Abstract
In construction technology, there are significant efforts to reduce environmental emissions, particularly NH3 and other pollutants. This study marks the first application of CaCO3 biomineralization biotechnology in microbially induced calcium carbonate precipitation (MICCP) to enhance mortar properties using the non-pathogenic Neisseria perflava strain SKC/VA-3, which employs carbonic anhydrase mechanisms. The results demonstrated that N. perflava could significantly improve the physical and mechanical characteristics of mortar. Incorporating N. perflava and calcium lactate pentahydrate resulted in a 20% increase in compressive strength and a 14% rise in indirect tensile strength of the mortar. Examination through scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM–EDS) revealed calcite formation within the microstructure of the bio-mortar. Additionally, self-healing assessments indicated that calcite precipitation, driven by bacterial metabolism, also occurred on the cracked surfaces of the bacterial mortar, suggesting potential for reduced maintenance and increased material longevity. This study provides the first report on the use of N. perflava for bio-mortar enhancement and represents a novel biotechnological approach to improving the properties of mortar and other cementitious materials. The utilization of N. perflava in bio-mortar represents a groundbreaking biotechnological advance, potentially enhancing mortar and other cement-based materials. This development contributes to sustainable, durable, and environmentally friendly construction technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cement-based materials are integral to the construction industry. However, their high demand leads to significant cement production, contributing markedly to increased CO2 emissions and energy consumption [1, 2]. Additional challenges include discontinuities arising from the composition of aggregates and water in the mix, which facilitate micro-crack formation [3]. To address these issues, various strategies have been employed, including the use of chemical admixtures (e.g., silica fume) and reinforcements (e.g., fibers) [4, 5]. Nonetheless, these conventional methods often incur relatively high costs. Consequently, researchers are exploring alternative approaches to reduce material and operational expenses, notably through biologically induced calcite biomineralization, commonly known as microbially induced calcium carbonate precipitation (MICCP) [6,7,8,9,10,11,12].
Bacillus spp. are the predominant calcite-precipitating bacteria employed in improving the properties of cementitious materials, as evidenced by numerous studies [6, 8, 11, 13, 14]. Additionally, the capability of other bacterial species such as Shewanella sp. [15], Enterococcus faecalis [16, 17], Sporosarcina pasteurii [18, 19], and Acinetobacter sp. [20] in enhancing the characteristics of cement-based materials has been documented. Despite these findings, the potential of other bacteria remains largely unexplored. Notably, certain Neisseria spp. may demonstrate the capability for calcium carbonate precipitation via carbonic anhydrase (CA) activity and the presence of calcium ions. It has been observed that Neisseria gonorrhoeae [21,22,23,24] and Neisseria sicca [25] can produce bicarbonate ions through CA activity. Carbonic anhydrase, a zinc-containing metalloenzyme, predominantly catalyzes the reversible hydration of carbon dioxide (CO2) to bicarbonate ion (HCO3−) in many organisms, especially in the prokaryotic domain [26, 27]. Furthermore, CA activity plays a crucial role in biomimetic CO2 sequestration, potentially leading to calcium carbonate formation in the presence of Ca2+ ions [13, 28, 29].
Correspondingly, Neisseria perflava, a member of the Neisseria genus, is identified as a Gram-negative, oxidase-positive, and catalase-positive bacterium. This species is commonly recognized as a commensal, coexisting symbiotically with humans, and is often found in the nasopharynx and oropharynx without being pathogenic or causing illness [30]. Neisseria perflava is part of the diverse non-pathogenic Neisseria species that make up the human pharyngeal microflora [30]. Studies have shown that Neisseria perflava is capable of producing carbonic anhydrase [31]. Furthermore, a study conducted by Sanders and Maren in 1967 [32] on the carbonic anhydrase activity in nine strains of Neisseria perflava, ascertained via the pH change technique, revealed that eight of these strains exhibited this enzymatic function. Nonetheless, the suitability of Neisseria perflava for application in MICCP remains unexplored.
Consequently, this study examined the capacity of the bacterium Neisseria perflava strain SKC/VA-3 to enhance mortar properties. The selection of Neisseria perflava strain SKC/VA-3 was based on its classification as a non-pathogenic bacterium [33, 34]. Calcium lactate pentahydrate served as the source of calcium and carbon due to its minimal negative impact on the strength of cement mortar, as documented by [35]. To analyze the microstructure of the bacterial mortar samples, scanning electron microscope-energy dispersive X-ray spectroscopy (SEM–EDS) was employed. Furthermore, visualization and characterization of self-healing, facilitated by bacterial metabolism, were also assessed using SEM–EDS.
