Abstract
In our previous study, the combination of two on-line sample preconcentration techniques, large-volume sample stacking with an electroosmotic flow (EOF) pump (LVSEP) and transient isotachophoresis (tITP), in microchip electrophoresis (MCE) was developed, which was named large-volume dual preconcentration by isotachophoresis and stacking (LDIS). LDIS was apparently effective for improving the sensitivity and the peak shape. In LDIS, however, there was a limit to the improvement of the sensitivity enhancement factor (SEF) since the amount of analytes to be concentrated was limited to the channel volume. To overcome this issue, in the present article, LDIS was coupled with field-amplified sample injection (FASI) technique on Y-shaped channel microchips. The use of a Y-channel in LDIS-FASI allowed consecutive LVSEP, FASI and tITP enrichments with a simple voltage control. In conventional LVSEP and LDIS analyses of a standard analyte, the SEFs were evaluated to be 2630 and 13,100, respectively, whereas in LDIS-FASI that was increased to 27,900 even at the FASI injection time of 0 s. To achieve higher SEFs, furthermore, the FASI injection time was increased to 150 s, resulting in the best SEF of 58,500. It should be emphasized that the peak width in LDIS-FASI was quite narrow, only 0.3–3.1 s, while in normal LVSEP that was 13 s. Furthermore, the LDIS-FASI technique was applied to the analysis of oligosaccharide mixture. Due to the focusing effect by LDIS-FASI, the resolutions were improved from 0.97–1.57 to 2.08–2.73.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capillary electrophoresis (CE) is an analytical technique that allows electrophoretic separation of ions by applying high voltages to both ends of the capillary. In CE, high-speed and highly efficient separations are achieved, so that CE has been applied to various analytical fields. Furthermore, with recent advancements in microfabrication technology, microchip electrophoresis (MCE) has also been progressed [1,2,3,4,5,6]. MCE is an analytical technique that conducts CE separation within microchannels fabricated on a small, few-centimeter square substrate. In MCE, faster separation with a small volume of samples can be realized, and it is expected to prevent sample contamination due to the disposability of the microchips. Hence, MCE is applicable to clinical tests and/or on-site analyses. However, both CE and MCE have the critical issue of low detection sensitivities. To overcome this problem, developments and applications of various on-line sample preconcentration techniques have been exhaustively pursued [7,8,9,10,11].
In our research group, the on-line sample preconcentration by large-volume sample stacking with an electroosmotic flow (EOF) pump (LVSEP) [12] has been applied to CE and MCE [13,14,15,16,17,18]. LVSEP allows highly efficient enrichments of a sample injected throughout the entire capillary/microchannel. In the LVSEP analyses, however, peak frontings have often been observed according to the band broadenings and the reversal of the moving direction after the stacking (Fig. 1) [13], resulting in reduced separation efficiency. To address this issue, a large-volume dual preconcentration by isotachophoresis and stacking (LDIS) technique which combines LVSEP with transient isotachophoresis (tITP) [19,20,21] has been developed and applied to CE [22, 23] and MCE [24]. In the application of the LDIS technique to MCE, a sample solution with low ionic strength is injected into the whole channel, and then a short plug of leading electrolytes (LE) is injected from the outlet reservoir (Fig. 1a). By adding a background electrolyte (BGE) solution containing terminating electrolytes (TE) to both inlet and outlet reservoirs and a negative voltage is applied, anionic analytes are concentrated by the field-amplified stacking. Since the low ionic strength sample is filled with in poly(vinyl alcohol) (PVA)-coated microchannel, a faster anodic EOF is generated to transport the stacked zone toward the inlet reservoir [13, 14]. When the enriched analyte zone approaches the inlet reservoir, a vacant sample matrix is discharged from the