Abstract
Paediatric acute respiratory distress syndrome (PARDS) is a manifestation of severe, life-threatening lung injury necessitating mechanical support. However, if inappropriately set and not tailored to the respiratory system mechanics of the individual patient, mechanical support of breathing can lead to ventilator-induced lung injury. High-frequency oscillatory ventilation (HFOV) is, at least theoretically, a justifiable mode to be considered to limit lung stress and strain, especially in patients severe PARDS. However, these theoretical benefits have not been translated into improved clinical outcomes. In addition, in adult ARDS HFOV is associated with harm. However, an important question is whether the results of the exisitng randomised clinical trials confirm that HFOV is not beneficial, and its use should be discouraged, or if it is a matter of how the oscillator was used that determins patient outcomes. Currently, HFOV is mainly used as a rescue mode of ventilation and titration of HFOV settings is mainly based on manufacturer’s recommendations, personal beliefs, and institutional preferences. We propose in this perspective a physiology-driven, open-lung strategy for paediatric HFOV for patients with moderate to severe lung disease to avoid injurious conventional ventilation settings, making use of lung recruitment manoeuvres, and setting high oscillatory frequencies to deliver the smallest distal pressure amplitudes. This approach has been shown feasible and safe in children, but needs evaluateion for efficacy. Future investigations should also explore HFOV weaning and monitoring during HFOV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paediatric acute respiratory distress syndrome (PARDS) is a manifestation of severe, life-threatening lung injury. The prevalence of PARDS may be as high as 10% of all children admitted to the paediatric intensive care unit (PICU) with mortality rates ranging up to 40–50% [1]. Mechanical ventilation (MV) is intimately linked with the daily care of PARDS patients and has added significantly to survival. However, if inappropriately set and not tailored to the respiratory system mechanics of the individual patient, MV can lead to ventilator-induced lung injury (VILI) and ventilator-induced diaphragmatic dysfunction (VIDD) [2, 3]. MV can cause lung injury by a variety of putative interacting pathways, primarily mediated by mechanical stress and strain (i.e., alveolar deformation) and cyclic alveolar opening and closure [4, 5]. With MV, the transpulmonary pressure reflects mechanical stress and change in volume in relation to its resting volume reflects lung strain. Considering these pathways, high-frequency oscillatory ventilation (HFOV) is, at least theoretically, a justifiable mode to be considered to limit lung stress and strain, especially in patients with moderate to severe lung disease. Data from animal studies have reported improved effects on oxygenation, lung compliance, attenuation of the pulmonary inflammation and histologic injury, and better alveolar stability with HFOV than conventional MV (CMV) [6,7,8].
Paediatric critical care clinicians may resort to HFOV despite the lack of high-quality paediatric evidence as reported in a systematic review [9, 10]. HFOV in adults is no longer recommended following the outcome of two large randomized clinical trials (RCT) of which particularly the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) raised concerns [11, 12]. This study was stopped prematurely after inclusion of 548 of 1200 planned subjects for increased mortality (47% vs. 35%) and worse secondary outcomes in the HFOV group [12].
An important question is whether the results of the paediatric (and adult) RCTs indeed confirm that HFOV is not beneficial and its use should therefore be discouraged, or if it is a matter of how the oscillator was used that determined patient outcomes [13, 14]. In other words, has HFOV been applied in its most optimal fashion, taking full advantage of the unique properties of this ventilatory mode?
What is the basis for a physiology-driven approach to HFOV titration?
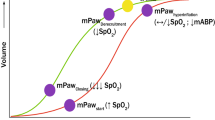
Minimising lung stress and strain during HFOV adheres to the same principles as for CMV. This means opening up the lung and keeping it open through (repeated) lung volume optimisation manoeuvres (LVOM) and delivering the smallest distending pressure that allow for sufficient gas exchange [15].
Lung volume is the main determinant of oxygenation during HFOV. Simplified, PaO2 increases linearly with lung volume up to a certain point when alveoli become overdistended [16]. Additional benefits include a better distal dampening of the pressure oscillations (i.e., lower lung stress) in a well-aerated lung compared with less dampened pressure oscillations in poorly aerated lungs, thereby not only delivering the smallest stroke volume but also decreasing the risk for conducting airways and alveoli to be exposed to higher injurious pressure swings [17]. Recruiting the lung at high oscillatory frequency allows for better lung recruitment and the delivered stroke volume to be more equally distributed as it becomes less dependent on regional compliance [18, 19]. Second, performing LVOMs promotes oscillating the patient on the deflation limb of the pressure – volume loop, thereby making use of hysteresis of the lungs and maintaining sufficient lung aeration at lower airway pressures [6, 13, 14, 20,21,22].