2 Materials and methods
2.1 Microbial cultures and growth media
In this study, Neisseria perflava strain SKC/VA-3, isolated from a mixed sample comprising crude oil (sourced from PT. Pertamina, Sukabumi, West Java) and rust deposits from petroleum pipelines (Metal Laboratory, Faculty of Mechanical and Aerospace Engineering, Bandung Institute of Technology), was utilized. The 16S rRNA gene sequence of this strain is registered under the GenBank/EMBL/DDBJ accession number MT229315. Cultivation of this bacterial species was conducted in a medium consisting of 1.5 g/L nutrient broth (Oxoid) and 3.2 mM calcium lactate pentahydrate (CaC6H10O6.5H2O; sourced locally in Bandung, West Java, Indonesia), following the protocol outlined in Syarif et al. [36]. Sterilization of the media was carried out at 121 °C for 15 min before inoculating with the bacterial culture (10% v/v). The cultures were then incubated under agitation at 180 rpm for 48 h at room temperature, followed by storage in a refrigerator prior to their use in bio-mortar specimen preparation. The concentration of bacterial cells, measured as colony-forming units (CFU) per milliliter, in the Neisseria perflava strain SKC/VA-3 culture, was determined using a serial dilution method, ranging from 10–5 to 10–9 [37]. These serially diluted samples were incubated on Nutrient Agar (NA) medium for a duration of 3 days, followed by a quantitative assessment of bacterial colonies. Utilizing this enumeration technique, the bacterial concentration of the Neisseria perflava strain SKC/VA-3 was estimated to be approximately 4.6 × 107 CFU/ml.
2.2 Preparation of mortar specimens
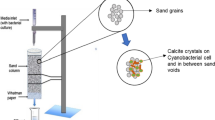
For the fabrication of all cylindrical mortar specimens, Ordinary Portland Cement (sourced from Indocement Ltd., Cirebon, Indonesia), fine aggregate (with a specific gravity of 2.685 and a fineness modulus of 3.39), and tap water were employed as the basic components. In this study, the mortar mix was formulated adhering to a ratio of 1:3 for cement to fine aggregate and a water-to-cement ratio of 0.5. Control specimens of ordinary mortar were produced using only the aforementioned mixture. In contrast, the bacterial mortar specimens in the experiment incorporated Neisseria perflava bacterial cells and calcium lactate pentahydrate (CaC6H10O6.5H2O), procured from a chemical supplier in Bandung, West Java, Indonesia. The bacterial cells, in their liquid form, were added as a partial substitute for water, constituting 10% v/v of the water content. The proportion of calcium lactate pentahydrate used was 0.5% of the cement's weight. Both sets of specimens were manually mixed and cast into PVC pipes with a diameter of 45 mm, followed by compaction using a tamping rod. Subsequently, they were immediately enveloped in plastic wrap and left to set for 1 day. Post 24 h, the specimens were demolded, cut to the desired dimensions, and submerged in tap water for a 7 day curing period. This study adopted a 7 day curing duration based on findings from previous research indicating that 70–90% of the compressive strength attainable in 28 days could be achieved within 7 days [38,39,40].
2.3 Ultrasonic pulse velocity measurement
For each set of control and bacterial mortar specimens, three cylindrical samples, each 4.5 cm in diameter and 9 cm in length, were prepared. Following a 7 day curing period in tap water, the specimens were removed, dried, and their dimensions measured in triplicate to calculate average values prior to testing. The testing procedure, adhering to ASTM C597 [41] standards, involved the use of a PUNDIT (Portable Unit for Non-Destructive Digital Indicated Testing) or an Ultrasonic tester E46. Before conducting measurements on the specimens, the PUNDIT was calibrated using a calibration cylinder with a known pulse propagation time of 56 μs. The average UPV for the three specimens in each of the control and bacterial mortar groups was then determined.
2.4 Porosity and water absorption measurement
The specimens utilized for porosity and water absorption tests were cylindrical, measuring 4.5 cm in diameter and 3 cm in length. Porosity was measured following the ISRM suggested method [42], and water absorption was determined in accordance with ASTM C642 [43]. After a 7-day immersion in tap water, three specimens from each of the control and bacterial mortar groups were extracted and oven-dried at 105 °C for 24 h to ascertain their oven-dry mass. These specimens were then submerged in tap water for another 24 h, removed, wiped, and weighed to obtain their saturated surface-dry mass. In the subsequent step, the specimens, secured with wire, were submerged in water to measure their apparent mass while immersed. The average values for porosity and water absorption for both control and bacterial mortar groups were then calculated.
2.5 Compressive and indirect tensile strength test
Compressive and indirect tensile strength tests were performed in triplicate, adhering to the methods suggested by ISRM [44, 45]. For compressive strength testing, specimens measuring 4.5 cm in diameter and 9 cm in height were utilized, while for the indirect tensile strength test (Brazilian test), specimens of the same diameter but 2.25 cm in height were employed. Following a 7-day period of wet curing, the specimens were removed, dried, and subjected to testing using a servo-controlled hydraulic compression tester (Hung Ta HT-8391). The loading rates applied were 6000 N/min for compressive strength and 2000 N/min for indirect tensile strength testing. The mean compressive and indirect tensile strengths for the three specimens in each group, both control and bacterial mortar, were subsequently calculated.
2.6 Microstructure observation
Microstructural and elemental analysis of the control and bacterial mortar specimens was conducted using Scanning Electron Microscope-Energy Dispersive X-ray Spectroscopy (SEM–EDS; JEOL JSM-J6510 A). Small fragments were extracted from both types of specimens and subjected to preparatory procedures before SEM–EDS analysis. This preparation involved chemical fixation using 2.5% glutaraldehyde for 24 h, followed by triple washing with phosphate buffer. Subsequent dehydration was carried out using a graded acetone series (25, 50, 75, and 100% concentrations for 15 min, 15 min, 24 h, and 15 min, respectively).