reservoir and the channel is filled with high ionic strength BGE, and as a result that the EOF is suppressed. Since the electrophoretic velocities of the analytes surpass the EOF rate, the migration direction of the stacked zone is reversed. In this situation, the analytes are sandwiched by LE and TE, so that isotachophoretic enrichments proceed as shown in Fig. 1c. Finally, when the LE is diluted by diffusion, tITP is finished, and the enriched analytes are electrophoretically separated during the migration toward the outlet reservoir. Due to dual preconcentration effects LVSEP and tITP, LDIS can improve both peak shapes and sensitivities. In our previous report [24], furthermore, it has been demonstrated that MCE-LDIS analyses can be realized using highly viscous running solutions containing high concentrations of sieving matrices, such as hydroxypropyl(methyl cellulose) (HPMC) [25, 26], since a pressure flow is generated during the injection of LE. Such gel electrophoretic matrices in MCE-LDIS enables highly sensitive separation of biomacromolecules such as proteins. Although highly efficient enrichments and improved peak shapes can be obtained in LDIS, a sensitivity enhancement factor (SEF) often reaches a ceiling because a sample volume for the preconcentration is limited to the entire channel volume. To increase the amounts of analytes to be concentrated, in our previous study, LVSEP was combined with a field-amplified sample stacking injection (FASI) [27, 28] on cross- and Y-channel microchips [29, 30]. Despite higher SEFs were obtained according to dual preconcentration effects by LVSEP and FASI, the problem on the peak frontings remained. Hence, in the present article, a novel on-line sample preconcentration technique by combining FASI with LDIS was developed. In LDIS-FASI, a dramatic increase in the SEF is expected without peak frontings since larger amounts of analytes can be provided from the inlet reservoir.
Schematics of LDIS-FASI in MCE. a Sample, LE and BGE injection into the Y-form microchannel and three reservoirs, and LVSEP preconcentration by applying dc voltage, b FASI preconcentration around the merging point in the Y-form channel, c switching the circuit for changing the float reservoir from B to S to start tITP. After finishing tITP, stacked analytes are separated by the electrophoretic migration toward the BW reservoir in the EOF-suppressed channel
In the application of LDIS-FASI to MCE, a Y-shaped microchannel is employed (Fig. 1d). In LDIS-FASI, the whole Y-channel is filled with a low ionic strength anionic sample solution. To inject a short LE plug from the outlet side of the Y-channel, the LE solution is poured to the buffer waste (BW) reservoir and stand for 30 s. After removing the LE solution from the reservoir BW, the buffer (B) and BW reservoirs are filled with high ionic strength BGE containing TE, and a sample solution is injected into the sample (S) reservoir. When a negative voltage is applied between the S and BW reservoirs, the analytes are enriched by field-amplified stacking (Fig. 1a), and the stacked zone is pushed back to the vicinity of the Y-junction by a faster EOF. As the vacant sample matrix is discharged from the channel, the EOF velocity gradually decreases. When the pressure flow and EOF rates balances each other, the movement of the stacked zone stops around the Y-junction, and the sample is further concentrated by FASI [31]. After the FASI enrichment, the electrical circuit is switched to apply the voltage between the B and BW reservoirs as shown in Fig. 1c. Since the stacked analytes zone is sandwiched between LE and TE, tITP proceeds to enrich remaining unconcentrated analytes. When the LE zone is diluted by a longitudinal diffusion, tITP is finished and the enriched analytes are separated by conventional zone electrophoretic mode. Since LDIS-FASI on Y-channel microchips would be operated only by a simple electric-circuit switching (Fig. 1b and c), complex voltage operations and expensive multichannel high voltage power supplies are not needed. As shown in Fig. 1d, Y-shaped microchips were fabricated to achieve LDIS-FASI analyses in MCE.
