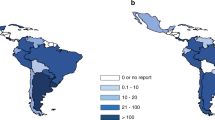
Absolute numbers ranged from 1 to 16 194 for all HSCT, autologous and allogeneic combined (Figure 1a); from 1 to 7275 for allogeneic HSCT; and from 2 to 8919 for autologous HSCT (Supplementary Figures 1a and b). Autologous HSCT were performed in 76 and allogeneic HSCT in 72 countries, with allogeneic transplantation from unrelated donors in 56 countries and from cord blood in 46 countries. Absolute numbers of unrelated donor HSCT ranged from 0 to 4217 and of cord blood transplants from 0 to 1176. Numbers of transplant centers ranged from 1 to 373 centers per country with team densities from 0.06 to 33.3 teams per 10 million inhabitants. The absolute numbers of HSCT in 2012 performed by individual teams ranged from 1 to 302. Transplant rates in 2012 for all HSCT (allogeneic and autologous combined) ranged from 0.1 to 1001, median 264.2 per 10 million inhabitants (Figure 1b). The corresponding rates for allogeneic transplantation range from 0.1 to 510 (median 101.7) and for autologous transplantation from 0.2 to 667 (median 162.5) (Supplementary Figures 1c and d).
Global HSCT activity in 2012. (a) Absolute number of HSCT (allogeneic and autologous combined) in participating countries by WHO regional offices area in 2012. Regions are colored by WHO regional offices area code (see text). Shades of colors reflect absolute transplant numbers. (b) Transplant rates of allogeneic and autologous HSCT combined/10 million population in participating countries by WHO regional offices area in 2012. Regions are colored by WHO regional offices area code (see text). (c) Use of allogeneic compared to autologous HSCT in participating countries by WHO regional offices area in 2012. Regions are colored by WHO regional offices area code (see text). Darker colors indicate preferred use of allogeneic HSCT. (d) Use of family donor HSCT in participating countries by WHO regional office areas in 2012. Regions are colored by WHO regional office area code (see text). Darker colors indicate preferred use of family donors compared with unrelated donor HSCT. (e) Use of HSCT for malignant indications compared with non-malignancies in participating countries by WHO regional office area in 2012. Regions are colored by WHO regional office area code (see text). Darker colors indicate preferred use of HSCT for malignant indications.
Allogeneic HSCT accounted for 46.8% (range 0–100%) of all HSCT in 2012, with a total of 15 countries performing more allogeneic than autologous HSCT (Figure 1c). Among the allogeneic HSCT, the proportion of unrelated donor transplants ranged from 0.83 to 83.3% (median 34.6%), with 23 countries performing more unrelated than family donor HSCT (Figure 1d).
Indications, donor type and stem cell source
The indications for HSCT in 2012 are listed in detail in Table 2. The most frequent main indication was a lymphoproliferative disorder with 36 243 patients (53.2% of all HSCT), 4322 (12%) of whom received allogeneic and 31 921 (88%) autologous HSCT. Within this group, the most frequent single indication was plasma cell disorders with 18 553 patients, 963 (5%) of whom received allogeneic and 17 590 (95%) autologous HSCT. Leukemia was the second most frequent indication with 24 280 patients (36% of the total), with 23 236 (96%) patients receiving allogeneic HSCT and 1044 (4%) autologous HSCT. Here, the most frequent single indication was acute myeloid leukemia (including other leukemia), with 12 254 patients in total (18% of all HSCT), 11 419 (93%) with an allogeneic and 835 (7%) with autologous HSCT. Non-malignant disorders comprised the third largest group with 4398 patients (6% of total HSCT), 4068 (93%) with allogeneic and 330 (7%) with autologous HSCT. This latter group of autologous HSCT for non-malignant disorders predominantly comprised of patients (N=239) with autoimmune disorders. The most frequent indication was bone marrow failure with 1986 patients, 1979 (99%) of whom received an allogeneic HSCT. A total of 3014 patients (4%) were treated for solid tumors, 2884 thereof (96%) with an autologous HSCT (Figures 2a and b).
