Abstract
The outcome of 55 children with severe aplastic anemia (SAA) who received a second hematopoietic stem cell transplantation (HSCT) was retrospectively analyzed using the registration data of the Japanese Society for Hematopoietic Cell Transplantation. The 5-year overall survival (OS) and failure-free survival (FFS) after the second transplantation were 82.9% (95% confidence interval (CI), 69.7–90.8)) and 81.2% (95% CI, 67.8–89.4), respectively. FFS was significantly better when the interval between the first and second transplantation was >60 days (88.9%; 95% CI, 73.0–95.7) than when it was ⩽60 days (61.4%; 95% CI, 33.3–80.5; P=0.026). All 12 patients who were conditioned with regimens containing fludarabine and melphalan were alive with hematopoietic recovery. These findings justify the recommendation of a second HSCT for children with SAA who have experienced graft failure after first HSCT.
Similar content being viewed by others
Introduction
Hematopoietic stem cell transplantation (HSCT) from a HLA-matched related donor (MRD) is the first-line treatment for children with severe aplastic anemia (SAA).1, 2, 3 HSCT from an HLA-matched unrelated donor (MUD) is indicated as a salvage treatment for children who are nonresponsive to immunosuppressive therapy.4, 5, 6 Although the overall survival (OS) following HSCT from both MRD and MUD has markedly improved and exceeds 90%, graft failure (GF) remains the most frequent cause of HSCT failure in children aplastic anemia (AA). Burroughs et al.7 reported the outcome of 148 children with SAA who received HSCT from an MRD at the Fred Hutchinson Cancer Center between 1971 and 2010. Overall, 30 patients (20%) rejected their first marrow. However, the incidence of GF remarkably decreased. The cumulative incidence of GF was 8% in 374 adolescent patients who received HSCT from an MRD between 2000 and 2009 in Europe.8 In a recent report from our country (Japan), the causes of treatment failure included GF in 12 patients (5.6%) among 213 children with SAA who received HSCT from an MRD between 1992 and 2009.9 A report from the National Marrow Donor Program showed that the probability of GF at 28 days and 1 year was 10% and 15%, respectively, in 195 children with AA receiving HSCT from an MUD.10 The only curative approach to overcome GF is a second allograft for patients failing to engraft after the first transplantation. However, information on second transplantations in patients with AA is scarce, especially in pediatric patients. We analyzed the outcomes of a second HSCT in children with AA registered in the Transplant Registry Unified Management Program (TRUMP) conducted by the Japanese Society for Hematopoietic Cell Transplantation.
Patients and methods
Between 1982 and 2012, 55 patients <15-years old who underwent a second HSCT were registered in the TRUMP database. Patients with a congenital bone marrow failure syndrome such as Fanconi's anemia were excluded from the analysis. Neutrophil engraftment was defined as achieving an absolute neutrophil count of >0.5 × 109/L for 3 consecutive days. GF was classified as primary GF when no hematopoietic recovery was observed until 21 days after transplantation and secondary GF as recurrent pancytopenia with hypocelullar marrow after initial hematopoietic recovery. Treatment failure was defined as death by all causes, GF and secondary malignancy. Survival curves were calculated using the Kaplan–Meier method. Comparisons between survival curves were performed using the log-rank test. Risk factors associated with OS and failure-free survival (FFS) were assessed using multivariate Cox regression model. Pair-wise comparisons of patient characteristics (co-variates) were performed using the χ2 test for categorical variables. Statistical significance was defined as P-value of <0.05. Data collected as of October 2013 were analyzed. The study was approved by the institutional review boards of Shizuoka Children's Hospital and by the JSHCT committee.
Results
The clinical characteristics of the 55 patients are summarized in Table 1. Among the 55 patients, 16 and 38 patients suffered from primary and secondary GF, respectively. Information on the time of GF was not available for one patient. The median age at the time of the second HSCT was 9 years (range 1–15). The male/female ratio was 27/28. The median interval between the first and second HSCT was 195 days (range 29–2463). The donor for the second HSCT was an MRD in 25 patients, a mismatched related donor in 16 patients, an MUD in 8 patients and unrelated cord blood in 6 patients. The stem cell source was bone marrow in 35 patients, peripheral blood in 12 patients and bone marrow/peripheral blood in 1 patient. Except for one patient, the donor of the first HSCT could be confirmed in all patients. Of the patients who underwent a second HSCT, 29 underwent HSCT from the same related donor, 11 underwent HSCT from a different related donor and 14 underwent HSCT from an unrelated donor. Except one patient, all patients with primary GF underwent second HSCT within 60 days after the first HSCT. They received second HSCT between 29 and 58 days after first HSCT. Nine patients underwent HSCT from the same related donor, 4 underwent HSCT from a different related donor and 3 underwent HSCT from an unrelated donor in patients with primary GF.
