Abstract
Background
Sepsis is the leading cause of death in hospitalized patients in the intensive care unit (ICU). Although substantial progress has been made in studies on the treatment of sepsis, the mortality rate remains extremely high. We have previously reported that electroacupuncture (EA) induced tolerance against sepsis, but the underlying mechanism remains unclear.
Methods
C57BL/6 mice were pretreated with EA before sepsis was induced by cecal ligation and puncture (CLP). Then the indexes associated with pulmonary edema and mortality were tested. And the changes of endogenous cholinergic anti-inflammatory pathway especially their typical receptor α7nAChR were detected. Finally, the mechanism of EA in sepsis was explored through regulating the expression of α7nAChR.
Results
The expression of α7nAChR was significantly decreased after sepsis, while EA prevented this reduction. Methyllycaconitine (MLA), an antagonist of α7nAChR, attenuated the beneficial effects of EA. On the other hand, as an α7nAChR agonist, GTS-21 produced similar protective effects against sepsis. Furthermore, the EA-induced enhancement of α7nAChR and inhibition of NF-κB expression in the lungs were reversed by MLA administration.
Conclusions
EA robustly protects the lungs against sepsis and inhibits NF-κB release by activating α7nAChR in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sepsis is the leading cause of mortality in noncoronary intensive care units (ICUs), and it kills over 250,000 patients annually and accounts for 9.3% of deaths in the United States [1, 2]. Among septic patients, the inflammatory cascade produces an uncontrolled inflammatory response, which is the major cause of tissue injury and poor prognosis [3]. Morbidity/mortality in sepsis is principally caused by injury and dysfunction of multiple organs, most commonly acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) [4,5,6,7]. However, efforts to block or inhibit inflammatory responses to improve septic outcomes have failed in clinical trials [8]. Electroacupuncture (EA), a potent endogenous protective strategy, activates several endogenous signaling pathways that result in tolerance against different kinds of injury. We previously reported that EA preconditioning, also known as EA pretreatment, induced robust neuroprotection against transient cerebral ischemic injury [9, 10]. However, the underlying mechanism of EA, especially in the area of sepsis, is unclear.
One of the hallmarks of sepsis is overwhelming inflammatory responses that cause multiple organ failure [11,12,13,14,15]. Acute microbial (bacterial or viral) infections responsible for sepsis cause severe inflammatory injury to the lungs, leading to the development of acute lung injury/inflammation (ALI) [16]. EA has been demonstrated to induce anti-inflammatory effects by inducing the endogenous cholinergic anti-inflammatory pathway in the brain [17, 18]. The cholinergic anti-inflammatory pathway plays an essential role in regulating systemic immunity through the autonomic nervous system, indicating high potential for clinical use in the treatment of hyperimmune inflammatory diseases [19]. The cholinergic anti-inflammatory pathway directly modulates the systemic response to pathogenic invasion through neural-immune interaction [19]. Using vagotomy, Tracey et al. found α7nAChR regulates immune response through the central nervous system-vagus nerve and acetylcholine pathway. It’s reported that the cholinergic anti-inflammatory signaling pathway was activated by selective activating α7nAChR, thereby modulating NF-κB and oxidative stress activity [20]. Furthermore, activation of α7nAChR protects against LPS-induced acute lung injury by inhibiting the TLR4/MyD88/NF-κB pathway [21]. However, whether the endogenous cholinergic anti-inflammatory pathway is involved in the ability of EA to protect against sepsis has not been elucidated.
Here, our goal is to reveal new pathways and therapeutic directions for sepsis treatment in clinical ICU patients by activating cholinergic anti-inflammatory pathways. We attempt to demonstrate that EA is an effective intervention against sepsis. Besides, we are trying to document whether α7nAChR is involved in lung injury induced by sepsis.
2 Materials and Methods
2.1 Experimental Animals and Drugs
The experimental protocol used in this study was approved by the Ethics Committee for Animal Experimentation of Fourth Military Medical University and was conducted according to the Guidelines for Animal Experimentation of Fourth Military Medical University. Male C57BL/6 mice weighing 20–25 g were housed under controlled conditions with a 12-h light/dark cycle, a temperature of 23 ± 1 °C. The mice were allowed free access to a standard rodent diet and tap water. Animals were randomly distributed in different experimental groups.