Experimental
Materials and chemicals
Poly(vinyl alcohol) (PVA, Mw 80,000, 88% hydrolyzed), hydroxypropyl(methyl cellulose) (HPMC, Mw 22,000) and 8-aminopyrene-1,3,6-trisulfonic acid trisodium salt (APTS) were obtained from Sigma-Aldrich (Tokyo, Japan), fluorescein sodium salt, maltotriose (G3), maltopentaose (G5) and maltoheptaose (G7) from Tokyo Chemical Industry (Tokyo, Japan), HEPES from DOJINDO (Kumamoto, Japan), PDMS base and curing reagent (Sylgard 184) from Dow Corning Toray (Tokyo, Japan), SU8-50 from MicroChem (Newton, MA, USA), NaCl and sodium dodecyl sulfate (SDS) were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). All solutions were prepared with deionized water purified by Auto-Still (WG510, Yamato Scientific, Tokyo, Japan) and Simplicity-UV (Merck Millipore, Billerica, MA, USA) systems, and filtered through a 0.45 μm pore membrane filter prior to use. For fluorescence labeling, 0.1 mL of 20 mM G3, G5 and G7 was mixed with 0.01 mL of 20 mM aqueous APTS and 0.02 mL of 0.1 M NaBH3CN in THF. The mixture was kept for 16 h at 37 °C. The obtained stock solution of 10 mM APTS-labeled G3–G7 was stored at −20 °C. In the MCE analysis, the stock solution was diluted to an appropriate concentration with deionized water. In the LVSEP, LDIS, LVSEP-FASI, and LDIS-FASI measurements, 1.5–2.0% HPMC in 10 mM HEPES buffer (pH 8.0) was mainly used as the BGE. In LDIS and LDIS-FASI, HEPES ions acted as TE, and 20 mM NaCl was injected as LE.
A PDMS microchannel was fabricated by the conventional soft lithography technique. Y-channel chip consisted of two 7.5 mm and one 52.5 mm long channels (125 μm width × 100 μm depth), respectively, as shown in Fig. 1d. After producing reservoir wells (2.5 mm diameter) with a piercer, these microchannels were directly bonded onto the slide-glass lids (76.2 × 25.4 mm) via plasma treatment (YHS-R, SAKIGAKE-Semiconductor, Kyoto, Japan). For the LVSEP/LDIS-FASI analysis, a PFA tube (I.D., 4.0 mm; 1.0 cm long) was adhered using PDMS as the adhesive agent onto the S and/or B reservoir wells for raising the liquid level (Fig. 1d). Fabricated PDMS-glass hybrid microchannels were coated with PVA to suppress the EOF by the vacuum-drying method [14]. The PVA-coated channel was washed with water, and finally conditioned with the BGS prior to use.
Apparatus and procedures
MCE experiments were performed on a fluorescence microscope (IX73, Olympus, Tokyo, Japan). A 130 W mercury lamp (SHI-1300L, Olympus) for excitation was introduced to the microscope, and irradiated to the microchannel through a dichroic filter (BP460-480GFP, Olympus) and an objective lens (LUCPLFLN 20 × , Olympus). Fluorescence from analytes collected by the same objective lens was passed through a dichroic filter (U-FBWA, Olympus), and led to a multichannel photodetector (PMA-12, Hamamatsu Photonics, Hamamatsu, Japan). All the analytes were detected by the fluorescence detection scheme at the point 10.0 mm from the anodic end of the separation channel. To evaluate the enrichment efficiency, the value of sensitive enhancement factor (SEF) was calculated by comparing the peak height obtained under the on-line sample preconcentration condition with the fluorescence intensity from the microchannel filled with a sample solution (no preconcentration) taking into account a dilution factor.
In the LDIS-FASI analysis on the Y-channel chips, a sample solution was manually injected into the entire channel using a microsyringe, and then the sample in the three reservoirs was removed using a micropipette. In Fig. 1a, 6.0 µL of the LE solution was added into the vacant BW reservoir to produce the liquid level of 1.4 mm, which was left for 30 s to introduce of the short LE plug by pressure flow. After the LE injection, the LE solution in the reservoir BW was aspirated using the micropipette, and then the B and BW reservoirs were filled with 6.0 μL of the BGE containing TE, whereas to the S reservoir 6.0 μL of the sample solution was loaded, whose liquid levels were defined as 0.0 mm. In the modified LDIS-FASI procedure, the application of 6.4 μL BGE by a microsyringe gives + 0.1 mm higher B liquid level, which produces the hydrodynamic pressure of 0.98 Pa (details shown later).