2.7 Self-healing visualization and SEM–EDS of the precipitates
Self-healing visualization was performed to examine the precipitates formed on the surfaces of the specimens. Post-indirect tensile strength testing, the fractured specimens from each batch were immediately visualized, marking this as the 0-day observation (Control shown in Fig. 5a and bacterial mortar in Fig. 5c). Detailed examination of specific areas (indicated by red rectangles in Fig. 5) was carried out using a trinocular stereomicroscope (SMZ-2 T, Nikon). Following the initial assessment, the specimens were submerged in tap water and re-examined after 21 days using the same method (Control depicted in Fig. 5b and bacterial mortar in Fig. 5d). The precipitates were then carefully extracted, chemically fixed, washed thrice with phosphate buffer, and dehydrated through a graded acetone series (25, 50, 75, 100%, each for 15 min, 15 min, 24 h, and 15 min, respectively), before being analyzed using SEM–EDS. The SEM–EDS analysis focused on verifying the presence of calcite precipitation on the surfaces of the bacterial specimens.
3 Results and discussion
3.1 Ultrasonic pulse velocity, porosity, and water absorption
Ultrasonic Pulse Velocity (UPV) measurements were conducted to assess the structural integrity and uniformity of the specimens. The results of these measurements are depicted in Fig. 1. Notably, the average UPV for the bacterial mortar specimens (incorporating N. perflava) was observed to be approximately 5% higher than that of the control, as indicated in Fig. 1. To evaluate the physical properties of the specimens, porosity and water absorption tests were performed. Figure 2a shows a marginal decrease (around 2%) in the average porosity of the bacterial mortar specimens, suggesting fewer voids compared to the control. This observation is consistent with the UPV data, where reduced void content correlates with decreased porosity and improved UPV. Additionally, a decrease in water absorption by approximately 7% was noted in the bacterial mortar, as illustrated in Fig. 2b.
3.2 Compressive and indirect tensile strength
Compressive and indirect tensile strength tests were performed to evaluate the mechanical properties of the specimens, as depicted in Fig. 3. The results, illustrated in Fig. 3a, indicate a 20% increase in the average uniaxial compressive strength of the bacterial mortar specimens, a slightly higher enhancement compared to previous studies involving Bacillus spp. as the calcite-precipitating agent. Additionally, as shown in Fig. 3b, there was a 14% increase in the average indirect tensile strength of the bacterial mortar specimens. This improvement in strength correlates with the reductions in porosity and water absorption (shown in Fig. 2a, b) and aligns with the observed increase in ultrasonic pulse velocity, indicating enhanced mechanical properties.
3.3 The microstructure inside the specimens and self-healing phenomenon
Figure 4a, b illustrate the microstructure of the control and bacterial mortar specimens, respectively. Notably, in the bacterial mortar containing N. perflava (Fig. 4b), white precipitates were observed occluding the voids, which were tentatively identified as calcium carbonate based on the EDS spectrum analysis (Fig. 4c). Additionally, the examination of the cracked specimens post 21-day immersion revealed further instances of calcium carbonate precipitation on the surface of the bacterial specimen. The control mortar specimen’s crack appeared unchanged from the initial state (Fig. 5a) to after the 21-day period (Fig. 5b). In contrast, the bacterial mortar specimen displayed white precipitates on its surface and within the crack after the 21-day immersion (Fig. 5d), which were confirmed as calcium carbonate by SEM (Fig. 6a) and EDS spectrum analysis (Fig. 6b).
3.4 Discussion
To date, no research has been conducted on the application of Neisseria perflava as a Microbially Induced Calcium Carbonate Precipitation (MICCP) agent. However, this study reveals that Neisseria perflava is capable of enhancing the mechanical properties of mortar, a prediction inferred from the carbonic anhydrase enzyme it produces. Smith and Ferry [26] reported that Neisseria perflava exhibits carbonic anhydrase activity, and Forkman [46] identified the location of carbonic anhydrase in the cell wall of Neisseria flava. Carbonic anhydrase (CA), a pivotal enzyme in various physiological processes, primarily catalyzes the interconversion of carbon dioxide (CO2) and water (H2O) into bicarbonate (HCO3−) and protons (H+), and facilitates the reversible hydration of CO2 to bicarbonate ions.
In the context of MICCP in this study, carbonic anhydrase aids in producing bicarbonate ions, which react with calcium ions to form calcite. This reaction contributes to an enhancement in the average ultrasonic pulse velocity (UPV) of bacterial mortar (incorporating N. perflava), approximately 5% higher than that of the control, as depicted in Fig. 1. The underlying mechanism involves CA activity from N. perflava, potentially leading to calcium carbonate (CaCO3) precipitation. This precipitation fills the voids or micro-cracks within the mortar, thereby increasing its density and resulting in a higher UPV. This aligns with previous studies that incorporated Bacillus sp. into mortar mixtures [47, 48].