C57BL/6 mice were randomly divided into the following groups: Sham, CLP, EA + CLP, EA-sham + CLP, MLA + EA + CLP, GTS-21 + CLP, and EA + GTS-21 + CLP. The EA + CLP group was started with electroacupuncture treatment five days before modelling, which selects the Zusanli points and the stimulation intensity of 1 mA, 2 Hz dense wave. This lasts for five days, of which half an hour per day. Mice were anesthetized with isoflurane during 30 min of electroacupuncture every day, and the mice were awake at the end of electroacupuncture, 30 min a day for 5 days. The EA-sham + CLP group only acupuncture points without electrical stimulation. We administered mice to GTS-21 or MLA treatment 30 min prior to electroacupuncture. All drugs were given through intraperitoneal injection.
The specific α7nAChR agonist 3-[2,4-dimethoxybenzylidene] anabaseine (GTS-21) was obtained from Abcam (London, UK). The drug was administered at a dose of 4 mg/kg according to a previous study [22]. The specific α7nAChR antagonist methyllycaconitine (MLA) was obtained from Sigma‑Aldrich (Darmstadt, Germany). The drug was administered at a dose of 5 mg/kg according to a previous study [23]. We administered mice to GTS-21 or MLA treatment 30 min prior to electroacupuncture [24]. All drugs were given through intraperitoneal injection.
2.2 Cecal Ligation and Puncture (CLP) Model
CLP is the most clinically relevant experimental model of sepsis because inflammatory responses are induced by both polymicrobial peritonitis caused by cecal puncture and necrotic tissue produced by cecal ligation. Briefly, anesthesia was induced with an intraperitoneal injection of pentobarbital sodium (50 mg/kg in saline). Then, a midline incision was made in the abdomen, and the cecum was isolated. We placed a 6–0 prolene ligature 5.0 mm from the cecal tip, away from the ileocecal valve; the ligated cecal stump was then punctured once with a 22-gauge needle, and the stool was extruded (1 mm). We then placed the cecum back into its normal intra-abdominal position and closed the abdomen with a running suture of 6–0 prolene. The abdominal wound was closed in two separate layers, the peritoneum and fascia, to prevent leakage of fluid. All animals received resuscitative normal saline (20 ml/kg body weight) immediately after surgery. Animals were subjected to a standard CLP procedure with a 50% average mortality rate, as noted in a previous study [25]. We administered mice to GTS-21 or MLA treatment starting 30 min prior to electroacupuncture. All drug treatments were given by intraperitoneal injection. After the end of the last EA pretreatment, the animals were subjected to CLP models for 24 h [18, 26]. The mice in the Sham group underwent a similar protocol without ligation.
2.3 Electroacupuncture
Electroacupuncture was performed by stimulating both limbs at the ST36 Zusanli acupoint by inserting each 12 mm unipolar stainless steel needle electrode (EL452, Biopac Systems, Goleta, CA) to a depth of approximately 3 mm at each acupoint. The ST36 Zusanli acupoint is located 2 mm lateral to the anterior tubercle of the tibia in the anterior tibial muscle and 4 mm distal to the lower point of the knee joint. This acupoint is located in the proximity of the common peroneal and tibial branches of the sciatic nerve [27, 28]. Briefly, the animals were anesthetized, and the Zusanli (ST36) acupoint was stimulated at an intensity of 1 mA and a frequency of 2 Hz for 30 min daily and received EA preconditioning for consecutive 5 days using the Hwato Electronic Acupuncture Treatment Instrument (Model No. SDZ-V, Suzhou Medical Appliances Co., Ltd., Suzhou, China). Sham treatments included the same procedure but used nonelectrical wood “toothpicks” instead of electrodes. The core temperature of all the mice was maintained at 37.0 ± 0.5 °C during EA by surface heating or cooling (Spacelabs Medical Inc., Redmond, WA).
2.4 Lung W/D Ratio
We used the ratio of lung wet to dry weight to judge the degree of pulmonary edema. The left lungs of mice were collected and weighed to obtain the “wet” weight. The “dry” weight of the lungs was obtained at 56 °C after multiple weighing at a constant weight. The wet/dry ratio (W/D) was quantified.