Of the 31 926 allogeneic HSCT, 7590 (24%) were bone marrow derived, 21 226 (66%) from PBSCs and 3110 (10%) from cord blood cells. Of the 36 220 autologous transplants, 290 (1%) were bone marrow, 35 920 (99%) peripheral blood and 10 cord blood derived. The proportion of allogeneic HSCT using bone marrow as the stem cell source was highest for non-malignant disorders (53%), followed by leukemias (20%) and was very low in lymphoproliferative disorders (15%; Table 2). The use of cord blood as a source for allogeneic HSCT was highest for patients with leukemia (71%), followed by those with non-malignant disorders (16%), lymphoproliferative disorders (10%) and solid tumors (1%).
Key developments from 2006 to 2012
Absolute transplant numbers increased from 46 563 (20 333 allogeneic, 26 230 autologous) in 2006 to 68 146 (31 926 allogeneic, 36 220 autologous) in 2012. This corresponds to an absolute increase of 21 583 total (11 593 allogeneic, 9990 autologous HSCT), and a relative increase of 46% total (57% allogeneic, 43% autologous). This growth was due firstly to an increase in the number of reporting teams from 1327 in 67 countries to 1566 in 77 reporting countries and secondly to an increase in transplant activity in previously established teams.3 There were some differences between the four WHO regions, with minimal increases in autologous HSCT being seen in the East Mediterranean/African region (Figure 3c). In general, transplant numbers increased in all four WHO regions for all main indications and both allogeneic and autologous donor types. However, there were differences in the extent of the changes and a few exceptions (Figures 3b and c). Most notably, the number of allogeneic HSCT increased to the greatest extent for leukemias and non-malignant disorders (Supplementary Figure 2). This is illustrated by the proportion of malignant indications compared with non-malignancies in the different participating countries (Figure 1e). The numbers of autologous HSCT for lymphomas and plasma cell disorders increased, whereas those for leukemias declined (Supplementary Figure 3). Allogeneic HSCT showed the same pattern in all four WHO regions, with a predominant increase in non-malignant disorders in the Eastern Mediterranean/Africa region. HSCTs from unrelated donors were again more frequent than from related donors (Figure 4a). Changes in the number of autologous HSCT (both increases and decreases) were largely limited to America and Europe. Overall, the observed increases did correlate with Gross National Income/cap of the participating countries, with a greater increase in countries with more resources. This gap continues to widen.
Global trends 2006–2012. (a) Increase in absolute numbers of global HSCT from 2006 to 2012. Colors depict allogeneic (blue), autologous (red) and total HSCT (green). (b) Increase in absolute numbers of allogeneic HSCT from 2006 to 2012 by WHO region (for definitions see text). Colors depict AMR/PAH (blue), SEAR/WPR (red), EUR (green) and EMR/AFR (purple). (c) Increase in absolute numbers of autologous HSCT from 2006 to 2012 by WHO region. Colors depict AMR/PAH (blue), SEAR/WPR (red), EUR (green) and EMR/AFR (purple). AMR/PAH=Region of the Americas; EMR/AFR=Eastern Mediterranean Region and African Region; EUR=European Region; SEAR/WPR=South-East Asia Region and Western Pacific.
Trends in use of allogeneic HSCT. (a) Global trends in use of family vs unrelated donors (left panel) and global trend in use of cord blood products or haploidentical donors (right panel). (b) Use of haploidentical HSCT (left panel) or unrelated cord blood products (right panel) by WHO region. AMR/PAH=Region of the Americas; EMR/AFR=Eastern Mediterranean Region and African Region; EUR=European Region; SEAR/WPR=South-East Asia Region and Western Pacific.