This study therefore provides direct evidence that Neisseria perflava, through its production of carbonic anhydrase, can be used for CaCO3 biomineralization to enhance mortar properties (as demonstrated in Figs. 1 and 3). The enzyme catalyzes the reversible hydration of CO2, an essential step in biomineralization, wherein organisms produce minerals [26]. The bacterial-induced precipitation of calcium carbonate leads to the formation of carbonate crystals, improving mortar's mechanical properties such as UPV and uniaxial compressive strength. In fact, bacterial mortar specimens showed a 20% increase in average uniaxial compressive strength compared to control specimens (Fig. 3). Due to CA activity stimulated by N. perflava metabolism, calcium carbonate deposits fill voids (as shown in Figs. 4, 5, and 6), reducing porosity and water absorption (Fig. 2). These deposits also impede water passage within the microstructure of bacterial mortar, decreasing water absorption [13, 18, 48, 49].
These findings suggest that Neisseria perflava exhibits a higher efficacy than previously studied Bacillus sp. in calcite precipitation. González et al. (2000) [50] reported only a 10% increase in uniaxial compressive strength using B. pseudofirmus after 7 days, whereas Vaezi et al. [51] observed a maximum 14% increase in compressive strength at the same age. Additionally, studies incorporating B. subtilis and calcium lactate in concrete reported only a 12% increase in compressive strength [52, 53].
Based on the results obtained from SEM–EDS analysis (Figs. 4–6), and considering that the pH of the mixture was maintained between 8 and 9 in this study [36], it can be deduced that the precipitation of calcium carbonate both within and on the surface of the bacterial mortar specimen is likely a consequence of the carbonic anhydrase (CA) activity resulting from the metabolic processes of Neisseria perflava. This deduction is further substantiated by the following chemical Eqs. (1–5):
In summary, this study represents the first report of the capability of Neisseria perflava to produce carbonic anhydrase, which significantly enhances the mechanical properties of mortar through CaCO3 biomineralization. This discovery holds substantial potential and offers advantageous prospects for MICCP, presenting an environmentally benign, sustainable, and cost-effective approach for addressing various engineering challenges, particularly in construction engineering and environmental applications. From a construction industry perspective, MICCP significantly enhances the durability, strength, and sustainability of concrete. This enhancement involves the role of specific bacteria, such as Neisseria perflava in this study, which precipitate calcium carbonate to seal micro-cracks within concrete structures. This biogenic precipitation not only improves the compressive strength and reduces the permeability of concrete but also extends the lifespan of the structures and mitigates environmental impacts compared to conventional concrete production methods, which are highly energy-intensive and major contributors to CO2 emissions. Furthermore, MICCP offers cost efficiencies by decreasing the necessity for frequent repairs, thereby reducing the lifecycle costs associated with building materials. The technology is also applicable in hydraulic engineering to enhance the properties of geomaterials and prevent erosion [54]. However, translating MICCP from laboratory settings to full-scale industrial applications presents significant challenges. Future research is essential to tailor the conditions for varied environmental settings and to develop more sustainable substrates, enhancing the overall feasibility and sustainability of MICCP in the construction sector.
Given the non-toxic nature of the end-products, in contrast to ammonia produced by certain bacterial strains, MICCP employing carbonic anhydrase catalysis emerges as a preferable method. Employing Neisseria perflava in MICCP technology paves the way for the development of innovative construction materials, expanding its utility in construction engineering and environmental contexts. However, comprehensive research is imperative to fully comprehend the complexities of utilizing Neisseria perflava for biomineralization within the construction industry. Biomineralization processes are notably influenced by environmental conditions such as pH and temperature. For instance, the optimal stability of carbonic anhydrase, a vital enzyme in biomineralization, has been identified at a pH of 7.5–9.0 and a temperature range of 35–50 °C [55]. Additionally, the biomineralization process is subject to alteration by various environmental factors, including the concentrations of calcium and other ions [56]. To expedite the scalability of MICCP applications using Neisseria perflava, further research is necessary to optimize the specific conditions conducive to optimal biomineralization by this bacterium in construction-related applications.
Regarding the use of endospores in MICCP processes, the application of non-endospore-forming bacteria, such as N. perflava, for MICCP in biomortar or bioconcrete offers numerous advantages and substantial challenges. These bacteria are selected for their capacity to rapidly initiate MICCP, owing to their vegetative state, which eliminates the need for a germination phase typical of endospores. They are metabolically active, synthesizing enzymes critical for transforming biochemical compounds into carbonate ions, thereby effectively facilitating calcium carbonate formation and enhancing the structural properties of the material [57]. However, the application of these bacteria in concrete environments encounters challenges due to their reduced resistance to extreme conditions such as high pH, dehydration, and nutrient deficiency. Their active metabolic state also renders them more susceptible during storage and transportation and heightens their sensitivity to environmental stressors such as temperature variations and UV radiation, potentially impairing their functionality. Additionally, there is an increased risk of contamination [58] and a necessity for additional protective measures to sustain their viability within concrete, complicating and elevating the cost of the process [59]. Despite these obstacles, non-endospore-forming bacteria demonstrate considerable potential for advancing MICCP applications, contingent upon careful management of their limitations.