2.5 Western Blot Analysis
At 24 h after surgery, the mice were deeply anesthetized, and the lungs were collected. The tissue was homogenized on ice in RIPA lysis buffer (Beyotime, Nantong, China) with 1 × Roche complete protease inhibitor cocktail and 1 mM phenylmethylsulfonylfluoride (PMSF). The following primary antibodies were used in this study: anti-α7nAChR rabbit polyclonal antibody (1:300 dilution, Abcam), anti-β-actin mouse monoclonal antibody (1:1000 dilution, Santa Cruz Biotechnology), and anti-NF-κB p65 mouse primary antibody (1:100 dilution, Cell Signaling Technology). Appropriate secondary horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibodies (1:5000 dilution, Pierce Biotechnology Inc.) were used. Semiquantitative analysis of the blots was performed using densitometry followed by quantification with the NIH image program (NIH Image Version 1.61). Each sample was subjected to immunoblotting three times, and the final optical density value (relative to that for the internal standard) represents the average of these three separate analyses. A total of 50 μg of protein was loaded onto a 10–15% sodium dodecyl sulfate/polyacrylamide gel and blotted onto nitrocellulose membranes after electrophoresis. The intensity of each band was quantified with Quantity One-4.2.3 software (Bio-Rad, Hercules, Calif) and normalized to β-actin by density analysis.
2.6 Double Immunofluorescence
At 24 h after surgery, the mice were deeply anesthetized and transcardially perfused with PBS and 4% paraformaldehyde. To ensure that homologous areas of injury were sampled between animals, parallel sets of sections from − 3.0 to − 5.0 mm from the Bregma (covering the infarct area) were used. The 12-μm thick coronal sections were incubated for 12 h at room temperature with the following primary antibodies: anti-α7nAChR rabbit polyclonal antibody (1:300 dilution, Abcam) and anti-CD68 mouse monoclonal antibody (1:500 dilution, Abcam). After washing three times with PBS, sections were incubated for 1 h at room temperature with Alexa Fluor 488-labeled goat anti-mouse IgG (1:1000 dilution, Abcam) and Alexa Fluor 594-labeled goat anti-rabbit IgG (1:1000 dilution, Abcam). Sections incubated without primary or secondary antibodies served as negative controls. Finally, the sections were observed, and images were captured using an Olympus BX-60 fluorescence microscope (Olympus Corporation, Japan).
2.7 Lung Histology and Grading
The lungs were removed from the mice and immediately fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained using hematoxylin and eosin (H&E). We observed histological changes in lung tissues under a light microscope at 400 × magnification, according to lung injury scores [29].
2.8 Peritoneal Lavage
The mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg in saline) and then fixed on the operating table, and the hair on the abdomens of the mice was cut with a curved shear and then wiped with 75% alcohol. The skin of the abdomen was lifted with forceps to inject the salt solution. Five milliliters of acidic buffer (PBS) was added, the abdomen of the mouse was gently massaged with a thumb for 1 min, and the PBS was removed; this procedure was repeated three times, and the PBS lavage fluid was returned to a 4 °C pre-cooled centrifuge tube. After centrifugation (1500 rpm, 4 °C, 10 min), the supernatant was stored in a − 80 °C refrigerator, and the expression levels of the proinflammatory factors TNF-α and IL-1β were measured by an ELISA kit.
2.9 Statistical Analysis
SPSS 19.0 for Windows (SPSS Inc., Chicago, IL) was used to conduct statistical analyses. All values except for lung injury scores are presented as the mean ± SEM and were analyzed by one-way analysis of variance. Between-group differences were detected with Tukey’s post hoc tests. Survival curves and comparisons between curves were assessed using the Mantel-Cox log-rank test. A P value of less than 0.05 was considered to be statistically significant.
3 Results
3.1 EA Improves Survival and Reduces Lung Injury in Septic Mice
First, we examined the protective effect of EA against sepsis in mice. For this purpose, we utilized a model of CLP-induced sepsis, as we described previously [30]. The survival rate of the mice was significantly decreased in the CLP group compared to the Sham group. EA significantly improved the survival of the mice at 7 days after sepsis (Fig. 1A). Since sepsis causes severe pulmonary edema, in the current study, we focused on the pathology of the lung after sepsis and EA. The ratio of wet to dry weight of lung tissue reflects the degree of pulmonary edema [31]. As shown in Fig. 1B, the ratio of wet to dry weight was relatively small in the Sham group, whereas it was significantly increased in the CLP group. EA significantly reduced the extent of lung edema in septic mice induced by CLP (Fig. 1B). Together, these findings reveal that EA improves survival and reduces lung injury in septic mice.