In LDIS-FASI, a voltage of −1.5 kV was applied with a high voltage power supply (HCZE-30PN0.25, Matsusada Precision, Kusatsu, Japan) through two platinum electrodes immersed in the S and BW reservoirs. During the LVSEP preconcentration step as shown in Fig. 1a, the current value was almost 0 µA since the almost entire channel was filled with the low-ionic strength sample solution. As the current reached 12 µA, which was 90% of the current obtained in the BGE entirely filled Y-channel, the enrichment by FASI would proceed around the Y-junction as shown in Fig. 1b. After the voltage switching time (tVS), the electric circuit was switched for the application of the negative potential between the B and BW reservoirs as shown in Fig. 1c. In the LVSEP-FASI measurements, the experimental procedures were same as those in LDIS-FASI without injecting the short plug of LE. In the conventional LDIS experiments, the voltage was applied between the B and BW reservoirs. Normal LVSEP was also conducted without the LE injection by only using the B and BW reservoirs as cathodic and anodic ends, respectively [13].
Results and discussion
Analytical performance of LDIS-FASI in MCE
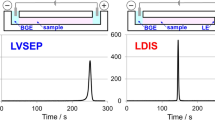
For comparison of the analytical performances of the LDIS-FASI analysis in MCE, LVSEP, LDIS and LVSEP-FASI analyses were carried out using fluorescein as a standard analyte. In the conventional LVSEP analysis, the sample was filled throughout the Y-form channel, and the voltage was applied between the B and BW reservoirs after filling the three reservoirs with the BGE [13]. As a result, a single peak of fluorescein was observed as shown in Fig. 2a, and the SEF obtained with LVSEP was estimated to be 2630. However, the peak was apparently broader, i.e., the peak width at half height (w1/2) was 13 s as summarized in Table 1. Furthermore, the peak asymmetry factor (AS) was 0.81, indicating slight fronting peak. This peak shape is quite characteristic in LVSEP, resulted from the reversal of the migration direction of the stacked zone in the microchannel [13].
Next, the LDIS analysis was conducted by injecting the LE solution from the BW reservoir [24]. First, the sample was injected into the entire Y-channel. After removing the sample from three reservoirs with a micropipette, 6.0 μL of LE was injected into the BW reservoir to create a liquid level difference of 1.4 mm, and it was left to stand for 30 s to inject a short plug of LE into the channel. At the liquid level of 1.4 mm, which was equivalent to the applied pressure of 13.7 Pa, the pressure flow velocity was calculated to be 0.14 mm/s from Hagen–Poiseuille equation. As a result, the injected length of the LE solution was estimated to be ca. 4.2 mm at the injection time of 30 s. After removing the LE solution from the BW reservoir, the BGE containing HEPES as TE was added to all three reservoirs. When the voltage was applied between the B and BW reservoirs, a sharp peak of fluorescein was observed as shown in Fig. 2b. The SEF was evaluated to be 13,100, which was significantly improved from 2630 in the LVSEP analysis. It should be noted that the peak width was very narrow, only 1.0 s. These results demonstrated that the LDIS was apparently effective for enhancing the peak heights and narrowing the peak width due to the preconcentration effect by tITP.
Furthermore, we investigated the on-line sample preconcentration by combining LVSEP/LDIS with FASI. In the LVSEP-FASI and LDIS-FASI analyses, the BGE was poured into the B and BW reservoirs, and the sample solution was added to the S reservoir. As the enrichment progressed by the application of the voltage, vacant sample matrix was discharged from the B or S reservoirs for LVSEP-FASI or LDIS-FASI, respectively, and the entire channel was filled with BGE, causing the current to increase from 0 to 13–14 μA. When the current reached ~ 90%, approximately 12 μA, the enrichment zone was presumed to be reached around the Y-junction as shown in Fig. 1b. We defined the duration from reaching 12 µA to switching the electric circuit as the voltage switching time (tVS). During the duration of tVS, the enrichment by FASI would predominantly proceed. Hence, the SEFs are expected to be increased upon increasing tVS.