During HFOV, the delivered stroke volume is, thus, not only influenced by compliance (Crs) and resistance of the respiratory system (Rrs), but also by the oscillator settings, such as oscillatory power (magnitude of membrane displacement), oscillatory frequency (Fosc, Hertz), I:E ratio, and position of the membrane, as well as endotracheal tube (ETT) length and diameter, and presence of ETT leakage [23,24,25,26,27]. The ETT constitutes the major workload to the oscillator with the stroke volume being proportional to the ETT inner cross-sectional area as the impedance of the ETT exceeds the impedance of the lung [28,29,30]. F is also a strong determinant of delivered stroke volume as changes in Fosc are inversely proportional to the distal oscillatory pressure amplitude (∆Pdistal) [31].
Translating physiology at the bedside
The obvious question is how to implement the physiologic basis for HFOV at the bedside (i.e., how to perform a LVOM and how to titrate F). The 2nd Paediatric Acute Lung Injury Consensus Conference (PALICC-2) recommends performing LVOMs after switching to HFOV [32]. But to date, the best approach remains to be determined. The only direction comes from one neonatal lamb model study investigating four different LVOMs approaches: a step-wise pressure increase over 6 min, a 20 s sustained dynamic inflation either once or repeated six times, and a standard approach (setting continuous airway pressure [CDP] direct at start) [33]. This study showed that a stepwise pressure increase produced the greatest increase in lung volume and resolution of atelectasis. We have shown a significant heterogeneity in lung behaviour during the incremental phase of a staircase incremental-decremental CDP titration in paediatric HFOV, further providing support for an individualised LVOM [34].
Titrating Fosc is traditionally accomplished according to the patient’s age, ventilator settings and observation of chest wiggle. Alternatively, it can be appreciated that titrating Fosc should be guided by the corner frequency (Fc) of the respiratory system calculated by 1/(2πRC), where R is resistance and C compliance [35]. Fc defines the optimal frequency at which there is adequate gas transport during HFOV in combination with the least injurious pressures and is influenced by the underlying disease process. Fc is increased in lung diseases characterized by short time constants and low compliance, such as in PARDS [36]. From a clinical perspective, this means that the initial Fosc should be as high as possible and then further titrated based on ventilation goals.
Oscillators are designed for the operator to set an oscillatory power that moves the piston forward and backward, generating a pressure amplitude. This pressure amplitude is known as the proximally measured ∆P (∆Pproximal) and is measured near the Y – piece of the ETT). While commonly used as setting parameter, ∆Pproximal should be interpreted as a monitoring parameter. As the ETT is the strongest resistor in the respiratory system during HFOV, ∆Pproximal does not reflect the alveolar ∆P (∆P distal) [37]. In principle, the higher the power the greater the ∆Pproximal and thus potentially the greater ∆Pdistal. However, In bench testing it was shown that combining high Fosc (15 Hz) and high power (set to achieve a ∆Pproximal of 90) resulted in a significantly smaller ∆Pdistal compared with low Fosc (5 Hz) and low power settings [38]. Furthermore, the ratio of ∆Pproximal over ∆Pdistal (the oscillatory pressure ratio) increased with increasing compliance [37]. From a clinical perspective, therefore, targeting the highest possible Fosc in combination with power settings aiming for ∆Pproximal of ∼ 90 appears to be physiologic. It would then be easier to stay within the limits of the safe zone (i.e., zone with the smallest risk of injurious hyperinflation or atelectasis) of oscillation on the deflation limb of the pressure – volume loop.
Can we explain why HFOV has not conferred outcome benefit?