3.2 EA Alleviates the Sepsis-Induced Downregulation of α7nAChR
The cholinergic anti-inflammatory pathway is an emerging discovery in the field of immunology and has been widely explored. It has been demonstrated that α7nAChR is a crucial regulator of the cholinergic anti-inflammatory pathway. As demonstrated by Western blot analysis, pulmonary α7nAChR expression was significantly reduced in septic mice, while EA reversed this reduction (Supplementary Fig. 1, Fig. 2A). In addition, our immunofluorescence staining showed that α7nAChR immunoreactivity colocalized with CD68, a molecule expressed on the surface of macrophages in the lung, and macrophage α7nAChR decreased after sepsis. In accordance with the Western blot results, EA increased α7nAChR expression in macrophages in the lungs (Fig. 2B, 2C). These results indicated that α7nAChR downregulation may be involved in lung injury in sepsis and that EA may be protective by reversing this downregulation.
EA pretreatment upregulated the expression of α7nAChR in the lungs of septic mice. A Immunoblot of α7nAChR in the lungs of each group of mice (n = 3 for each group). B Immunofluorescence of α7nAChR in the lungs of each group of mice. C Statistical analysis of macrophages double-labeled for CD68 and α7nAChR in the lungs of each group of mice (n = 3 for each group). Red represents CD68 expressed by macrophages, and the green represents α7nAChR. Scale bars = 50 µm. *P < 0.05 vs. Sham; #P < 0.05 vs. CLP
3.3 MLA, an Inhibitor of α7nAChR, Attenuates the Protective Effect of EA in Septic Mice
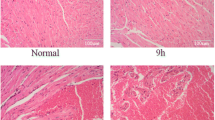
To confirm the crucial role of α7nAChR in the protective effect of EA, we treated mice with MLA, an inhibitor of α7nAChR (Supplementary Fig. 2). We found that MLA attenuated the protective effects of EA on mouse survival (Fig. 3A). The CLP group showed perivascular edema and peribronchial infiltration, and the alveolar spaces were filled with alveolar macrophages and inflammatory cells (Fig. 3B). In addition, there was a significant increase in the lung pathological scores in the CLP group compared with the Sham group (Supplementary table 1). Compared to the CLP group, the EA + CLP group significantly reduced perivascular edema, peribronchial inflammation and macrophage infiltration in the alveolar space. Moreover, MLA treatment significantly reversed the protective effect of EA on the lung tissue of septic mice (Fig. 3B, Supplementary table 1). Meanwhile, EA + Sham and MLA + Sham have no effect on survival and histopathological changes of lung for the mice without sepsis (Supplementary Figs. 4, 5 and Supplementary table 3). These results indicate that α7nAChR plays an essential role in the protection against sepsis induced by EA.
α7nAChR inhibitors reversed the protective effect of EA pretreatment against sepsis. A Survival curve 7 days after surgery in four groups of mice (n = 20 for each group). B Lungs were removed for histopathologic examination using hematoxylin and eosin staining (n = 3 for each group), EA pretreatment reduced perivascular edema, peribronchial inflammation and macrophage infiltration in the alveolar space in septic mice, yet MLA reversed the protective effect of EA. Scale bars = 200 µm. *P < 0.05 vs. Sham; #P < 0.05 vs. CLP
3.4 GTS-21, an Agonist of α7nAChR, Mimics the Protective Effect of EA
To further validate the relationship between EA and the cholinergic anti-inflammatory pathway, we used GTS-21, an agonist of α7nAChR (Supplementary Fig. 3). Survival analysis indicated that GTS-21 significantly improved survival after sepsis, with an effect as strong as that of EA. When GTS-21 and EA were simultaneously applied to septic mice, the survival rate of septic mice was almost the same as that observed after EA or GTS-21 treatment alone (Fig. 4A). There were a large number of infiltrating inflammatory cells in the CLP group compared with the Sham group. In addition, the lung injury score was significantly increased in the CLP group compared with the Sham group. However, the number of infiltrating inflammatory cells decreased in the GTS-21 + CLP and GTS-21 + EA + CLP groups, with a significantly decreased lung injury score compared with the CLP group (Fig. 4B, Supplementary table 2). Meanwhile, GTS-21 + Sham has no effect on survival and histopathological changes of lung for the mice without sepsis (Supplementary Fig. 4, 5 and Supplementary table 3). These results suggest that activation of α7nAChR is an essential mediator of the protective effect of EA.