At tVS of 0 s, a higher peak was obtained with LDIS-FASI (Fig. 2d) relative to that with the LDIS analysis (Fig. 2b). In LDIS-FASI, the SEF was calculated to be 27,900, while in the conventional LDIS that was 13,100. Despite tVS was 0 s in LDIS-FASI, the SEF was improved from 13,100 to 27,900, indicating that the enrichment by FASI gradually proceeded as the current rose to 12 μA. Although the peak width was slightly increased to 2.2 s due to the larger amount of the analytes provided by FASI, AS was calculated to be 1.0, showing the symmetrical peak. In LDIS-FASI, unconcentrated analytes derived from the band broadening of the stacked zone during the LVSEP and FASI processes would be finally focused by tITP as shown in Fig. 1c, giving a symmetrical peak. Such narrow and symmetrical peaks should be effective for improving the resolutions in LDIS-FASI.
To clarify the contribution of tITP, the analytical performances in LDIS-FASI were compared with those in LVSEP-FASI [30]. As shown in Fig. 2c and d, the peak height in LVSEP-FASI was apparently lower than that in LDIS-FASI in spite of the same tVS. As summarized in Table 1, the SEF in LVSEP-FASI was 3520, approximately one-eighth of that in LDIS-FASI. Although the peak width in LVSEP-FASI was increased only 1.5-fold from 2.2 s to 3.1 s, the SEF was decreased to one-eighth, suggesting that the amount of the analytes injected by FASI in LDIS-FASI was ca. five times larger than that in LVSEP-FASI. This would be due to a larger difference in the electric field strength in LDIS-FASI, i.e., FASI occurred at the sample/BGE containing TE (10 mM HEPES) boundary in LVSEP-FASI, whereas in LDIS-FASI, it proceeded at the sample/LE (20 mM NaCl) interface. Such greater difference in the electric field led to more efficient electrophoretic sample injection in LDIS-FASI. Despite the fivefold increase in the injected amount of the analytes in LDIS-FASI relative to LVSEP-FASI, the peak width was decreased from 3.1 s to 2.2 s. The narrower peak should be obtained by the preconcentration effect of tITP in LDIS-FASI. Therefore, these results demonstrated that tITP progressed by the injection of LE contributed not only to narrowing the peak width but also to improving the enrichment efficiency.
In our previous report on LVSEP-FASI, it was demonstrated that effective enrichments could be achieved by applying a weak pressure with raising the liquid level of the reservoir S about 2 mm higher during the run [29, 30]. However, in the present LDIS-FASI analysis, when the liquid level in the reservoir S was raised 1 mm higher, the SEF was decreased from 27,900 to 4840, and further raising the level to 2 mm resulted in a significant decrease of the SEF to 770 (data not shown). Such larger difference in the effect of the pressure flow from the reservoir S might be due to the injection of LE in LDIS-FASI. As previously reported, a pressure flow is generated when LE is injected from the reservoir BW in the conventional LDIS [24]. This weak pressure flow from the reservoir BW assists the introduction of the BGE into the microchannel during the LVSEP step (Fig. 1a). According to the pressure flow generated by the LE injection, a highly viscous BGE containing high concentration of sieving polymers could be employed to the LDIS analysis, resulting in the improvement of the SEF due to the suppression of the band broadening [24]. Hence, the application of the weak pressure from the reservoir S by raising the liquid level should counteract that from the reservoir BW, inhibiting the first enrichment step by the field-amplified sample stacking (Fig. 1a).
Considering the principle of LDIS-FASI, a weak pressure flow from the reservoir S should be needed at the FASI stage as shown in Fig. 1a. When the velocity of the stacked zone is balanced with the pressure flow, the movement of the analyte zone is almost stopped, and then the FASI enrichment is progressed [31]. Hence, we investigated the possibility that the liquid level of the reservoir S might rise as the vacant sample matrix was discharged during the LVSEP stage. Assuming that 90% of the sample solution in the Y- channel was drained into the reservoir S by LVSEP, the liquid level is calculated to be raised to 0.12 mm. This suggests that a weak pressure flow may be generated from the reservoir S at the FASI stage as shown in Fig. 1b. In LDIS-FASI, therefore, the above mechanism that automatically increased the liquid level of the S reservoir would contribute to the progress of the enrichment at the FASI stage.