This study demonstrates that the bacterium Neisseria perflava can produce CaCO3, enhancing mortar properties through biomineralization. This bacterium shows considerable commercial potential for use in MICCP within construction engineering. Although Gram-negative bacteria do not form endospores like Gram-positive bacteria, their outer membrane provides several advantages for survival in the heterogeneous concrete matrix, thus effectively contributing to MICCP and improving mortar durability. These advantages include: (1) acting as a protective barrier against environmental stresses such as pH fluctuations, temperature variations, and the presence of toxic compounds [60]; (2) facilitating the acquisition and utilization of nutrients from the concrete matrix, ensuring bacterial survival and functionality in MICCP [60]; (3) serving as nucleation sites for calcium carbonate precipitation, thereby enhancing MICCP efficiency [60]; (4) aiding bacterial adhesion to the concrete matrix and biofilm formation, which provide additional protection and facilitate nutrient uptake [61]. As there are no prior reports on using Gram-negative bacteria to enhance mortar properties through MICCP, further research should assess the viability of these bacteria post-curing to confirm their effectiveness within the concrete matrix during MICCP processes. Therefore, due to the benefits provided by the outer membrane of Gram-negative bacteria such as Neisseria perflava, these bacteria can effectively survive and contribute to MICP within the heterogeneous concrete matrix through various mechanisms, even without the presence of endospores.
The application of non-pathogenic Neisseria perflava for MICCP to enhance mortar properties presents multiple advantages over traditional MICCP mechanisms and established bio-concrete techniques. Neisseria perflava efficiently utilizes carbonic anhydrase to convert CO2 into bicarbonate ions, significantly enhancing calcium carbonate precipitation. Its ureolytic activity facilitates the hydrolysis of urea into ammonia, which raises the pH and further promotes calcium carbonate precipitation. The calcium carbonate formed acts as a protective barrier, shielding the bacteria from environmental stresses and providing essential calcium ions for growth. Additionally, Neisseria perflava’s capacity to acquire and utilize nutrients from the concrete matrix enhances its survival and effectiveness in MICCP. It also forms synergistic relationships with other microorganisms within the concrete, boosting both its survival and functional contribution to MICCP. The scalability and cost-effectiveness of using Neisseria perflava make it suitable for large-scale construction projects. Moreover, the environmentally sustainable nature of the MICCP process with Neisseria perflava reduces reliance on traditional chemicals and minimizes the carbon footprint of construction projects by incorporating carbonic anhydrase for carbon capture sequestration. The process also enhances the self-healing properties of mortar, thereby reducing the need for maintenance and repairs. By leveraging these benefits, employing non-pathogenic Neisseria perflava for MICCP offers a promising strategy to enhance mortar properties while ensuring safety, sustainability, and cost-effectiveness.
During the study on the use of Neisseria perflava, a Gram-negative bacterium, to enhance mortar properties through calcium carbonate precipitation, several potential challenges were identified, including: (1) determining the optimal amount of bacterial inoculum to be added to the mortar; (2) identifying the optimal nutrient concentrations for bacterial growth; (3) the need for precise control of environmental conditions such as temperature, pH, and nutrient availability to support bacterial activity through carbonic anhydrase mechanisms; (4) potential increases in costs associated with large-scale bacterial cultivation and developing efficient methods for incorporating bacteria into mortar mixtures. These challenges can be addressed by future research aimed at confirming whether Gram-negative bacteria can provide benefits comparable to those of Gram-positive bacteria and potentially replace them in specific MICCP applications. For instance, in the crack repair process, Gram-negative bacteria may be more suitable than Gram-positive bacteria due to their ability to rapidly initiate MICCP processes. Unlike endospores, which require a germination phase, Gram-negative bacteria are in a vegetative state, making them metabolically active and capable of synthesizing enzymes that convert biochemical compounds into carbonate ions, thereby effectively facilitating calcium carbonate formation and enhancing the structural properties of the material.
Therefore, this study assesses the efficacy of employing Neisseria perflava for MICCP to enhance mortar properties, marking a pioneering application of Gram-negative bacteria in construction engineering, contrasting with uses of Gram-positive strains documented in existing construction biotechnology research. Notably, Sarkar et al. [62] have shown that incorporating 30% class C fly ash and Bacillus cohnii endospores—a Gram-positive bacterium—into concrete not only boosts self-healing properties but also enhances durability and cuts carbon emissions by 39%, thereby marking a significant leap in sustainable construction practices. Similarly, Sarkar et al. [63] reported that the integration of Bacillus Cohnii endospores into an alkali-activated fly ash-based composite substantially improved crack healing and durability, decreased porosity, and enhanced resistance to environmental degradation, affirming its viability for modern construction. Moreover, Sarkar et al. (64) demonstrated that Bacillus cohnii, through microbial-induced calcite precipitation, effectively seals cracks and restores structural integrity, thereby increasing mechanical strength by up to 60% and enhancing durability, which confirms the dual sealing and healing functionalities of this biologically augmented concrete.
Furthermore, the potential scalability and feasibility of implementing CaCO3 biomineralization biotechnology using Neisseria perflava strain SKC/VA-3 in construction projects present a promising outlook but also poses several challenges. Key issues include the scalability of bacterial cultivation, requiring optimization of growth conditions and cost-effective production methods that integrate into existing construction processes. Additionally, the MICCP technology must align with current construction practices without disrupting schedules or necessitating extensive training. Long-term viability and performance under varying environmental conditions, including freeze–thaw cycles and chemical exposure, need thorough evaluation. Regulatory and environmental considerations are crucial, particularly concerning the safety and ecological impacts of using bacterial agents. Economic viability is essential, with a detailed cost–benefit analysis needed to assess potential savings from reduced maintenance. Public perception and acceptance are vital; effective communication strategies are necessary to educate stakeholders about the benefits and safety of MICCP technology. Addressing these challenges through further research and pilot projects is essential for successful technology integration into the industry.