We note with special interest the adoption of emerging new technologies (Figures 4a and b). For instance, the number of HSCT from mismatched family or 'haploidentical' donors increased from 1186 (6%) in 2006 to 3171 in 2012, when they constituted 10% of all allogeneic HSCT (Figure 4a). The proportion of haploidentical HSCT was highest in the South-East Asia/Western Pacific region (1540; 48.6%), followed by Europe and the Americas (949 (29.9%) and 629 (19.8%)) and the Eastern Mediterranean/Africa region (53; 1.8%). The frequency in individual countries ranged from 0.9% (Hungary and Hong Kong) to 36% (Costa Rica and Uruguay) (excluding Nigeria, where only one haploidentical HSCT was performed). The number of unrelated cord blood HSCT increased from 1722 (8.5%) in 2006 to 2859 in 2012, representing 9.0% of all allogeneic HSCT. The proportion was highest in the South-East Asia/Western Pacific region (1366; 15.7%), followed by the Americas (851; 9.7%), Europe (596; 4.5%) and the Eastern Mediterranean/Africa region (46; 3.9%; Figures 4b).
SWOT analysis
The results of the SWOT analysis are presented in Table 1. All responders viewed the special position occupied by WBMT as a Non-Governmental global organization in official relations with WHO to be a major strength and underlined the necessity to take advantage of this unique position. They were all concerned with the slow and still limited data collection without outcome data and considered this to be the major weakness of WBMT. There is a window of opportunity to become a key data provider for health-care professionals, patients, competent authorities and Health Technology Assessment agencies. All respondents viewed the limited level of funding as major threat for the organization.
Discussion
Five years after its formation, WBMT has established itself as a global umbrella organization for HSCT. It has accomplished one of the prime prerequisites of WHO’s guiding principles on cell, organ and tissue transplants: to collect, analyze and disseminate information on global transplantation activity.1 More than 1500 teams from 75 countries over all five continents contributed to this survey. The results demonstrate a continuing rise in the number of patients worldwide treated with HSCT for all donor types and main indications. In part, this increase reflects the adoption of novel emerging technologies, specifically the use of haploidentical family donors or the use of unrelated cord blood products.14, 15, 16, 17 As also shown, the absolute numbers of HSCT continue to rise faster in countries that already have higher transplant rates and this gap continues to widen.3
The report was accompanied by a SWOT analysis conducted by experienced participants representing the contributing transplant organizations. The findings revealed the complexity of the underlying structure of a voluntary global non-profit organization network. The successful establishment of WBMT as a global player is its major strength and provides the basis for its development as a major information platform for professionals, national and regional transplant organizations, patients, competent authorities and funding bodies. The partially incomplete and erratic reporting of data with the associated delay between transplant and data submission, analysis and publication, together with the lack of outcome data present a major current weakness. Failure to account for patients who crossed borders and received their HSCT in a foreign country might represent a limitation to the analysis as well. Lack of regional organizations in particular regions was considered a clear limitation to the global survey. Starting a few years ago, the Latin America Blood and Bone Marrow transplantation group (LABMT) and the African Blood and Bone Marrow transplantation group (AFBMT) has been collecting data with the help of the WBMT, checking the completeness of the reports and the inclusion of all transplant centers. Both societies could provide standardized patient-specific data including outcome in the near future, which will be used to cross-check the survey and help to establish national registries and national organizations. The insecure financial situation at all levels presents the major threat. On balance, however, the special position that the WBMT has come to occupy as the sole coordinator of global information in this area has created a unique opportunity to build up a truly comprehensive worldwide network of standardized and quality controlled data collection and analysis. In this way, it represents a major step towards the realization of WHO’s principle that 'data collection and data analysis remain integral parts of the therapy'. This includes regular information on both patients and donors.18, 19 WBMT will continue to interact with competent authorities to pursue the goal that costs and reimbursement for an HSCT should include a component for comprehensive data and quality management. If successful, WBMT could become a role model for similar initiatives covering other complex medical therapies in the future.