Effect of voltage switching time on SEF in LDIS-FASI
To improve the SEF in LDIS-FASI, tVS was extended. When tVS was increased from 0 to 60 s, a sub-peak at 305 s appeared before the main peak as shown in Fig. 3a. The peak splitting was also observed in our previous LVSEP-FASI report on the Y-channel chip [30], suggesting that the observed electropherogram would be due to the leakage of the stacked zone toward reservoir B from the Y-junction by the weak pressure flow during the FASI stage as illustrated in the inset of Fig. 3a. When the leaked analytes moved along with the main enriched zone to the detection point after the voltage switching, the sub-peak should be observed on the Y-channel chips. To eliminate the leakage, the suppression of the inflow by applying a weak pressure through slightly raising the liquid level of the reservoir B. As shown in Fig. 3b, LDIS-FASI at tVS of 60 s was conducted with the liquid level of 0.1 mm at the B reservoir, leading to the elimination of sub-peaks, the improvement in SEF from 37,000 to 41,000, and the reduction of the peak width from 0.8 to 0.4 s. The obtained results indicated a successful suppression of the leakage toward the B reservoir since the pressure flow generated from the liquid level of 0.1 mm at the B reservoir would be balanced with that of 0.12 mm at the S reservoir automatically raised during the LVSEP step as discussed in the previous section. When the B liquid level was raised to 0.25 mm, on the other hand, the SEF was decreased to 330 (data not shown). Thus, the liquid level of 0.1 mm at the B reservoir would be optimal for suppressing the leakage around the Y-junction.
Effect of the liquid level of the reservoir B in LDIS-FASI: a 0 and b 0.1 mm. Sample, 50 ppb fluorescein dissolved in deionized water; BGE, 1.5% HPMC in 10 mM HEPES (pH 8.0); tVS, 60 s. Other conditions are as in Fig. 2d
Under 0.1 mm of the B liquid level condition, furthermore, the peak height of fluorescein was gradually increased upon increasing tVS from 0 to 150 s as shown in Fig. 4a–c. As summarized in Table 2, the SEFs were calculated to be 21,200, 35,800, and 58,500 at tVS of 0, 90 and 150 s, respectively. It should be emphasized that, at tVS of 0 ~ 150 s, the peak width was quite narrow, ranging only from 0.3 to 1.1 s, indicating the efficient focusing by FASI and tITP. At tVS of 180 s, on the other hand, the SEF was decreased to 43,600. Under the long tVS condition, the peak width was increased from 1.1 to 1.7 s. This suggested that excessive amounts of analytes injected by FASI caused incomplete enrichment by tITP, resulting in the decrease in SEF as shown in Fig. 4d.
LDIS-FASI analyses of fluorescein at tVS of a 0, b 90, and c 150 s. d Effect of tVS on SEF. Conditions are as in Fig. 3b
Application to oligosaccharide analysis
To evaluate the separation ability of LDIS-FASI, an oligosaccharide mixture containing APTS-labeled G3, G5 and G7 was analyzed. Prior to LDIS-FASI, the LVSEP analysis of the oligosaccharide mixture was carried out. As shown in Fig. 5a, three peaks for G3, G5 and G7 were detected in LVSEP. However, all of three peaks were quite broader. Due to the broader peaks, the resolution between G5 and G7 fell below 1.0 as summarized in Table 3, leading to a partial separation. In contrast, when the LDIS-FASI analysis was conducted at tVS of 150 s, extremely sharp and higher peaks were obtained as shown in Fig. 5b. As demonstrated in Table 3, the SEFs were improved from 370–850 to 830–2530 compared with the conventional LVSEP. These narrower peaks resulted in the resolution exceeding 1.5 both for G3–G5 and G5–G7, achieving the complete separations. By utilizing the three consecutive focusing by LVSEP, FASI and tITP in LDIS-FASI, higher and narrower peaks could be obtained, which demonstrated that LDIS-FASI should be effective for improving the separation and the sensitivity.