Several explanations can be proposed why HFOV has not been shown to improve clinically relevant patient outcomes. First, patient selection is an important feature. Many negative trials in critical care can be explained by the so-called heterogeneity of treatment effect (HIE) [39]. HIE refers to some patients having benefit from the tested intervention, whereas others experience harm leading to an indifferent trial outcome. However, the indications for HFOV are ill-defined in the medical literature and are usually dictated by clinician preferences and institutional beliefs. In general, HFOV is often considered a rescue mode of ventilation when conventional MV (CMV) fails. But, on the other hand, it could be argued that HFOV should be considered early in the PARDS trajectory to minimise VILI and prevent more “toxic” ventilator settings (e.g., plateau pressure > 28–32 cmH2O and driving pressure > 15 cmH2O). Nonetheless, there are at present virtually no data supporting this concept, except for one small observational study of 26 patients reporting significantly higher 30-day survival rates (58.8% vs. 12.5%) when HFOV was employed within the first 24 h of MV rather than as rescue intervention [40]. In the 1994 paediatric RCT by Arnold and colleagues, duration of CMV before enrolment was mean ± standard deviation (SD) 80 ± 81 vs. 143 ± 240 h for the HFOV group, thus the exposure to potentially injurious MV was longer in the HFOV group [41]. The OSCILLATE trial enrolled subjects within 72 h of ARDS diagnosis, but at the same time subjects could have been on the ventilator for up to 14 days prior to randomization making the true effects of early HFOV less clear [12]. Aside from timing, metrics for oxygenation such as the oxygenation index (OI) or the PaO2 /FiO2 ratio as estimates of PARDS severity, are often used in the decision-making to switch to HFOV. In two paediatric RCTS OI > 13 and 15 were used as inclusion criterium, but so far, no appropriate threshold has been identified nor validated [41, 42]. Re-evaluation of OSCILLATE showed that a mortality benefit of HFOV could only be expected in adults with severe ARDS (i.e., PaO2/FiO2 < 100) [43], suggesting that HFOV as alternative intervention should be considered at least in those with the most severe lung injury. This warrants further investigations as such observations for children are unavailable.
Second, there are different LVOMs reported in the paediatric and adult trials. These included incrementally titrating the continuous distending pressure (CDP) to achieve SaO2 ≥ 90% with FiO2 ≤ 0.6 or a sustained inflation (SI) of 30–40 cmH2O for 20–40 s [12, 41, 44, 45]. However, limiting the LVOM to only the incremental phase excludes making use of hysteresis of the lungs. By design, SIs ignore individual patient’s pathophysiology and respiratory system mechanics. It may be thus be surmised that both LVOM approaches used may have resulted in over- or under-aerated lungs in some patients [14].
Third, the HFOV strategy (including titrating Fosc and power) employed in these trials may be either been poorly detailed or poorly adhered. Reported Fosc varied between 5 and 12 Hz, and ∆Pproximal was primarily used as the setting parameter targeting either chest wiggle or a pre-specified multiplication of the pressures on CMV [41, 42, 44, 45]. Similar criticisms can be made to three paediatric observational studies [46,47,48].
Fourth, it cannot be ruled out that improved understanding of lung-protective ventilation has made CMV much safer than a few decades ago. Lastly, while generally the most commonly available oscillator was tested, in one paediatric trial a neonatal hybrid oscillator was tested (with limit capabilities in children > 6 kg) [44].
A physiologic approach to HFOV in PARDS
Integrating the working principles of HFOV as outlined above, we propose a physiology-driven, open-lung strategy for paediatric HFOV (Fig. 1). Patients should be considered for HFOV early (arbitrarily defined as 72–96 h) after endotracheal intubation or the onset of moderate lung disease including PARDS in tracheostomy patients to avoid (potentially) injurious conventional ventilation settings based on the second iteration of the Paediatric Acute Lung Injury Consensus Conference (PALICC-2) on the diagnosis and management of PARDS, including Pplat < 28 cmH2O (< 32 cmH2O in case of increased chest wall elastance), adequate PEEP and driving pressure < 15 cmH2O) [1]. While it is not yet defined how to quantify at the bedside Fc, from a pragmatic perspective we propose as initial oscillator settings Fosc > 10–12 Hz and power titrated to ∆Pproximal 70–90 cm H2O for sufficient ventilation. These settings are irrespective of age or weight. Then, a staircase incremental-decremental CDP titration should be performed, identifying the optimal CDP for the individual patient and oscillation on the deflation limb of the pressure – volume loop. Previously, we have reported that increasing CDP up to 34–38 cmH2O is necessary during this LVOM, with the initial optimal CDP being 28–32 cmH2O [34]. Oxygenation is then managed by changes in CDP and ventilation by F titration (and not ∆P). When stabilized, twice daily CDP challenges should be performed for aggressive HFOV weaning in addition to commonly used approaches including small decreases in CDP if the FiO2 is above a pre-defined threshold and the SpO2 is in an acceptable range. With this CDP challenge, the CDP is decreased until the SpO2 also decreases, indicating the onset of lung derecruitment. Subsequently, the CDP is over a period (at least 5–10 min) increased to its initial value before the manoeuvre and then reduced again to approximately 2 cmH2O above the CDP when lung decruitment started.