Of the 29 patients who underwent HSCT from the same related donor, 22 engrafted following the second HSCT. Moreover, among the 54 patients with confirmed preconditioning of the second HSCT, 14 received fludarabine (FLU)+cyclophosphamide (CY)±irradiation (11 with irradiation and 3 without irradiation)±antithymocyte globulin (ATG; 9 with ATG and 5 without ATG; the FLU+CY regimen), 12 received FLU+melphalan (MEL)±irradiation (9 with irradiation and 3 without irradiation)±ATG (7 with ATG and 5 without ATG; the FLU+MEL regimen), 20 received CY±irradiation±ATG (the CY regimen) and the remaining 9 received other various conditioning regimens (others). We screened anti-HLA antibodies in 6 patients but none of them were positive.
With median follow-up times of >4 years after the second transplantation, 45 of 55 patients were alive. Causes of death included GF in 4 patients, interstitial pneumonia in 2 patients, secondary malignancy in 2 patients, infection in 1 patient and multiple organ failure in 1 patient.
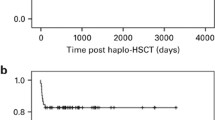
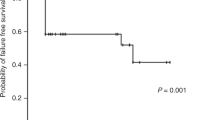
The 5-year OS and FFS of all patients were 82.9% (95% confidence interval (CI), 69.7–90.8)) and 81.2% (95% CI, 67.8–89.4), respectively. (Figure 1). The 5-year OS of patients in the interval between the first and second HSCTs within and beyond 60 days was 67.0% (95% CI, 37.7–84.9) and 88.9% (95% CI, 73.0–95.7), respectively (P=0.12). The 5-year FFS of patients in the interval between the first and second HSCT within and beyond 60 days was 61.4% (95% CI, 33.3–80.5) and 88.9% (95% CI, 73.0–95.7), respectively (P=0.026; Figure 2a). The 5-year FFS of patients with primary and secondary GF were 68.8% (40.5–85.6) and 86.3% (70.0–94.1), respectively (P=0.14). All 12 patients who received the FLU+MEL regimen were alive and without GF. The total dose of MEL varied from 80 mg/m2 to 180 mg/m2. Six patients received a total dose of 80 mg/m2. Each of the two patients received a total dose of 120, 140 and 180 mg/m2.
The effect of the interval between the first and second HSCT. (a) The OS rates when the interval was within 60 days (n=16, solid line) and beyond 60 days (n=38, dotted line) were 67.0% (95% CI, 37.7–84.9) and 88.9% (95% CI, 73.0–95.7), respectively (P=0.121). (b) The FFS rates when the interval was within 60 days (n=16, solid line) and beyond 60 days (n=38, dotted line) were 61.4% (95% CI, 33.3–80.5) and 88.9% (95% CI, 73.0–95.7), respectively (P=0.0259).
The transplantation outcomes of the second HSCT are shown in Table 2. Grades II–IV acute GvHD, limited chronic GvHD and extensive chronic GvHD were observed in 12, 6 and 7 patients, respectively. Among 27 patients whose results of chimerism analysis were available, 12 showed mixed chimera and 15 showed complete donor-type chimera. Six of 16 patients with primary GF experienced second GF. On the other hand, only 3 of 38 patients with secondary GF following the first HSCT experienced GF after the second HSCT (P=0.0067). Univariate and multivariate analysis resulted in no significant differences in OS and FFS in age, primary vs secondary GF after first HSCT, donor type, conditioning regimen, the use of irradiation, ATG/ALG and GvHD prophylaxis. The 5-year FFS of patients in the interval between the first and second HSCT within 60 days was lower than beyond 60 days (P=0.036). Three patients developed secondary malignancy. The cumulative incidence of secondary malignancy was 7.9% (0.0–18.5) at 10 years and 15.5% (0.0–31.5) at 15 years. Patients who developed osteosarcoma and secondary leukemia died, whereas a patient suffering from thyroid cancer is alive without disease.
