Abstract
It has been suggested that the chelating agent 2-(2-(1-thiophene-2-yl) ethylidene) hydrazinyl) benzoic acid (TEHBA) be utilized to extract, separate and measure platinum(IV) by UV–visible spectrophotometry at the microgram level. Following 5 min of heating the reaction mixture in a water bath, Pt(IV)-TEHBA complex formed. This complex was formed in the presence of potassium iodide solution with a molar absorption coefficient 1.9 × 103 dm3 mol−1 cm−1. At 420 nm, the substance exhibited the greatest absorption. As Beer’s law described, the Pt(IV)-TEHBA complex for platinum(IV) has a beer’s range of 10–50 μg cm−3. It was determined that the proportion ratio of the Pt(IV)-TEHBA complex was 1:1 after its extraction. Despite the investigation of interference from various ions, it was ascertained that the method exhibited selectivity exclusively towards platinum(IV). The trace amounts of platinum(IV) were extracted and quantified from synthetic mixtures representing alloys, binaries and ternary synthetic mixtures. The process of extracting platinum(IV) from pharmaceutical samples involves the implementation of a specific method. Moreover, the procedure exhibits a progressive segregation of palladium(II), platinum(IV) and nickel(II) while also boasting its ease of operation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Platinum is an extremely valuable mineral. An increase in its utilization has been observed as time has passed. Platinum exhibits several noteworthy attributes such as resistance to elevated temperatures, a high melting point and corrosion resistance [1]. Also, platinum serves a substantial role in the fields of biology and ecology. It is utilized as a catalyst in a huge variety of chemical reactions. The utilization of platinum-based catalysts in automobile exhaust gases effectively eliminates carbon monoxide, and unburned hydrocarbons from fuel and nitrogen oxides [2]. Platinum alloys also find application in dental and medical devices owing to platinum's corrosion resistance and alloying capability [3].
As a metallic element, platinum presents no health hazards. In therapeutic contexts, the "cis-platin" denotes cis-dichlorodiammineplatinum(II), a square planer platinum complex. Particularly advised to treat testicular cancer, minor lung cancer, cervical cancer, and ovarian cancer [4]. Furthermore, cancer can be managed with a variety of additional platinum-based pharmaceutical agents, including carboplatin and oxaliplatin. Paclitaxel and carboplatin are frequently administered concurrently for the management of lung cancer [5]. Oxaliplatin, a platinum compound of the third generation, is an essential constituent in the therapeutic management of cervical cancer [6]. Nedaplatin, Lobaplatin, and Heptaplatin [7,8,9] are three additional platinum-based drugs that serve a critical function as compounds against cancer.
Various analytical techniques, including inductively coupled plasma atomic emission spectrophotometry (ICP–AES) [10, 11], ICP mass spectroscopy (ICP–MS) [12], electrothermal atomic absorption spectrometry [13, 14], graphite furnace atomic absorption spectrometry [15], neutron activation analysis [16], capillary electrophoresis [17, 18] and others, have been utilized to identify platinum from environmental samples. Numerous reports detail the use of chromogenic reagents in spectrophotometric determinations of platinum(IV). Illustrative instances of such reagents comprise 2-nitrobenzaldehydethiocarbohydrazone [19], o-methylphenyl thiourea [20], p-methylphenyl thiourea [21], glyoxal (p-anisyl)-thiosemicarbazone [22], 4-(4′-nitrobenzylideneimino)-3-methyl-5-mercapto-1,2,4-triazole [23], thiosemicarbazone containing benzoaldehyde in the presence of a 4-[N,N-(diethyl)amino] group [24]. Although there have been descriptions of mercapto carboxylic acids [25] and leuco xylene cyanol FF [26] but high selectivity reagents have been reported only rarely. Table 1 displays the outcomes of the comparative analysis between the recently developed methodology and established techniques utilized for the extractive identification of platinum(IV).
By employing 2-(2-(1-thiophene-2-yl) ethylidene) hydrazinyl) benzoic acid (TEHBA) as an innovative reagent, this study aims to develop a spectrophotometric methodology for the detection of platinum(IV) at a micro level. A straightforward, discerning and quick methodology for the separation and spectrophotometric quantification of platinum(IV) is described in the current study. Additionally, this methodology exhibits notable levels of sensitivity, selectivity and precision. Furthermore, the research displays the feasibility of the established methodology in terms of platinum separation and quantification across an extensive range of matrices, including an authentic sample.