Lessons from the neonatal experience
HFOV has been studied extensively in neonates. Although most neonatal RCTs did not report improved outcomes with HFOV, it remains a well-established ventilation mode for pre-term and term born infants with severe respiratory failure [49, 50]. Nineteen RCTS in pre-term born infants comparing HFOV with CMV have been performed; there was no reduction in mortality or the incidence of bronchopulmonary dysplasia (BPD) between HFOV and CMV after analysis in an individual patient data meta-analysis [49]. Consequently, also the neonatal evidence for the ideal HFOV strategy remains scarce. Interestingly, interpretation of this meta-analysis is similar to adult and paediatric experiences hampered by the HFOV strategy [50]. Also in neonates, inappropriate use of pressure and lack of recruitment manoeuvres influenced the outcome of the RCTs [50]. Notably, the 2019 European Consensus Guidelines on the Management of neonatal respiratory distress syndrome (RDS) recommends an open lung approach on initiation of HFOV, mirroring the same recruitment manoeuvre we have adopted of older children and adults [51]. Setting Fosc in neonates also follow the same principles for older children and adults (i.e., driving by resonance and corner frequency); on a physiologic base in neonatal patients the initial Fosc might be closer to 15 Hz than the commonly used 10–12 Hz [52].
Ongoing and future research directions
Does the physiology-based approach improve outcomes?
We have reported feasibility and safety of the physiology-driven, open-lung strategy for paediatric HFOV in non-cardiac and cardiac children with acute respiratory failure [53, 54]. Nonetheless, it needs to be determined if this approach confers benefit in clinically relevant outcomes in PARDS. Currently, the global 2 × 2 factorial adaptive PRone and OScillation PEdiatric Clinical Trial (PROSpect) is enrolling children with high moderate-to-severe PARDS (i.e., OI ≥ 12 / OSI ≥ 10), randomizing them to CMV or HFOV and prone versus supine positioning (NCT03896763) to test the hypothesis amongst others that HFOV improves VFD by at least two days.
Spontaneous breathing and going back to CMV
Almost all patients transitioned to HFOV will have high-moderate to severe PARDS. In those patients spontaneous breathing may be unwanted as vigorous breathing coming from increased respiratory drive to normalize gas exchange potentially can aggravate underlying lung injury (patient self-inflicted lung injury) due to increased global and regional lung stress and strain [4, 55, 56]. Controversy exists regarding the optimal pharmacological approach to control spontaneous breathing, whether that be deep sedation alone or in combination with neuromuscular blockade. No data exist to conclusively support either approach. However, when the underlying disorder starts to resolve and spontaneous breathing is preferable, transition back to CMV should be considered as the imposed work of breathing may be substantial in HFOV as there is no demand flow system [57]. While there is no ideal CDP identified to transition to CMV, this occurs when CDP of 15–20 cm H2O, oxygenation is stable (i.e., FiO2 < 0.6), and ETT suction is well-tolerated for most patients.
Can HFOV weaning be improved?
In three observational studies it was reported that patients managed with HFOV experienced a longer total ventilation time, even after adjusting for disease severity [46,47,48]. While much can be said about the methodology of these three reports and lack of explaining of the HFOV strategy employed, the observations made are compatible with the fact that bedside clinicians tend to feel more comfortable weaning CMV using the readily available information about pressures and volume as guidance. With the current HFOV devices, there is limited information that can assist in HFOV weaning other than SpO2 and results from blood gas analysis and chest radiographs. In the OSCILATE trial, a CDP/FiO2 table was used, mirroring the much used PEEP/FIO2 table that was developed by the ARDS Network [12]. However, such a CDP/FiO2 table has not been physiologically validated. In fact, there are no data supporting the relationship between CDP and FiO2 in terms of lung mechanics or lung aeration proposed by such as table.
Can monitoring during HFOV be improved?
Currently, available paediatric and adult HFOV devices offer little monitoring possibilities except for CDP, Fosc, ∆P, inspiratory time and sometimes stroke volume. Modern neonatal oscillators incorporate stroke volume measurement and display an index of ventilation (DCO2) and some even have forced oscillation technique build in to measure respiratory system reactance, which may help the bedside clinician in titrating HFOV settings [50]. It is clear that there is much room for improvement in patient monitoring during paediatric and adult HFOV. Electrical impedance tomography (EIT) is a promising monitoring tool that has become more available outside research settings over the past few years. EIT is an imaging modality that estimates the electrical properties inside an object from measurements made on its surface. It involves injecting low currents through electrodes placed on the surface and measuring the resulting electrode voltages change. These measurements are then used to compute the electrical conductivity and permittivity distributions within the object. EIT can identify global and regional changes in lung volume during the LVOM and thereby aid in balancing lung under- and overinflation [58]. So far, the paediatric literature on usefulness of EIT to guide HFOV titration is limited.