WBMT and its member organizations also realize and acknowledge the major threats. The rapid dissemination of emerging trends carries an inherent risk of encouraging the spread of a particular practice before sufficient evidence is available to support the value of these novel approaches. This carries the risk of supporting 'bubbles' that may subsequently burst. The past experience with autologous HSCT for breast cancer serves as a strong reminder of the potential dangers in this respect.20
What are the major consequences of this report? National and regional transplant organizations are urged to perform the appropriate randomized studies early in the course of new trends, but requirements may well differ from region to region. There are clear indications that economically disadvantaged countries and those just beginning HSCT programs concentrate on non-malignant disorders as the main indications. Most likely, cost benefit evaluations are more favorable in these cases and there is no need for the expensive and intensive pretreatment that is required for acute leukemias. On the other hand, a focus on non-malignant indications increases the need to establish regional bone marrow harvest centers to provide the optimal stem cell source and to safeguard donors. Furthermore, the role of cord blood within transplant programs might be markedly different in countries with lower HLA genetic disparity or for those with a high frequency of pediatric patients. The skewed disease distribution among the cord blood recipients indeed suggests that there were more pediatric than adult patients in this group. This is consistent with the general experience with cord blood transplants.17 Similarly, the role of haploidentical HSCT might be viewed differently in countries with one child families, whereas in Europe and the United States, the role of autologous HSCT for some indications might need to be re-evaluated.
In summary, the present survey serves as a role model for organ transplantation and other stem cell transplants21 by providing information on current status and current trends. It illustrates the strengths of this medical technology while also helping to identify the major urgent needs.
Participating organizations
European data were derived from the European Group for Blood and Marrow Transplantation (EBMT) database for the years 1965–1989 and from the EBMT annual activity survey office from 1990.20 Non-European data were initially provided by the Center for International Blood and Marrow Transplant Research (CIBMTR) starting in 1964. They have been supplemented or replaced by the surveys of the Asian Pacific Blood and Marrow Transplantation Group (APBMT) since 1974, the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR) since 1992, the Eastern Mediterranean Blood and Marrow Transplantation Group (EMBMT) since 1984, the Canadian Blood and Marrow Transplantation Group (CGBMT) since 2002, the Latin American Blood and Marrow Transplantation Group (LABMT) since 2009 and the African Blood and Marrow Transplant Group (AFBMT) since 2010. Unrelated donor and cord blood information were derived from the World Marrow Donor Association (WMDA) and Bone Marrow Donors Worldwide (BMDW).
References
World Health Organization. WHO guiding principles on human cell, tissue and organ transplantation. Transplantation 2010; 90: 229–233.
White SL, Hirth R, Mahíllo B, Domínguez-Gil B, Delmonico FL, Noel L et al. The global diffusion of organ transplantation: trends, drivers and policy implications. Bull World Health Organ 2014; 92: 826–835.
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624.
Gratwohl A, Baldomero H, Gratwohl M, Aljurf M, Bouzas LF, Horowitz M et al. Worldwide Network of Blood and Marrow Transplantation (WBMT). Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a Global Observational Study. Haematologica 2013; 98: 1282–1290.
Gratwohl A, Pasquini MC, Aljurf M, Yoshiko A, Helen B, Lydia F et al. For the Worldwide Network of Blood and Marrow Transplantation WBMT. One million haemopoietic stem- cell transplants: a retrospective observational study. Lancet Haematol 2015; 2: e91–100.
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant 2015; 50: 1037–1056.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P et al. European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant 2015; 50: 476–482.
Hequet O . Hematopoietic stem and progenitor cell harvesting: technical advances and clinical utility. J Blood Med 2015; 6: 55–67.
Kurata M, Yanagisawa A, Atsuta Y, Sakamaki H, Kato K, Ichinohe T et al. Hematopoietic cell transplantation in Japan: nationwide survey 2013. Rinsho Ketsueki 2014; 55: 2381–2399.