Experimental
Instrumentation
The absorbance measurements were conducted using quartz cells with a diameter of 1 cm and a digital spectrophotometer model SL 244 manufactured by ELICO. To conduct the weighing, a Contech model CA-123 electronic balance was utilized. After undergoing the calibration process, the glassware underwent a cleaning procedure which entailed submerging it in a nitric acid solution that had been acidified, followed by two rinses with distilled water.
Reagents
A generic solution of platinum(IV) was made via dissolving 1.0 g of platinum chloride (H2PtCl6⋅xH2O) in 20 cm3 of hydrochloric acid solution (1.0 mol dm−3). Subsequently, this solution was diluted to the designated volume in a graduated flask comprising 250 cm3 of water, until it achieved gravimetric standardization [36]. The working solution of platinum(IV) with a concentration of 50 μg cm−3 was produced via dilution of a generic solution with distilled water. The chromogenic reagent 2-(2-(1-(thiophene-2-yl) ethylidene) hydrazinyl) benzoic acid (TEHBA) (Fig. 1) was synthesized following the methodology that was proclaimed [37]. To prepare the stock solution of the reagent, which had a concentration of 0.1 mol dm−3, 0.65 g of TEHBA dissolved in 25.0 cm3 of dimethylformamide (DMF). A TEHBA working reagent solution with a concentration of 0.001 mol dm−3 was prepared in DMF through the dilution of the stock solution to the desired degree. To conduct interference analysis, a multitude of metal ions were employed. Prior to preparing these solutions, the corresponding compounds were dissolved in diluted hydrochloric acid or distilled water and the resultant mixture was subsequently diluted to the desired concentration. Alkali metals were produced by dissolving metal ions in water that had undergone double distillation. Furthermore, interfering ion solutions were prepared to facilitate investigations into their applications.
Recommended method
A mixture of 50 μg Pt(IV), 2 cm3 of 0.001 mol dm−3 TEHBA reagent and an adequate volume of potassium iodide solution (0.1 mol dm−3) was transferred in a standard graduated flask with a capacity of 10 cm3 and diluted to desired level with distilled water. This solution was retained in the hot water bath for 5 min. After heating, once the mixture had reached room temperature, it was transferred into a 125 cm3 separatory funnel and equilibrated for ten seconds with 10 cm3 of cyclohexanone. Following the unimpeded separation of the two layers, the organic layer contained the platinum(IV)-TEHBA complex was characterized by its yellow hue. The organic layer was dehydrated with anhydrous sodium sulfate. After dehydration it was added to a 25 cm3 graduated flask. To achieve the desired concentration, cyclohexanone was utilized. Upon comparison with the reagent blank, which was prepared in a similar way, the absorbance wavelength of the Pt(IV)-TEHBA complex was greatest at 420 nm.
Result and discussion
Absorption spectra
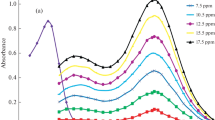
The absorption wavelength of the yellow Pt(IV)-TEHBA complex was ascertained greatest at 420 nm, whereas the reagent blank did not demonstrate any impact at this specific wavelength. This indicates that only one compound was generated under these experimental conditions, as the absorption curve remains unchanged (Fig. 2).
Effect of potassium iodide concentration
Formation of the Pt(IV)-TEHBA complex is highly dependent on the potassium iodide concentration. Platinum(IV) forms chelate with reagent (TEHBA), forms ionic unstable chelate and iodide make it stable is the role of potassium iodide. A KI concentration was varied throughout the duration of the investigation from 0.01 to 0.2 mol dm−3. Rise in KI concentration results in a corresponding rise in absorbance; nevertheless, beyond 0.1 mol dm−3, there is no substantial alteration observed. Consequently, a KI concentration of 0.1 mol dm−3 was employed for subsequent investigations (Fig. 3).
Effect of extraction solvents
To determine the suitability of various solvents for extracting complexes quantitatively toulene, xylene, ethyl acetate, n-butanol, cyclohexane, isoamyl alcohol, chloroform and cyclohexanone were evaluated as potential extraction solvents. Cyclohexanone was identified as the most appropriate choice based on its observation of the greatest complex absorbance at 420 nm (Fig. 4).
Effect of reagent (TEHBA) concentration
It was determined that a concentration of 0.001 mol dm−3 of TEHBA in DMF was sufficient to induce the formation of a complex between Pt(IV) and TEHBA. Additionally, it was observed that further adjustments to the concentration of the reagent did not produce any statistically notable deviation from the result. Considering the findings presented, it has been confirmed that the optimal concentration of TEHBA in DMF for the ongoing inquiry is 0.001 mol dm−3 (Fig. 5).