Lung ultrasound is becoming increasing popular and is readily available. The point of care ultrasound (POCUS) Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) recommends that lung ultrasound is helpful to semi-quantitatively evaluate lung aeration and thereby help the management of respiratory intervention in ARDS in neonates and PARDS [59]. Similar to EIT, the paediatric literature on usefulness of lung ultrasound to guide HFOV titration is very limited.
Respiratory Inductance Plethysmography (RIP) is a non-invasive method for monitoring respiratory function by measuring the movement of the chest and abdominal wall. The technology involves the use of inductive bands placed around the chest and abdomen, which detect changes in their circumference with amongst others changes in lung volumes. These changes are then converted into electrical signals that can be analysed to provide information on the breathing pattern, tidal volume, and other aspects of respiratory function. Nonetheless, its use is cumbersome and not readily available outside research settings [60].
Conclusions
Despite theory and positive experimental studies, the use of HFOV has not been shown to result in improved clinical outcomes. Continued use has been scrutinised because of the suggestions of increased harm with this ventilator mode in one adult RCT. However, there are strong arguments that HFOV has not been applied in its most optimal fashion, taking full advantage of the unique properties of this ventilator mode and individual patient respiratory system mechanics. We propose a physiology-driven, open-lung approach that is currently being tested in a large RCT for efficacy. Future work should also include improving patient monitoring during HFOV.
Data availability
Not applicable.
Code availability
Not applicable.
References
Emeriaud G, Lopez-Fernandez YM, Iyer NP, Bembea MM, Agulnik A, Barbaro RP, Baudin F, Bhalla A, Brunow de Carvalho W, Carroll CL, Cheifetz IM, Chisti MJ, Cruces P, Curley MAQ, Dahmer MK, Dalton HJ, Erickson SJ, Essouri S, Fernandez A, Flori HR, Grunwell JR, Jouvet P, Killien EY, Kneyber MCJ, Kudchadkar SR, Korang SK, Lee JH, Macrae DJ, Maddux A, Modesto IAV, Morrow BM, Nadkarni VM, Napolitano N, Newth CJL, Pons-Odena M, Quasney MW, Rajapreyar P, Rambaud J, Randolph AG, Rimensberger P, Rowan CM, Sanchez-Pinto S, Ward S, Watson RS, Yehya N, Zimmerman JJ (2023) Khemani, RG, Second Pediatric Acute Lung Injury Consensus Conference Group on behalf of the Pediatric Acute Lung, I, Sepsis Investigators, N Executive Summary of the Second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr Crit Care Med 24: 143–168
Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32:24–33
Pinhu L, Whitehead T, Evans T, Griffiths M (2003) Ventilator-associated lung injury. Lancet 361:332–340
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, Yoshida T, Vaporidi K, Grieco DL, Schepens T, Grasselli G, Spadaro S, Dianti J, Amato M, Bellani G, Demoule A, Fan E, Ferguson ND, Georgopoulos D, Guerin C, Khemani RG, Laghi F, Mercat A, Mojoli F, Ottenheijm CAC, Jaber S, Heunks L, Mancebo J, Mauri T, Pesenti A, Brochard L (2020) Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med 202:950–961
Karageorgos V, Proklou A, Vaporidi K (2022) Lung and diaphragm protective ventilation: a synthesis of recent data. Expert Rev Respir Med 16:375–390
Froese AB (1997) High-frequency oscillatory ventilation for adult respiratory distress syndrome: let’s get it right this time! Crit Care Med 25:906–908
Ng J, Ferguson ND (2017) High-frequency oscillatory ventilation: still a role? Curr Opin Crit Care
Imai Y, Slutsky AS (2005) High-frequency oscillatory ventilation and ventilator-induced lung injury. Crit Care Med 33:S129–S134
Rowan CM, Klein MJ, Hsing DD, Dahmer MK, Spinella PC, Emeriaud G, Hassinger AB, Pineres-Olave BE, Flori HR, Haileselassie B, Lopez-Fernandez YM, Chima RS, Shein SL, Maddux AB, Lillie J, Izquierdo L, Kneyber, Yehya N (2020) Early Use of Adjunctive therapies for Pediatric Acute Respiratory Distress Syndrome: a PARDIE study. Am J Respir Crit Care Med 201:1389–1397 MCJ, Smith, LS, Khemani, RG, Thomas, NJ