α7nAChR agonist improves survival and reduces lung injury in mice. A Survival curve 7 days after surgery in five groups of mice (n = 20 for each group). B Lungs were removed for histopathologic examination using hematoxylin and eosin staining (n = 3 for each group). The GTS-21 and EA pretreatment decreased the number of infiltrating inflammatory cells in septic mice. Scale bars = 200 µm. *P < 0.05 vs. Sham; #P < 0.05 vs. CLP
3.5 EA Alleviates Inflammatory Responses and Attenuates NF-κB Activity in Septic Mice
In the early stage of sepsis, the body releases pro-inflammatory cytokines, such as TNF-α and IL-1β, which cause proinflammatory hyperplasia and systemic inflammatory response syndrome [32]. EA at the ST36 Zusanli acupoint reduced the CLP-induced levels of pro-inflammatory cytokines analyzed in peritoneal lavage fluid, including TNF-α and interleukin-1β (IL-1β) (Fig. 5A, B), while increasing the release of anti-inflammatory factor IL-10 (Fig. 5C). Compared with CLP groups, GTS-21 significantly down-regulated the levels of TNF-α, IL-1β, and increased the release of IL-10. Moreover, MLA treatment significantly reversed the anti-inflammatory effect of EA (Fig. 5D, E, F). Our Western blot analysis showed that EA reduced the expression level of NF-κB p65 in the lung. However, MLA treatment reversed this downregulation (Fig. 5H). In addition, GTS-21 and EA significantly reduced the expression level of NF-κB p65 in the lungs of septic mice (Fig. 5I).
EA pretreatment regulates the balance between pro-inflammatory factors and anti-inflammatory cytokines through α7nAChR. Representative ELISA analysis of proinflammatory factors TNF-α (A, D), IL-1β (B, E), and anti-inflammatory cytokines IL-10 (C, F) levels in peritoneal lavage fluid (n = 4 for each group). (H, I) Detection of NF-κB p65 levels in the lung by Western blotting (n = 3 for each group). *P < 0.05 vs. Sham; #P < 0.05 vs. CLP
4 Discussion
Sepsis usually occurs after trauma, burns, bleeding, or abdominal surgery and progresses to multiple organ failure. The hallmark of sepsis is systemic inflammatory response syndrome, during which the lung is one of the first and most severely affected organs [33]. Current treatments focus on broad-spectrum antibiotics and restoring tissue oxygenation to reduce mortality, but mortality in sepsis patients is still as high as 30% [34, 35]. The cholinergic anti-inflammatory pathway is a neural α7nAChR-dependent mechanism that suppresses the innate inflammatory response [36, 37] and provides a novel opportunity for improving sepsis therapy. However, whether the cholinergic anti-inflammatory pathway confers a protective effect against sepsis induced by EA is still unclear.
CLP can simulate a polymicrobial infectious focus within the abdominal cavity, followed by bacterial translocation into the blood compartment, which then triggers a systemic inflammatory response [30]. This model perfectly mimics the pathophysiology of sepsis inside the human body and has been considered the gold standard for sepsis research [38]. Therefore, in the current study, we used the CLP model to investigate the protective effect of EA and the underlying mechanism.
Studies have reported that EA with stimulation of the Zusanli acupoint (ST36) reduced serum TNF-α levels in septic rats, which was achieved by a catecholamine-dependent mechanism. Additionally, recent research indicates that the specific molecular mechanism of this effect stimulated by electroacupuncture may be related to the NF-κB signaling pathway [39, 40]. The previous study in our lab reported that EA could attenuate cerebral ischemic injury via regulation of α7nAChR-mediated inhibition of HMGB1 release in rats [18]. However, our study is the first to use EA treatment, agonist and antagonist of α7nAChR in CLP induced sepsis. We demonstrate α7nAChR is a crucial protective determinant induced by electroacupuncture against sepsis. Furthermore, we are the first to demonstrate electroacupuncture increased α7nAChR expression and therefore inhibit NF-κB activity in the septic lung. In the present study, we found that α7nAChR protein expression significantly decreased in septic mice, while electroacupuncture reversed this reduction. In addition, our immunofluorescence staining showed that α7nAChR immunoreactivity colocalized with macrophage immunoreactivity, indicating the effect of electroacupuncture on α7nAChR expression was relevant. These results are consistent with the possibility that α7nAChR downregulation plays a role in producing lung injury in sepsis and that EA may exert protective effects by preventing this mechanism. Under accordance with these hypotheses, the current study demonstrated that EA increased pulmonary α7nAChR expression. In addition, the α7nAChR antagonist MLA attenuated the beneficial effects of EA on survival and pulmonary edema. Further, activation of α7nAChR with GTS-21 significantly improved survival and reduced pulmonary edema. These results suggest that activation of α7nAChR is an essential mediator of the protective effect of EA.