Discussion
GF is the most serious complication following HSCT for SAA. According to recent reports, GF has decreased to <10% among patients with SAA receiving bone marrow transplantation from both MRD and MUD. Meanwhile, the outcome in patients with GF is dismal if they do not receive a second HSCT. Only patients with autologous recovery may survive without a second HSCT. The EBMT surveillance estimated that the cumulative incidence of autologous recovery was 4.2% with an OS of 84%.11 Considering the low incidence of autologous recovery, a second transplantation is a realistic approach for patients who fail to engraft after the first transplantation.
In this study, we aimed to identify risk factors affecting the outcome of a second HSCT for GF in patients with SAA. Although 30 of 55 patients received the second HSCT from an alternative donor, the 5-year OS and FFS of all patients were 82.9% (95% CI, 69.7–90.8) and 81.2% (95% CI: 67.8–89.4), respectively, which were favorable compared with data from previous reports, in which patients received transplants from HLA-matched sibling donors. Especially, both OS and FFS in the interval between the first and second HSCT beyond 60 days were as high as 88.9% (95% CI, 73.0–95.7). Most patients who received the second HSCT within 60 days suffered from primary GF. McCann et al.12 reported that patients receiving a second BMT within 60 days from the first BMT had a significantly poorer outcome. de Medeiros et al.13 also reported that a longer inter-transplant interval (>90 days) was a significant factor for better OS in 34 patients who received a second transplantation for SAA. Horan et al.14 reported that a shorter inter-transplant interval (<3 months) and a poor performance score (<90) at a second HSCT were associated with high mortality. When the second HSCT is performed relatively shortly after the first HSCT, the patient has little time to recover from the toxicity and the myelosuppressive effects of conditioning, and consequently, the risk of death from infections or organ dysfunction may increase. Recent advances in supportive care may overcome these complications and improve the outcome of primary GF.
In the setting of a second HSCT, whether a different family donor should be used and the regimen that should be employed require clarification. In a report by de Mederios et al.13, 25 of 35 patients received transplants from the same sibling donor. In a report by Horan et al.14, 146 of 166 patients received the transplant from the same donor, and it was concluded that using a different sibling donor for the second transplantation conferred no detectable advantage. In the present study, 23 of 29 patients who received transplants from family donors had received a graft from the same donor as in the first HSCT and 21 exhibited engraftment. These results show the effectiveness of using the same donor for the second HSCT.
Although conditioning regimens have been intensified to overcome immunological barriers for engraftment, these are not always associated with a reduction in GF and an improved OS. Especially in patients with primary GF, infections and organ dysfunction augment regimen-related toxicities in those who receive aggressive conditioning regimens. The use of high-dose alkylating agents and TBI are not recommended in patients with AA, especially in children.15, 16, 17 Recent studies demonstrated the usefulness of a reduced-intensity regimen containing FLU to reduce the incidence of GF and improve OS in patients with SAA receiving the first HSCT from an alternative donor.15 In our analysis, half of the patients were conditioned with FLU-containing regimens. Notably, all 12 patients who received the FLU+MEL containing regimen were alive with full engraftment. Unrelated cord blood transplantation has extended the availability of treatment in patients with SAA in an absence of a suitable donor, but most reports have shown a poor outcome and high incidence of GF.18 However, Yamamoto et al.19 reported sustained engraftment in 11 of 12 patients with SAA who received unrelated cord blood transplantation with the FLU+MEL+low-dose TBI regimen. Taken together, the FLU+MEL+ATG+low-dose TBI regimen may be the most promising regimen for patients who receive a second HSCT for SAA.
In conclusion, we report a favorable result of a second HSCT in children with SAA with a high OS and FFS. Although this retrospective registry-based analysis had several limitations, a FLU+MEL+ATG+low-dose TBI regimen may sustain lasting engraftment and result in a promising outcome in patients with SAA receiving a second HSCT. Prospective studies are warranted to confirm these observations.
References
Korthof ET, Békássy AN, Hussein AA . On behalf of the SAA-WP of the EBMT. Management of acquired aplastic anemia in children. Bone Marrow Transplant 2013; 48: 191–195.
Scheinberg P . Aplastic anemia: therapeutic updates in immunosuppression and transplantation. Hematology Am Soc Hematol Educ Program 2012; 2012: 292–300.
Kikuchi A, Yabe H, Kato K, Koh K, Inagaki J, Sasahara Y . Long-term outcome of childhood aplastic anemia patients who underwent allogeneic hematopoietic SCT from an HLA-matched sibling donor in Japan. Bone Marrow Transplant 2013; 48: 657–660.
Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A et alSevere Aplastic Anemia Working Party of the European Blood and Marrow Transplant Group. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2007; 92: 11–18.