Effect of heating time
By heating the reaction mixture to a temperature of 70 °C for a duration of 1–30 min, the Pt(IV)-TEHBA complex was formed in 5 min of heating time. The absorption remains unchanged in the presence of supplementary heat. As a result, the heating period utilized for the entirety of the investigation was 5 min (Fig. 6).
Analytical figures of merit
Beer's law was observed to be adhered within a concentration range of 10–50 μg cm−3(Fig. 7). Following determination, the molar absorption coefficient and Sandell's sensitivity were assessed and discovered to be 1.9 × 103 dm3 mol−1 cm−1 and 0.010 μg cm−2, correspondingly. The value of the correlation coefficient (R2) for the Pt(IV)-TEHBA complex was determined to be 0.99. The intercept value of the line was 0.005 and the slope was 0.010. Consequently, the quantification of Pt(IV) in samples can be achieved through the utilization of the linear equation y = 0.010x + 0.005. Ringbom's plot exhibited a sigmoid form, distinguished by a rectilinear segment spanning intermediate absorbance values of 20–45 μg cm−3. The slope value of the graph was 0.6904. The graph of the logarithm of the platinum(IV) concentration (C) relative to (1 − T), (where T represents the transmittance values) was identified as the optimal concentration range (Fig. 8). A summary of the analytical parameters that were executed previously is presented in Table 2.
Stoichiometry of Pt(IV)-TEHBA complex
The Jobs continuous variation method was employed to determine the stoichiometry of the Pt(IV)-TEHBA complex. The objective was achieved through the utilization of a concentration versus absorbance to represent the ratio of metal concentration to the sum of metal concentration and ligand (Fig. 9). In the mole ratio approach, the concentration of metal to ligand is plotted against absorbance which provides additional support for this discovery (Fig. 10). As a consequence, it is highly probable that the extracted compound has 1:1 stoichiometry.
Interference study
An investigation was undertaken to assess the effects of several interfering ions on the process of separating and quantifying the Pt(IV)-TEHBA complex. The selective nature of the method was demonstrated by the relative error of ± 0.2% in the detection of platinum(IV) concentration (50 μg cm−3). The proposed methodology illustrates the upper limit of tolerance for a specified quantity of foreign ions. The efficacy of EDTA in eliminating interference caused by chromium(III) and cobalt(II) was ascertained, while tartrate demonstrated success in eliminating interference caused by thallium(III) (Table 3).
To the solution containing 50 μg cm−3 of Pt(IV), different amounts of foreign ions were introduced (100 mg for anions and 10 mg for cations). At first, a significant amount of foreign ion was added to 10 cm3 graduated flask containing Pt(IV) solution and as per the recommended procedure, 2 cm3 of 0.001 mol dm−3 TEHBA reagent and adequate volume of potassium iodide solution (0.1 mol dm−3) were added and diluted to the desired level with distilled water. This solution was heated in hot water bath for 5 min. After heating and extraction in cyclohexanone, the absorption measurements were carried out at 420 nm. When interference was strong, the trials were repeated with gradually lower amounts of foreign ion. The proposed methodology illustrates the upper limit of tolerance for a specified quantity of foreign ions. Interference caused by chromium(III) and cobalt(II) was removed by masking agent EDTA, while tartrate was also used as a masking agent for eliminating interference caused by thallium(III). The masking agents, EDTA and tartrate were added to the working solution before the addition of the reagent solution. The masking agents form complexes with the interfering metal ions for preventing them from complexing with TEHBA, due to this interference removed.
Detection limit, accuracy and precision
To evaluate the precision and repeatability of the proposed methodology, the absorbances of ten sample combinations that exhibited high similarity to each other were measured. A set of five measurements was compiled for the purpose of determining the mean and relative standard deviation. The relative value of the standard deviation was 0.35%. From the results, it was concluded that the presented approach was precise, selective, and accurate. The platinum(IV) limit of detection (LOD) was 0.023 μg cm−3. A relationship was measured between the quantity and the limit of quantitation (LOQ) by using the standard deviation.
Applications
Extraction of platinum(IV) from binary synthesized mixtures
The devised methodology exhibits the capacity to effectively facilitate the spectrophotometric detection and separation of platinum(IV) from blended components. Manganese(II), nickel(II), magnesium(II) and cobalt(II) were detected in the aqueous phase after the extraction of platinum(IV). For the evaporation of the aqueous layer, 3 cm3 of concentrated hydrochloric acid were utilized. After the solution had cooled, conventional techniques were employed to analyze the metal ions that had been combined with the dissolved residue in water [38] (Table 4).