The cholinergic anti-inflammatory pathway is a neurohumoral regulatory pathway mediated by the vagus nerve and plays an essential role in the regulation of the inflammatory response, in which α7nAChR plays a vital role. Stimulation of the vagus nerve causes the release of acetylcholine, which acts on α7nAChR, inhibits the release of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β on the surface of macrophages, and exerts anti-inflammatory effects [37]. In the early stage of sepsis, anti-inflammatory and pro-inflammatory responses are initiated simultaneously, and immune system activation and immunosuppression occur simultaneously. IL-10 also stimulates the proliferation of regulatory T cells that help balance immune responses, including pathogen clearance, without excessive inflammation or damage to self tissues. IL-10 is produced by Th2 cells after 24–48 h, and then plays a role in inhibiting and terminating the inflammatory response, which can reduce the release of pro-inflammatory cytokines such as IL-6, which is of great significance for the control of sepsis [41,42,43]. Accordingly, our results indicated that EA at the ST36 Zusanli acupoint reduced the CLP-induced levels of all the cytokines analyzed in peritoneal lavage fluid, including TNF-α and IL-1β, while increasing the release of anti-inflammatory factor IL-10. Furthermore, our Western blot analysis showed that EA reduced the expression level of NF-κB p65, but MLA treatment upregulated its expression. In addition, GTS-21 and EA significantly reduced the expression level of p65 in the lungs of septic mice.
The transcription of proinflammatory cytokines always contributes to uncontrolled cytokine release. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a ubiquitous transcription factor that plays a key role in regulating the immune response against infection [44]. When the body is stimulated by certain factors, such as ultraviolet light, cytokines, and growth factors, NF-κB is activated. Studies have shown that the inhibition of inflammation induced by α7nAChR on the surface of macrophages is achieved by inhibiting the nuclear translocation of NF-κB [25]. On the basis of these findings, we verified that EA reduces pro-inflammatory factor release and attenuates NF-κB activity in septic mice. Furthermore, our Western blot analysis showed that EA reduced the expression level of NF-κB p65, but MLA treatment upregulated its expression. In addition, GTS-21 and EA significantly reduced the expression level of p65 in the lungs of septic mice.
In summary, we demonstrated for the first time that EA protects against sepsis by activating the cholinergic anti-inflammatory pathway, which reveals new avenues and great therapeutic potential for the treatment of sepsis in clinical ICU patients. Due to the invasive nature of electroacupuncture stimulation, it is not widely used clinically. In the early stage of our department, the application of electroacupuncture to clinical patients used the method of transcutaneous electrical acupoint stimulation. Because of its non-invasiveness and good patient compliance, significant effects have been observed in the research on postoperative nausea and vomiting, postoperative delirium, etc. Therefore, we believe that transcutaneous electrical acupoint stimulation is the main intervention method for electroacupuncture widely used in clinical research on sepsis.
Availability of Supporting Data
The data used to support the findings of this study are included in the article.
References
de Pablo R, et al. Role of circulating lymphocytes in patients with sepsis. Biomed Res Int. 2014;2014: 671087.
Perman SM, Goyal M, Gaieski DF. Initial emergency department diagnosis and management of adult patients with severe sepsis and septic shock. Scand J Trauma Resusc Emerg Med. 2012;20:41.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51.
Husak L, et al. National analysis of sepsis hospitalizations and factors contributing to sepsis in-hospital mortality in Canada. Healthc Q. 2010;13 Spec No:35–41.
Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50.
Sandrock CE, Albertson TE. Controversies in the treatment of sepsis. Semin Respir Crit Care Med. 2010;31(1):66–78.