Junqueira FMD, Nadal JAH, Brandao MB, Nogueira RJN, de Souza TH (2021) High-frequency oscillatory ventilation in children: a systematic review and meta-analysis. Pediatr Pulmonol 56:1872–1888
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, Arabi YM, Baroncelli F, Beitler JR, Bellani G, Bellingan G, Blackwood B, Bos LDJ, Brochard L, Brodie D, Burns KEA, Combes A, D’Arrigo S, De Backer D, Demoule A, Einav S, Fan E, Ferguson ND, Frat JP, Gattinoni L, Guerin C, Herridge MS, Hodgson C, Hough CL, Jaber S, Juffermans NP, Karagiannidis C, Kesecioglu J, Kwizera A, Laffey JG, Mancebo J, Matthay MA, McAuley DF, Mercat A, Meyer NJ, Moss M, Munshi L, Myatra SN (2023) Ng Gong, M, Papazian, L, Patel, BK, Pellegrini, M, Perner, A, Pesenti, A, Piquilloud, L, Qiu, H, Ranieri, MV, Riviello, E, Slutsky, AS, Stapleton, RD, Summers, C, Thompson, TB, Valente Barbas, CS, Villar, J, Ware, LB, Weiss, B, Zampieri, FG, Azoulay, E, Cecconi, M, European Society of Intensive Care Medicine Taskforce on, A ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 49: 727–759
Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart (2013) High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 368:795–805 TE, Slutsky, AS, Meade, MO, Investigators, OT, Canadian Critical Care Trials, G
Kneyber MC, Markhorst DG (2016) Do we really know how to use high-frequency Oscillatory Ventilation in critically Ill children? Am J Respir Crit Care Med 193:1067–1068
Kneyber MC, Markhorst DG (2016) Any trial can (almost) kill a good technique. Intensive Care Med 42:1092–1093
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Suzuki H, Papazoglou K, Bryan AC (1992) Relationship between PaO2 and lung volume during high frequency oscillatory ventilation. Acta PaediatrJpn 34:494–500
Pillow JJ (2005) High-frequency oscillatory ventilation: mechanisms of gas exchange and lung mechanics. Crit Care Med 33:S135–S141
Bauer K, Brucker C (2009) The role of ventilation frequency in airway reopening. JBiomech 42:1108–1113
Tsuzaki K, Hales CA, Strieder DJ, Venegas JG (1993) Regional lung mechanics and gas transport in lungs with inhomogeneous compliance. JApplPhysiol 75:206–216
Markhorst DG, van Genderingen HR, van Vught AJ (2004) Static pressure-volume curve characteristics are moderate estimators of optimal airway pressures in a mathematical model of (primary/pulmonary) acute respiratory distress syndrome. Intensive Care Med 30:2086–2093
Tingay DG, Mills JF, Morley CJ, Pellicano A, Dargaville PA (2006) The deflation limb of the pressure-volume relationship in infants during high-frequency ventilation. AmJRespirCrit Care Med 173:414–420
Goddon S, Fujino Y, Hromi JM, Kacmarek RM (2001) Optimal mean airway pressure during high-frequency oscillation: predicted by the pressure-volume curve. Anesthesiology 94:862–869
Pillow JJ, Sly PD, Hantos Z, Bates JH (2002) Dependence of intrapulmonary pressure amplitudes on respiratory mechanics during high-frequency oscillatory ventilation in preterm lambs. PediatrRes 52:538–544
van Genderingen HR, Versprille A, Leenhoven T, Markhorst DG, van Vught AJ, Heethaar RM (2001) Reduction of oscillatory pressure along the endotracheal tube is indicative for maximal respiratory compliance during high-frequency oscillatory ventilation: a mathematical model study. Pediatr Pulmonol 31:458–463
Slutsky AS, Kamm RD, Rossing TH, Loring SH, Lehr J, Shapiro AH, Ingram RH Jr., Drazen JM (1981) Effects of frequency, tidal volume, and lung volume on CO2 elimination in dogs by high frequency (2–30 hz), low tidal volume ventilation. JClinInvest 68:1475–1484
Scalfaro P, Pillow JJ, Sly PD, Cotting J (2001) Reliable tidal volume estimates at the airway opening with an infant monitor during high-frequency oscillatory ventilation. Crit Care Med 29:1925–1930