Extraction of platinum(IV) from ternary synthesized mixtures
50 μg of Pt(IV) solution and a specific amount of other metal ions were mixed together. The developed procedure was applied to separate and determine the platinum(IV). The findings that were observed were precise and platinum-selective (Table 5).
Extraction of platinum(IV)from synthesized mixtures of alloys
The utilized methodology was designed for the purpose of extractive spectrophotometric identification of platinum(IV) from synthesized mixtures that represent alloys. As a result of the formation of the various alloys, assortments of synthetic combinations were generated. The Oakay alloy, the platinum alloy, the iridium mineral and the platinum–iridium alloy were prepared as per their composition. The process of micro-level separation of platinum(IV) was conducted following the conventional methodology (Table 6).
Extraction and separation of platinum(IV) from pharmaceutical sample
By isolating platinum from cisplatin injections of actual samples, the applicability of the proposed method was assessed. 4 cm3 of hydrochloride acid and 4 cm3 of 1 mg cm−3 of cis-platin were mixed together. This mixture was evaporated by heating. After the evaporation of the acid contained within, again 2 cm3 of hydrochloric acid was reintroduced to obtain the residue. The residue that was left over after the acid underwent evaporation was dissolved in a 10 cm3 graduated flask [39]. The platinum was isolated and analyzed using the proposed methodology. It was determined that at 420 nm mixture exhibited the greatest absorption. The obtained results were selected and found to be in outstanding agreement with the standard value specified on the product (Table 7).
Sequential extraction of palladium(II), platinum(IV) and nickel(II)
A procedure was developed to separate, extract and quantify palladium(II), platinum(IV) and nickel(II) as shown in Scheme 1. Palladium(II) was separated in cyclohexanone by using 2 cm3 of 0.001 mol dm−3 TEHBA in the presence of an acidic buffer medium. Concurrently, platinum(IV) and nickel(II) were evaporated which were present in the aqueous layer. After the remnant underwent evaporation, it was subsequently dissolved in water. For platinum(IV) extraction, 1 cm3 of KI (0.1 mol dm−3) and 2 cm3 of TEHBA reagent (0.001 mol dm−3) in DMF was transferred to the flask and was diluted to 10 cm3 using water. After heating and extracted in cyclohexanone, the final detection wavelength of the Pt(IV)-TEHBA complex was 420 nm. The nickel(II)-containing aqueous solution was evaporated until it became moist desiccated. Following the dilution of the residue in 10 cm3 of distilled water, nickel(II) was ascertained using a spectrophotometer [38] (Table 8).
Conclusion
A stable complex is formed when TEHBA and platinum(IV) are introduced into KI media. In contrast to the aforementioned spectrophotometric methodologies, the developed approach distinguishes itself through its efficacy in platinum(IV) separation, extraction, and quantification. For the procedure to be successful, a low reagent concentration (0.001 mol dm3) and a brief equilibrium period are required. Platinum(IV) has been successfully isolated and quantified from synthetic alloys, genuine samples, binary and ternary mixtures and synthetic alloys using the methodology that was developed. The successive metal extraction of palladium(II), platinum(IV) and nickel(II) was accomplished.
Data availability
Data are accessible on request.