3.5 Conclusion
Based on the results of this study, several key findings regarding the improvement of mortar properties using the Neisseria perflava strain SKC/VA-3 can be concluded as follows:
-
Enhancement in physical and mechanical properties: The incorporation of Neisseria perflava strain SKC/VA-3 with calcium lactate pentahydrate led to improved physical and mechanical properties of mortar. Specifically, there was a 5% increase in ultrasonic pulse velocity (UPV), indicating denser and more coherent material properties.
-
Reduction in porosity and water absorption: The study noted a decrease in both porosity and water absorption rates of the mortar, by 2% and 7% respectively. This suggests a tighter microstructure and improved resistance against moisture penetration, which are crucial for the durability of construction materials.
-
Increase in strengths: There was a significant enhancement in the compressive strength and indirect tensile strength of the mortar, by 20 and 14% respectively. These improvements in strength attributes are indicative of a more robust material, capable of withstanding greater loads and stresses.
-
Microstructural evidence of calcium carbonate formation: Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM–EDS) analysis confirmed the formation of calcium carbonate within the mortar's microstructure. This biogenic calcification is critical for the healing and reinforcement of the material.
-
Self-healing capability: The study observed self-healing capabilities in the mortar, with visible calcium carbonate precipitation on the surface cracks after 21 days. This feature enhances the longevity and sustainability of the mortar by reducing the need for manual repairs and maintenance.
Overall, the current study suggests that Neisseria perflava strain SKC/VA-3 holds significant potential for use in bio-mortar applications, highlighting its role in advancing eco-friendly construction techniques through microbial biomineralization.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Worrell E, Price L, Martin N, Hendriks C, Meida LO. Carbon dioxide emission from the global cement industry. Annu Rev Energy Environ. 2001;26:303–29.
Chen C, Habert G, Bouzidi Y, Jullien A. Environmental impact of cement production: detail of the different processes and cement plant variability evaluation. J Clean Prod. 2010;18:478–85.
Bu J, Tian Z, Zheng S, Tang Z. Effect of sand content on strength and pore structure of cement mortar. J Wuhan Univ Technol, Mater Sci Edition. 2017;32:382–90.
Goldman A, Bentur A. The influence of microfillers on enhancement of concrete strength. Cem Concr Res. 1993;23:962–72.
Thomas J, Ramaswamy A. Mechanical properties of steel fiber-reinforced concrete. J Mater Civ Eng. 2007;19:385–92.
Ramachandran SK, Ramakrishnan V, Bang SS. Remediation of concrete using microorganisms Mater J. 2001;98(1):3–9. https://doi.org/10.14359/10154. https://www.concrete.org/publications/internationalconcreteabstractsportal.aspx?m=details&ID=10154&m=details&ID=10154.
Sarayu K, Iyer NR, Murthy AR. Exploration on the biotechnological aspect of the ureolytic bacteria for the production of the cementitious materials—a review. Appl Biochem Biotechnol. 2014;172:2308–23.
Achal V, Mukherjee A. A review of microbial precipitation for sustainable construction. Constr Build Mater. 2015;93:1224–35.
Andalib R, Abd Majid MZ, Hussin MW, Ponraj M, Keyvanfar A, Mirza J, Lee HS. Optimum concentration of Bacillus megaterium for strengthening structural concrete. Constr Build Mater. 2016;118:180–93.
Ameri F, Shoaei P, Bahrami N, Vaezi M, Ozbakkaloglu T. Optimum rice husk ash content and bacterial concentration in self-compacting concrete. Constr Build Mater. 2019;222:796–813.
Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Macias Franco MR, Narayanasamy R, Balagurusamy N. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front Mater. 2019;6:1–15.
Singh H, Gupta R. Cellulose fiber as bacteria-carrier in mortar: self-healing quantification using UPV. J Build Eng. 2020;28:101090.
Achal V, Pan X. Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr Microbiol. 2011;62:894–902.
Jongvivatsakul P, Janprasit K, Nuaklong P, Pungrasmi W, Likitlersuang S. Investigation of the crack healing performance in mortar using microbially induced calcium carbonate precipitation (MICP) method. Constr Build Mater. 2019;212:737–44.
Ghosh P, Mandal S, Chattopadhyay BD, Pal S. Use of microorganism to improve the strength of cement mortar. Cem Concr Res. 2005;35:1980–3.
Irwan JM, Anneza LH, Othman N, Alshalif AF, Zamer MM, Teddy T. Mechanical properties of concrete with enterococcus faecalis and calcium lactate. Proc Eng. 2017;171:592–7.
Alshalif AF, Juki MI, Othman N, Al-Gheethi AA, Khalid FS. Improvement of mechanical properties of bio-concrete using Enterococcus faecalis and Bacillus cereus. Environ Eng Res. 2019;24:630–7.
Chahal N, Siddique R, Rajor A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of concrete incorporating silica fume. Constr Build Mater. 2012;37:645–51.