Aljurf M, Rizzo JD, Mohty M, Hussain F, Madrigal A, Pasquini MC et al. Challenges and opportunities for HSCT outcome registries: perspective from international HSCT registries experts. Bone Marrow Transplant 2014; 49: 1016–1021.
Casebeer A . Application of SWOT analysis. Br J Hosp Med 1993; 49: 430–431.
Yamazaki H, Slingsby BT, Takahashi M, Hayashi Y, Sugimori H, Nakayama T . Characteristics of qualitative studies in influential journals of general medicine: a critical review. Biosci Trends 2009; 3: 202–209.
Reisner Y, Aversa F, Martelli MF . Haploidentical hematopoietic stem cell transplantation: state of art. Bone Marrow Transplant 2015; 50 (Suppl 2): S1–S5.
Ballen KK, Gluckman E, Broxmeyer HE . Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013; 122: 491–498.
Petrini C . Umbilical cord blood banking: from personal donation to international public registries to global bioeconomy. J Blood Med 2014; 5: 87–97.
Kekre N, Antin JH . Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood 2014; 124: 334–343; Erratum in: Blood 2015; 125: 1048.
Halter JP, van Walraven SM, Worel N, Bengtsson M, Hägglund H, Nicoloso de Faveri G et al. Allogeneic hematopoietic stem cell donation-standardized assessment of donor outcome data: a consensus statement from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Bone Marrow Transplant 2013; 48: 220–225.
Kodera Y, Yamamoto K, Harada M, Morishima Y, Dohy H, Asano S et al. Japan Society for Hematopoietic Cell Transplantation. PBSC collection from family donors in Japan: a prospective survey. Bone Marrow Transplant 2014; 49: 195–200.
Vogl DT, Stadtmauer EA . High-dose chemotherapy and autologous hematopoietic stem cell transplantation for metastatic breast cancer: a therapy whose time has passed. Bone Marrow Transplant 2006; 37: 985–987.
Martin I, Ireland H, Baldomero H, Passweg J . The survey on cellular and engineered tissue therapies in Europe in 2012. Tissue Eng Part A 2015; 21: 1–13.
Acknowledgements
The cooperation of all participating teams, countries and organizations with their staff is greatly appreciated. Specifically the following: ABMTRR, APBMT, Aichi Medical School, CBMTG, CIBMTR, Medical College of Wisconsin, EBMT: Co-ordination offices in Barcelona, Paris and London and the Austrian Registry (ASCTR), the Czech BMT Registry, the French Registry (SFGM), the German Registry (DRST), the Italian Registry (GITMO), the Dutch Registry (HOVON), the Spanish BMT Registry (GETH), the Swiss Registry (SBST), the Turkish BMT Registry and the British Registry (BSBMT), EMBMT, SBTMO, LABMT, AFBMT, WMDA and Eurocord. Funding was provided by the participating organizations. The WBMT activity survey office is funded by the EBMT and the University of Basel. Funding was solely to support the study; no individual payment was made to any of the persons involved in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Niederwieser, D., Baldomero, H., Szer, J. et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant 51, 778–785 (2016). https://doi.org/10.1038/bmt.2016.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.18
- Springer Nature Limited
This article is cited by
-
Effects of pulmonary rehabilitation in hematopoietic stem cell transplantation recipients: a randomized controlled study
Supportive Care in Cancer (2024)
-
The effect of conditioning regimen and prescribed medications on hyposalivation in haematopoietic cell transplantation (HCT) patients: an 18-month prospective longitudinal study
Clinical Oral Investigations (2023)
-
Coexistence of HLA and KIR ligand mismatches as a risk factor for viral infection early after cord blood transplantation
Bone Marrow Transplantation (2022)
-
Life expectancy and burden of late complications after reduced intensity conditioning allogeneic transplantation
Bone Marrow Transplantation (2022)
-
Feasibility of a Digital Storytelling Intervention for Hematopoietic Cell Transplant Patients
Journal of Cancer Education (2022)