Marsh JC, Pearce RM, Koh MB, Lim Z, Pagliuca A, Mufti GJ et alBritish Society for Blood and Marrow Transplantation, Clinical Trials Committee. Retrospective study of alemtuzumab vs ATG-based conditioning without irradiation for unrelated and matched sibling donor transplants in acquired severe aplastic anemia: a study from the British Society for Blood and Marrow Transplantation. Bone Marrow Transplant 2014; 49: 42–48.
Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Burroughs LM, Woolfrey AE, Storer BE, Deeg HJ, Flowers ME, Martin PJ et al. Success of allogeneic marrow transplantation for children with severe aplastic anaemia. Br J Haematol 2012; 158: 120–128.
Dufour C, Pillon M, Passweg J, Socié G, Bacigalupo A, Franceschetto G et al. Outcome of aplastic anemia in adolescence: a survey of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica 2014; 99: 1574–1581.
Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor vs immunosuppressive therapy. Haematologica 2014; 99: 1784–1791.
Perez-Albuerne ED, Eapen M, Klein J, Gross TJ, Lipton JM, Baker KS et al. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. Br J Haematol 2008; 141: 216–223.
Piccin A, McCann S, Socié G, Oneto R, Bacigalupo A, Locasciulli A et al. Aplastic Anaemia Working Party of the European Group for Blood and Marrow Transplantation. Survival of patients with documented autologous recovery after SCT for severe aplastic anemia: a study by the WPSAA of the EBMT. Bone Marrow Transplant 2010; 45: 1008–1013.
McCann SR, Bacigalupo A, Gluckman E, Hinterberger W, Hows J, Ljungman P et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant 1994; 13: 233–237.
de Medeiros CR, Bitencourt MA, Medeiros BC, Ioshizumi L, Pasquini R . Second bone marrow transplantation for severe aplastic anemia: analysis of 34 cases. Bone Marrow Transplant 2001; 28: 941–944.
Horan JT, Carreras J, Tarima S, Camitta BM, Gale RP, Hale GA et al. Risk factors affecting outcome of second HLA-matched sibling donor transplantations for graft failure in severe acquired aplastic anemia. Biol Blood Marrow Transplant 2009; 15: 626–631.
Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica 2010; 95: 976–982.
Marsh JC, Gupta V, Lim Z, Ho AY, Ireland RM, Hayden J et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft versus host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood 2011; 118: 2351–2357.
Samarasinghe S, Steward C, Hiwarkar P, Saif MA, Hough R, Webb D et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br J Haematol 2012; 157: 339–346.
Yoshimi A, Kojima S, Taniguchi S, Hara J, Matsui T, Takahashi Y et al. Japan Cord Blood Bank Network. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant 2008; 14: 1057–1063.
Yamamoto H, Kato D, Uchida N, Ishiwata K, Araoka H, Takagi S et al. Successful sustained engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with severe aplastic anemia. Blood 2011; 117: 3240–3242.
Acknowledgements
We thank all participating doctors and patients who were involved in the Japanese Hematopoietic Cell Transplantation Registry. This work was supported in part by a Research Grant for Allergic Disease and Immunology from the Japanese Ministry of Health, Labor and Welfare.
Author Contributions
KazK and SK designed the research, analyzed the data and wrote the manuscript. HM, NY, RK, HY, KT, KKoh, YT, YC, KeiK, YH, MI, HS, KKawa and KojiK collected and managed the clinical data and discussed the results. RS supervised the data analyses.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kudo, K., Muramatsu, H., Yoshida, N. et al. Second allogeneic hematopoietic stem cell transplantation in children with severe aplastic anemia. Bone Marrow Transplant 50, 1312–1315 (2015). https://doi.org/10.1038/bmt.2015.153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.153
- Springer Nature Limited
This article is cited by
-
Second allogeneic hematopoietic stem cell transplantation in patients with inborn errors of immunity
Bone Marrow Transplantation (2023)
-
Outcome of Second Allogeneic HSCT for Patients with Inborn Errors of Immunity: Retrospective Study of 20 Years’ Experience
Journal of Clinical Immunology (2023)
-
Successful outcomes of second hematopoietic stem cell transplantation for graft failure in pediatric patients with severe aplastic anemia
Scientific Reports (2022)
-
Non-myeloablative conditioning for second hematopoietic cell transplantation for graft failure in patients with non-malignant disorders: a prospective study and review of the literature
Bone Marrow Transplantation (2017)