Hosseini Balam N, Mostofinejad D, Eftekhar M. Effects of bacterial remediation on compressive strength, water absorption, and chloride permeability of lightweight aggregate concrete. Constr Build Mater. 2017;145:107–16.
Li M, Fang C, Kawasaki S, Huang M, Achal V. Bio-consolidation of cracks in masonry cement mortars by Acinetobacter sp. SC4 isolated from a karst cave. Int Biodeterior Biodegradation. 2019;141:94–100.
Liu Z, Bartlow P, Dilmore RM, Soong Y, Pan Z, Koepsel R, Ataai M. Production, purification, and characterization of a fusion protein of carbonic anhydrase from Neisseria gonorrhoeae and cellulose binding domain from Clostridium thermocellum. Biotechnol Prog. 2009;25:68–74.
Gonzalez JM, Fisher SZ. Carbonic anhydrases in industrial applications. In: Frost SC, McKenna R, editors. Carbonic anhydrase: mechanism, regulation, links to disease, and industrial applications. Subcellular Biochemistry, Dordrecht: Springer. 2014;75:405–26. https://doi.org/10.1007/978-94-007-7359-2_20.
Ghelani AD, Bhagat CB, Dudhagara PR, Gondalia SV, Patel RK. Biomimetic sequestration of CO2 using carbonic anhydrase from calcite encrust forming marine actinomycetes. Sci Int. 2015;3:48–57.
Kim IG, Jo BH, Kang DG, Kim CS, Choi YS, Cha HJ. Biomineralization-based conversion of carbon dioxide to calcium carbonate using recombinant carbonic anhydrase. Chemosphere. 2012;87:1091–6.
Adler L, Brundell J, Falkbring SO, Nyman PO. Carbonic anhydrase from Neisseria sicca, strain 6021. Bacterial growth and purification of the enzyme. Biochem Biophys Acta. 1972;284:298–310.
Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev. 2000;24:335–66.
Vinoba M, Bhagiyalakshmi M, Jeong SK, Yoon YI, Nam SC. Capture and sequestration of CO2 by human carbonic anhydrase covalently immobilized onto amine-functionalized SBA-15. J Phys Chem C. 2011;115:20209–16.
Bond GM, Stringer J, Brandvold DK, Simsek FA, Medina MG, Egeland G. Development of integrated system for biomimetic CO2 sequestration using the enzyme carbonic anhydrase. Energy Fuels. 2001;15:309–16.
Ozdemir E. Biomimetic CO2 sequestration: 1. Immobilization of carbonic anhydrase within polyurethane foam. Energy Fuels. 2009;23:5725–30.
Diallo K, Trotter C, Timbine Y, Tamboura B, Sow SO, Issaka B, Dano ID, Collard J-M, Dieng M, Diallo A, Mihret A, Ali OA, Aseffa A, Quaye SL, Bugri A, Osei I, Gamougam K, Mbainadji L, Daugla DM, Gadzama G, Sambo ZB, Omotara BA, Bennett JS, Rebbetts LS, Watkins ER, Nascimento M, Woukeu A, Manigart O, Borrow R, Stuart JM, Greenwood BM, Maiden MCJ. Pharyngeal carriage of Neisseria species in the African meningitis belt. J Infect. 2016;72:667–77.
Veitch FP, Blankenship LC. Carbonic anhydrase in bacteria. Nature. 1963;197:76–7.
Sanders E, Maren TH. Inhibition of carbonic anhydrase in Neisseria: effects on enzyme activity and growth. Mol Pharmacol. 1967;3:204–15.
Mandrell RE. Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infect Immun. 1992;60:3017–20.
Ilina EN, Borovskaya AD, Malakhova MM, Vereshchagin VA, Kubanova AA, Kruglov AN, Svistunova TS, Gazarian AO, Maier T, Kostrzewa M, Govorun VM. Direct bacterial profiling by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for identification of pathogenic Neisseria. J Mol Diagn. 2009;11:75–86.
Jonkers HM, Thijssen A, Muyzer G, Copuroglu O, Schlangen E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng. 2010;36:230–5.
Syarif R, Rizki IN, Wattimena RK, Chaerun SK. Selection of bacteria inducing calcium carbonate precipitation for self-healing concrete application. Curr Res Biosci Biotechnol. 2019;1(1):26–30.
Chaerun SK, Tazaki K, Okuno M. Montmorillonite mitigates the toxic effect of heavy oil on hydrocarbon-degrading bacterial growth: implications for marine oil spill bioremediation. Clay Miner. 2013;48:639–54.
Elyamany HE, Abd Elmoaty AEM, Elshaboury AM. Setting time and 7-day strength of geopolymer mortar with various binders. Constr Build Mater. 2018;187:974–83.
Mehta PK, Monteiro PJM. Concrete: microstructure, properties and materials McGraw-Hill. 4th ed. New York: McGraw-Hill Publishing; 2014.
Mondal S, Ghosh AD. Investigation into the optimal bacterial concentration for compressive strength enhancement of microbial concrete. Constr Build Mater. 2018;183:202–14.
ASTM C 597. 2016. Standard Test Method for Pulse Velocity Through Concrete. West Conshohocken, PA.