Hamel DS, Katz AL, Craig DM, Davies JD, Cheifetz IM (2005) Carbon dioxide elimination and gas displacement vary with piston position during high-frequency oscillatory ventilation. RespirCare 50:361–366
Gavriely N, Solway J, Loring SH, Butler JP, Slutsky AS, Drazen JM (1985) Pressure-flow relationships of endotracheal tubes during high-frequency ventilation. JApplPhysiol 59:3–11
Niederer PF, Leuthold R, Bush EH, Spahn DR, Schmid ER (1994) High-frequency ventilation: oscillatory dynamics. Crit Care Med 22:S58–S65
Hirao O, Iguchi N, Uchiyama A, Mashimo T, Nishimura M, Fujino Y (2009) Influence of endotracheal tube bore on tidal volume during high frequency oscillatory ventilation: a model lung study. MedSciMonit 15:MT1–MT4
Wong R, Deakers T, Hotz J, Khemani RG, Ross PA, Newth CJ (2017) Volume and pressure delivery during Pediatric high-frequency Oscillatory Ventilation. Pediatr Crit Care Med 18:e189–e194
Fernandez A, Modesto V, Rimensberger PC, Korang SK, Iyer NP, Cheifetz IM, Second Pediatric Acute Lung Injury Consensus Conference of the Pediatric Acute Lung, Sepsis Investigators I (2023) N Invasive Ventilatory Support in Patients With Pediatric Acute Respiratory Distress Syndrome: From the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 24: S61-S75
Pellicano A, Tingay DG, Mills JF, Fasulakis S, Morley CJ, Dargaville PA (2009) Comparison of four methods of lung volume recruitment during high frequency oscillatory ventilation. Intensive Care Med 35:1990–1998
de Jager P, Burgerhof JGM, Koopman AA, Markhorst DG, Kneyber MCJ (2019) Lung volume optimization maneuver responses in Pediatric high frequency Oscillatory Ventilation. Am J Respir Crit Care Med
Venegas JG, Fredberg JJ (1994) Understanding the pressure cost of ventilation: why does high-frequency ventilation work? Crit Care Med 22:S49–S57
Kneyber MC, van Heerde M, Markhorst DG (2012) Reflections on pediatric high-frequency oscillatory ventilation from a physiologic perspective. Respir Care 57:1496–1504
van Genderingen HR, van Vught AJ, Duval EL, Markhorst DG, Jansen JR (2002) Attenuation of pressure swings along the endotracheal tube is indicative of optimal distending pressure during high-frequency oscillatory ventilation in a model of acute lung injury. Pediatr Pulmonol 33:429–436
Van de Kieft M, Dorsey D, Morison D, Bravo L, Venticinque S, Derdak S (2005) High-frequency oscillatory ventilation: lessons learned from mechanical test lung models. Crit Care Med 33:S142–147
Khan YA, Fan E, Ferguson ND (2021) Precision Medicine and Heterogeneity of Treatment Effect in therapies for ARDS. Chest 160:1729–1738
Fedora M, Klimovic M, Seda M, Dominik P, Nekvasil R (2000) Effect of early intervention of high-frequency oscillatory ventilation on the outcome in pediatric acute respiratory distress syndrome. BratislLekListy 101:8–13
Arnold JH, Hanson JH, Toro-Figuero LO, Gutierrez J, Berens RJ, Anglin DL (1994) Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med 22:1530–1539
Samransamruajkit R, Prapphal N, Deelodegenavong J, Poovorawan Y (2005) Plasma soluble intercellular adhesion molecule-1 (sICAM-1) in pediatric ARDS during high frequency oscillatory ventilation: a predictor of mortality. Asian PacJAllergy Immunol 23:181–188
Meade MO, Young D, Hanna S, Zhou Q, Bachman TE, Bollen C, Slutsky AS, Lamb SE, Adhikari NKJ, Mentzelopoulos SD, Cook DJ, Sud S, Brower RG, Thompson BT, Shah S, Stenzler A, Guyatt G, Ferguson ND (2017) Severity of Hypoxemia and Effect of high-frequency Oscillatory Ventilation in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 196:727–733
El-Nawawy A, Moustafa A, Heshmat H, Abouahmed A (2017) High frequency oscillatory ventilation versus conventional mechanical ventilation in pediatric acute respiratory distress syndrome: a randomized controlled study. Turk J Pediatr 59:130–143
Samransamruajkit R, Rassameehirun C, Pongsanon K, Huntrakul S, Deerojanawong J, Sritippayawan S, Prapphal N (2016) A comparison of clinical efficacy between high frequency oscillatory ventilation and conventional ventilation with lung volume recruitment in pediatric acute respiratory distress syndrome: a randomized controlled trial. Indian J Crit care Medicine: peer-reviewed Official Publication Indian Soc Crit Care Med 20:72–77