a Conventional LVSEP and b LDIS-FASI analyses of oligosaccharides. Sample, 1.0 μM APTS-G3, G5 and G7 dissolved in deionized water. Other conditions are as in Fig. 4c
Conclusion
In this study, the combination of LDIS with FASI was investigated in MCE to overcome the limitation that the amount of the analytes for the enrichment is restricted by the channel volume. To realize LDIS-FASI with the simple voltage operations, the Y-form channel microchips were employed. Among three on-line sample preconcentration techniques, i.e., LVSEP, LDIS and LDIS-FASI, the LDIS-FASI analysis showed the most significant improvements in the SEF, peak width, and peak shape. Furthermore, the SEF in LDIS-FASI was superior to that in LVSEP-FASI, suggesting that the preconcentration by tITP contributed not only to the peak narrowing but also to the SEF improvement in LDIS-FASI. To improve the SEF, the voltage switching time was increased in LDIS-FASI. When tVS was increased from 0 to 150 s, the SEF was increased from 21,200 to 58,500, demonstrating that the combination of FASI significantly contributed to the improvement of the SEF. In the LDIS-FASI analysis of oligosaccharides, extremely sharp peaks were obtained due to the focusing effect by tITP, resulting in the baseline separations. In the present stage, however, the RSDs of the SEF were poor as demonstrated in Tables. To achieve accurate and precise analyses, internal standards should be included in the sample solution to improve the RSDs. Our proposed LDIS-FASI in MCE should be effective for the application to various samples, especially for on-site analyses in environmental and clinical fields.
Data availability
The data supporting the findings of this study are available within the paper. Data will be made available on reasonable request.
Abbreviations
- LDIS:
-
Large-volume dual preconcentration by isotachophoresis and stacking
- LVSEP:
-
Large-volume sample stacking with an electroosmotic flow pump
- tITP:
-
Transient isotachophoresis
- FASI:
-
Field-amplified sample injection
- LE:
-
Leading electrolyte
- TE:
-
Terminating electrolyte
- HPMC:
-
Hydroxypropyl(methyl cellulose)
- PVA:
-
Poly(vinyl alcohol)
- EOF:
-
Electroosmotic flow
- BGE:
-
Background electrolytes
- MCE:
-
Microchip electrophoresis
- SEF:
-
Sensitivity enhancement factor
References
R.L. Nouwairi, K.C. O’Connell, L.M. Gunnoe, J.P. Landers, Anal. Chem. (2021). https://doi.org/10.1021/acs.analchem.0c04596
B.M. De Castro Costa, S. Griveau, F. d’Orlye, F. Bedioui, J.A.F. da Silva, A. Varenne, Electrochimica Acta. (2021). https://doi.org/10.1016/j.electacta.2021.138928
E. Naghdi, G.E. Moran, M.E. Reinau, W. De Malsche, C. Neusüß, Electrophoresis (2023). https://doi.org/10.1002/elps.202200179
A.M. Zeid, A. Abdussalam, S. Hanif, S. Anjum, B. Lou, G. Xu, Electrophoresis (2023). https://doi.org/10.1002/elps.202200082
M. Jozanović, I. Pukleš, N. Sakač, E. Carrilho, A. Kilár, B. Matasović, M. Samardžić, M. Budetić, F. Kilár, TrAC Trends Anal. Chem. (2023). https://doi.org/10.1016/j.trac.2023.117111
V. Kašička, Electrophoresis (2024). https://doi.org/10.1002/elps.202300152
Y. Wen, J. Li, J. Ma, L. Chen, Electrophoresis (2012). https://doi.org/10.1002/elps.201200240