ISRM. Suggested methods for determining water content, porosity, density, absorption and related properties and swelling and slake-durability index properties. Int J Rock Mechan Min Sci Geomechan Abstr. 1977;16:143–51.
ASTM C642. 2013. Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. West Conshohocken, PA.
ISRM. Suggested methods for determining the uniaxial compressive strength and deformability of rock materials. Int J Rock Mechan Min Sci Geomech. 1979;16:137–40.
ISRM. Suggested methods for determining tensile strength of rock materials. Int J Rock Mech Min Sci Geomech. 1978;15:99–103.
Forkman A. The location of carbonic anhydrase in a strain of Neisseria flava. Acta Pathol Microbiol Scand Section B Microbiol Immunol. 1972;80B:460–6.
Xu J, Yao W. Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem Concr Res. 2014;64:1–10.
Saxena S, Tembhurkar AR. Biotechnological approach for enhancing the properties of mortar using treated wastewater. Proc Inst Civil Eng—Eng Sustain. 2020;173:97–106.
Saxena S, Tembhurkar AR. Microbiological induced calcium carbonate process to enhance the properties of cement mortar. Mater Today: Proc. 2020;21:1350–4.
González Á, Parraguez A, Corvalán L, Correa N, Castro J, Stuckrath C, González M. Evaluation of Portland and Pozzolanic cement on the self-healing of mortars with calcium lactate and bacteria. Constr Build Mater. 2020;257:119558.
Vaezi M, Zareei SA, Jahadi M. Recycled microbial mortar: effects of bacterial concentration and calcium lactate content. Constr Build Mater. 2020;234:117349.
Khaliq W, Ehsan MB. Crack healing in concrete using various bio influenced self-healing techniques. Constr Build Mater. 2016;102:349–57.
Vijay K, Murmu M. Effect of calcium lactate on compressive strength and self-healing of cracks in microbial concrete. Front Struct Civ Eng. 2019;13:515–25.
Zhang K, Tang C-S, Jiang N-J, Pan X-H, Liu B, Wang Y-J, Shi B. Microbial-induced carbonate precipitation (MICP) technology: a review on the fundamentals and engineering applications. Environ Earth Sci. 2023;82:229.
Bhagat C, Dudhagara P, Tank S. Trends, application and future prospectives of microbial carbonic anhydrase mediated carbonation process for CCUS. J Appl Microbiol. 2018;124:316–35.
Arias D, Cisternas L, Rivas M. Biomineralization mediated by ureolytic bacteria applied to water treatment: a review. Crystals. 2017;7:345.
Chuo SC, Mohamed SF, Mohd Setapar SH, Ahmad A, Jawaid M, Wani WA, Yaqoob AA, Mohamad Ibrahim MN. Insights into the current trends in the utilization of bacteria for microbially induced calcium carbonate precipitation. Materials. 2020;13:4993.
Romero-Rodríguez A, Ruiz-Villafán B, Martínez-de La Peña CF, Sánchez S. Targeting the impossible: a review of new strategies against endospores. Antibiotics. 2023;12:248.
Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Macias Franco MR, Narayanasamy R, Balagurusamy N. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front Mater. 2019;6:126.
Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053.
Charpentier LA, Dolben EF, Hendricks MR, Hogan DA, Bomberger JM, Stanton BA. Bacterial outer membrane vesicles and immune modulation of the host. Membranes. 2023;13:752.
Sarkar M, Maiti M, Mandal S, Xu S. Enhancing concrete resilience and sustainability through fly ash-assisted microbial biomineralization for self-healing: from waste to greening construction materials. Chem Eng J. 2024;481:148148.
Sarkar M, Maiti M, Malik MA, Xu S. Evaluation of the crack-healing performance and durability of bacteria integrated alkali-activated fly ash composites. J Build Eng. 2022;54:104642.
Sarkar M, Maiti M, Xu S, Mandal S. Bio-concrete: unveiling self-healing properties beyond crack-sealing. J Build Eng. 2023;74:106888.
Acknowledgements
The authors express gratitude to the leaders of the Geomechanics & Mine Equipment Laboratory and the Hydrogeology and Hydrogeochemistry Laboratory at Institut Teknologi Bandung (ITB) for providing essential facilities and instruments for this research. Our appreciation also extends to the students and staff of the Geomicrobiology-Biomining & Biocorrosion Laboratory, Geomechanics & Mine Equipment Laboratory, and Hydrogeology and Hydrogeochemistry Laboratory at ITB for their invaluable cooperation and support. Additionally, this research was funded by the LPPM-ITB, under the ITB 2021 Research Program (Grant No. 144/IT1.B07.1/TA.00/2021).
Author information
Authors and Affiliations
Contributions
R.S. carried out the experiments under the guidance of S.K.C. and R.K.W. All authors (R.S., R.I.C., S.K.C., S.H.P., R.K.W.) contributed to writing and reviewing the manuscript prior to submission. R.I.C. and S.K.C. thoroughly revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syarif, R., Chaerun, R.I., Chaerun, S.K. et al. Construction biotechnology: improving mortar properties through calcium carbonate precipitation using a novel strain of the bacterium Neisseria perflava. Discov Civ Eng 1, 49 (2024). https://doi.org/10.1007/s44290-024-00047-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44290-024-00047-1