Gupta P, Green JW, Tang X, Gall CM, Gossett JM, Rice TB, Kacmarek RM, Wetzel RC (2014) Comparison of High-Frequency Oscillatory Ventilation and Conventional Mechanical Ventilation in Pediatric Respiratory Failure. JAMA pediatrics
Bateman ST, Borasino S, Asaro LA, Cheifetz IM, Diane S, Wypij D, Curley MA, Investigators RS (2016) Early high-frequency Oscillatory Ventilation in Pediatric Acute Respiratory failure. A propensity score analysis. Am J Respir Crit Care Med 193:495–503
Wong JJ, Liu S, Dang H, Anantasit N, Phan PH, Phumeetham S, Qian S, Ong JSM, Gan CS, Chor YK, Samransamruajkit R, Loh TF, Feng M, Lee JH, Pediatric N (2020) The impact of high frequency oscillatory ventilation on mortality in paediatric acute respiratory distress syndrome. Crit Care 24: 31
Cools F, Askie LM, Offringa M, Asselin JM, Calvert SA, Courtney SE, Dani C, Durand DJ, Gerstmann DR, Henderson-Smart DJ, Marlow N, Peacock JL, Pillow JJ, Soll RF, Thome UH, Truffert P, Schreiber MD, Van Reempts P, Vendettuoli V, Vento G, Pre V (2010) Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients’ data. Lancet 375:2082–2091
Hibberd J, Leontini J, Scott T, Pillow JJ, Miedema M, Rimensberger PC, Tingay DG (2023) Neonatal high-frequency oscillatory ventilation: where are we now? Archives of disease in childhood Fetal and neonatal edition
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL (2019) European Consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 115:432–450
Zannin E, Dellaca RL, Dognini G, Marconi L, Perego M, Pillow JJ, Tagliabue PE, Ventura ML (2017) Effect of frequency on pressure cost of ventilation and gas exchange in newborns receiving high-frequency oscillatory ventilation. Pediatr Res 82:994–999
de Jager P, Kamp T, Dijkstra SK, Burgerhof JGM, Markhorst DG, Curley MAQ, Cheifetz IM, Kneyber MCJ (2019) Feasibility of an alternative, physiologic, individualized open-lung approach to high-frequency oscillatory ventilation in children. Ann Intensiv Care 9:9
de Jager P, Curley MAQ, Cheifetz IM, Kneyber MCJ (2023) Hemodynamic effects of a high-frequency Oscillatory Ventilation Open-Lung Strategy in critically Ill Children with acquired or congenital Cardiac Disease. Pediatr Crit Care Med 24:e272–e281
Yoshida T, Fujino Y, Amato MB, Kavanagh BP (2017) Fifty years of Research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med 195:985–992
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y (2012) Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 40:1578–1585
van Heerde M, van Genderingen HR, Leenhoven T, Roubik K, Plotz FB, Markhorst DG (2006) Imposed work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 10:R23
Frerichs I, Amato MB, van Kaam AH, Tingay DG, Zhao Z, Grychtol B, Bodenstein M, Gagnon H, Bohm SH, Teschner E, Stenqvist O, Mauri T, Torsani V, Camporota L, Schibler A, Wolf GK, Gommers D, Leonhardt S, Adler A, Ts group (2017) Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 72:83–93
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D (2020) International evidence-based guidelines on point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and neonatal intensive care (ESPNIC). Crit Care 24:65
Wolf GK, Arnold JH (2005) Noninvasive assessment of lung volume: respiratory inductance plethysmography and electrical impedance tomography. Crit Care Med 33:S163–169
Funding
None.
Author information
Authors and Affiliations
Contributions
All drafted the work or reviewed it critically for important intellectual content; all provided final approval of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Jager, P., Markhorst, D.G., Cheifetz, I.M. et al. Clinical implications of a physiologic approach to paediatric high-frequency oscillatory ventilation. Intensive Care Med. Paediatr. Neonatal 2, 27 (2024). https://doi.org/10.1007/s44253-024-00050-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00050-5