F. Kitagawa, K. Otsuka, J. Chromatogr. A (2014). https://doi.org/10.1016/j.chroma.2013.10.066
R.L.C. Voeten, I.K. Ventouri, R. Haselberg, G.W. Somsen, Anal. Chem. (2018). https://doi.org/10.1021/acs.analchem.8b00015
A.S. John, M.M. Sidek, L.Y. Thang, S. Sami, H.Y. Tey, H.H. See, J. Chromatogr. A (2021). https://doi.org/10.1016/j.chroma.2020.461868
F. Kitagawa, Y. Soma, Chromatography (2022). https://doi.org/10.1016/j.chroma.2013.10.066
Y. He, H.K. Lee, Anal. Chem. (1999). https://doi.org/10.1021/ac981100e
T. Kawai, K. Sueyoshi, F. Kitagawa, K. Otsuka, Anal. Chem. (2010). https://doi.org/10.1021/ac1008145
F. Kitagawa, S. Nakagawara, I. Nukatsuka, Y. Hori, K. Sueyoshi, K. Otsuka, Anal. Sci. (2015). https://doi.org/10.2116/analsci.31.1171
F. Kitagawa, S. Kinami, Y. Takegawa, I. Nukatsuka, K. Sueyoshi, T. Kawai, K. Otsuka, Electrophoresis (2017). https://doi.org/10.1002/elps.201600184
F. Kitagawa, Y. Niimiya, I. Nukatsuka, Chromatography. (2024). https://doi.org/10.15583/jpchrom.2021.008
F. Kitagawa, A. Hayashi, I. Nukatsuka, Chromatography. (2022). https://doi.org/10.15583/jpchrom.2021.018
F. Kitagawa, Y. Takahashi, I. Nukatsuka, Chromatography. (2023). https://doi.org/10.15583/jpchrom.2023.019
A.R. Timerbaev, T. Hirokawa, Electrophoresis (2006). https://doi.org/10.1002/elps.200500320
Z. Xu, A.R. Timerbaev, T. Hirokawa, J. Chromatogr. A (2009). https://doi.org/10.1016/j.chroma.2008.10.077
Z. Malá, P. Gebauer, TrAC Trends Anal. Chem. (2023). https://doi.org/10.1016/j.trac.2022.116837
T. Kawai, N. Ota, A. Imasato, Y. Shirasaki, K. Otsuka, Y. Tanaka, J. Chromatogr. A (2018). https://doi.org/10.1016/j.chroma.2018.06.034
T. Kawai, N. Ota, K. Okada, A. Imasato, Y. Owa, M. Morita, M. Tada, Y. Tanaka, Anal. Chem. (2019). https://doi.org/10.1021/acs.analchem.9b01578
F. Kitagawa, K. Takahashi, R. Osanai, R. Sasaki, T. Kawai, Anal. Sci. (2024). https://doi.org/10.1007/s44211-024-00597-5
M. Jabasini, Y. Murakami, N. Kaji, M. Tokeshi, Y. Baba, Biol. Pharm. Bull. (2006). https://doi.org/10.1248/bpb.29.595
S. Štěpánová, V. Kašička, J. Sep. Sci. (2017). https://doi.org/10.1002/jssc.201600962
R.L. Chien, D.S. Burgi, J. Chromatogr. A (1991). https://doi.org/10.1016/0021-9673(91)80066-P
D.S. Lian, S.J. Zhao, J. Li, B.L. Li, Anal. Bioanal. Chem. (2014). https://doi.org/10.1007/s00216-014-8062-7
F. Kitagawa, T. Ishiguro, M. Tateyama, I. Nukatsuka, K. Sueyoshi, T. Kawai, K. Otsuka, Electrophoresis (2017). https://doi.org/10.1002/elps.201700155
F. Kitagawa, T. Yonekura, I. Nukatsuka, Anal. Sci. (2024). https://doi.org/10.1007/s44211-023-00456-9
T. Kawai, M. Ueda, Y. Fukushima, K. Sueyoshi, F. Kitagawa, K. Otsuka, Electrophoresis (2013). https://doi.org/10.1002/elps.201200615
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP18K05162 and JP22K05149.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitagawa, F., Sato, S., Suzuki, T. et al. Combination of on-line sample preconcentration by large-volume dual preconcentration by isotachophoresis and stacking (LDIS) with field-amplified sample injection (FASI) on Y-channel microchips. ANAL. SCI. (2024). https://doi.org/10.1007/s44211-024-00647-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44211-024-00647-y