Abraham E, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock NORASEPT II Study Group. Lancet. 1998;351(9107):929–33.
Wang Q, et al. Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett. 2005;381(1–2):158–62.
Wang Q, et al. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40(6):2157–64.
Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11(2):56–63.
Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–4.
Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–24.
Ulloa L, et al. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15(16):1918–35.
Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52.
Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):282.
Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6(3):251–7.
Wang Q, et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through alpha7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation. 2012;9:24.
Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62.
Terrando N, et al. Stimulation of the α7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol Med. 2015;20(1):667–75.
Zi SF, et al. Dexmedetomidine-mediated protection against septic liver injury depends on TLR4/MyD88/NF-κB signaling downregulation partly via cholinergic anti-inflammatory mechanisms. Int Immunopharmacol. 2019;76: 105898.
Khan MA, et al. Lipopolysaccharide upregulates α7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock. 2012;38(2):213–9.
Liu J, et al. Electroacupuncture attenuates learning and memory impairment via activation of α7nAChR-mediated anti-inflammatory activity in focal cerebral ischemia/reperfusion injured rats. Exp Ther Med. 2017;14(2):939–46.
Tyagi E, et al. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int. 2010;56(1):135–42.
Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–21.
Jiang T, et al. Electroacupuncture attenuated cerebral ischemic injury and neuroinflammation through α7nAChR-mediated inhibition of NLRP3 inflammasome in stroke rats. Mol Med. 2019;25(1):22.
Sorkin LS, Yaksh TL. Behavioral models of pain states evoked by physical injury to the peripheral nerve. Neurotherapeutics. 2009;6(4):609–19.
Kagitani F, et al. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol. 2005;55(3):149–55.
Zhong WT, et al. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia. 2013;90:132–9.
Zhang X, et al. Bakuchiol Protects Against Acute Lung Injury in Septic Mice. Inflammation. 2017;40(2):351–9.
Clark SB and Soos MP. Noncardiogenic Pulmonary Edema. In: StatPearls. 2019, StatPearls Publishing StatPearls Publishing LLC.: Treasure Island (FL)
Dinarello CA. The interleukin-1 family: 10 years of discovery. Faseb J. 1994;8(15):1314–25.
Zhou J, et al. miR-206 regulates alveolar type II epithelial cell Cx43 expression in sepsis-induced acute lung injury. Exp Ther Med. 2019;18(1):296–304.
Dellinger RP, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35(6):1599–608.
Wang H, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265(6):663–79.
Rittirsch D, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–6.
Villegas-Bastida A, et al. Electrical stimulation at the ST36 acupoint protects against sepsis lethality and reduces serum TNF levels through vagus nerve- and catecholamine-dependent mechanisms. Evid Based Complement Alternat Med. 2014;2014: 451674.
Yim YK, et al. Electro-acupuncture at acupoint ST36 reduces inflammation and regulates immune activity in Collagen-Induced Arthritic Mice. Evid Based Complement Alternat Med. 2007;4(1):51–7.
Feng M, et al. Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. J Clin Lab Anal. 2016;30(6):1037–43.
Henry CJ, et al. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23(3):309–17.
Sanjabi S, et al. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–53.
Miri S, Rasooli A, Brar SK. Data on changes of NF-κB gene expression in liver and lungs as a biomarker and hepatic injury in CLP-induced septic rats. Data Brief. 2019;25: 104117.
Funding
National Nature Science Foundation of China (81871603); National Natural Science Shanxi Province Natural Science Basic Research Program-Key Projects (2018JZ8004).
Author information
Authors and Affiliations
Contributions
ZF and XZ designed the experiments. XS and LD performed the research. ZF, BS and YC provided experimental and conceptual advice. ZF and XS analysed the data and wrote the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval and Consent to Participate
The experimental protocol used in this study was approved by the Ethics Committee for Animal Experimentation of Fourth Military Medical University and was conducted according to the Guidelines for Animal Experimentation of Fourth Military Medical University, Xi’an, China. Mice received humane care in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, X., Du, L., Fan, Z. et al. Electroacupuncture Alleviate Lung Injury of Sepsis Through α7nAChR and NF-κB Signaling Pathway. Intensive Care Res 2, 34–43 (2022). https://doi.org/10.1007/s44231-022-00008-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-022-00008-1