References
J.D. Lee, Concise Inorganic Chemistry (Blackwell Science Ltd, Oxford, 1996)
G.C. Bond, F.R. Hartley, Chemistry of the Platinum Group Metals: Recent Developments (Elsevier, Amsterdam, 1991)
A.R. Pillai, P.P. Ouseph, K.K. Ramachandran, Indian J. Chem. 36, 1103–1105 (1997)
B. Anilanmert, G. Yalçin, F. Arioz, E. Dolen, Anal. Lett. 34, 113–123 (2001)
H. Calvert, Inorganica Chim. Acta 498, 118987 (2019)
P. Comella, R. Casaretti, C. Sandomenico, A. Avallone, L. Franco, L. Ther. Clin. Risk Manag. 5, 229–238 (2009)
M. Shimada, H. Itamochi, J. Kigawa, Cancer Manag Res 5, 67–76 (2013)
M.J. McKeage, Expert Opin. Investig. Drugs 10, 119–128 (2001)
J.W. Lee, J.K. Park, S.H. Lee, S.Y. Kim, Y.B. Cho, H.J. Kuh, Anticancer Drugs 17, 377–384 (2006)
A.V. Dyachkova, T.M. Malutina, T.Y. Alekseeva, Y.A. Karpov, Inorg. Mater. 48(14), 1272–1278 (2012)
P. Petrova, S. Velichkov, N. Velitchkova, I. Havezov, N. Daskalova, Spectrochim. Acta B At. Spectrosc. 65(2), 130–136 (2010)
K. Yamada, N. Kato, A. Takagi, M. Koi, H. Hemmi, Anal. Bioanal. Chem. 382, 1702–1707 (2005)
N.N. Meeravali, K. Madhavi, R. Manjusha, S.J. Kumar, Talanta 118, 37–44 (2014)
B. Godlewska-Żyłkiewicz, J. Malejko, B. Leśniewska, A. Kojło, Mikrochim. Acta 163, 327–334 (2008)
J. Ye, S. Liu, M. Tian, W. Li, B. Hu, W. Zhou, Q. Jia, Talanta 118, 231–237 (2014)
B. Rietz, K. Heydorn, A. Krarup-Hansen, Trace Elements Electrocytes 19(1), 38–41 (2002)
A.R. Timerbaev, A. Küng, B.K. Keppler, J. Chromatogr. A 945(1–2), 25–44 (2002)
Z. Huang, A.R. Timerbaev, B.K. Keppler, T. Hirokawa, J. Chromatogr. A 1106(1–2), 75–79 (2006)
S.B. Zanje, A.N. Kokare, V.J. Suryavanshi, G.D. Kore, B.T. Khogare, M.A. Anuse, J. Trace Elements Anal. 24(1), 1–24 (2016)
S.R. Kuchekar, Y.S. Shelar, S.H. Han, Braz. J. Anal. Chem. 3(10), 421–428 (2012)
S.R. Kuchekar, S.D. Bhumkar, H.R. Aher, S.H. Han, Anal. Chem. Lett. 9(6), 775–788 (2019)
M. Eliyas, Oxid. Commun. 41(1), 161–175 (2018)
A.B. Shaikh, U.B. Barache, A.S. Lawand, G.S. Kamble, M.L. Gaur, A.H. Gaikwad, Spectrochim. Acta-A Mol. Biomol. Spectrosc. 285, 121918 (2023)
P.P. Naik, J. Karthikeyan, A.N. Shetty, Environ. Monit. Assess. 171, 639–649 (2010)
G. Crisponi, F. Cristiani, V. Nurchi, R. Pinna, T. Pivetta, Polyhedron 19(24–25), 2435–2440 (2000)
H. Revanasiddappa, K.T. Kumar, Anal. Bioanal. Chem. 375, 319–323 (2013)
A.O. Dhokte, M.K. Lande, B.R. Arbad, J. Korean Chem. Soc. 56(1), 58–61 (2012)
A.A. Amirh, Jordan J. Chem. 2(2), 183–197 (2007)
A.S. Gupta, V.D. Barhate, Orient. J. Chem. 26(1), 70–73 (2009)
P. Ratre, D. Kumar, Int. J. Emerg. Technol. Comput. Appl. Sci. 5(4), 421–429 (2013)
M.I. Toral, P. Richter, N. Lara, M.T. Escudero, C. Soto, Anal. Lett. 33(1), 93–109 (2000)
Y. Terada, A. Harada, K. Saito, S. Murakami, A. Muromatsu, Bunseki Kagaku 52(9), 725–730 (2003)
A.S. Amin, M.Y. Nassar, Anal. Chem. Lett. 7(1), 128–141 (2017)
Y.R. Bazel, T.A. Kulakova, Y.I. Studenyak, R. Serbin, S. Rednik, V. Andruch, J. Anal. Chem.m. 67, 519–526 (2012)
D. Ma, Y. Li, K. Ma, J. Li, J. Chen, J. Yan, Y. Wang, Talanta 53(5), 937–941 (2001)
N.H. Furman, J.W. Frank, Standard Methods of Chemical Analysis, 6th edn. (Robert E. Krieger Publishing Company, Florida, 1962)
P.V. Rao, S. Ammanni, S. Gajula, J. Ind. Council. Chem. 28(1), 95–101 (2011)
E.B. Sandell, Colorimetric Determination of Traces of Metals, 3rd edn. (Interscience Publishers, New York, 1958)
M.Y. Khuhawar, G.M. Arain, Talanta 66(1), 34–39 (2005)
Acknowledgements
The Pravara Rural Education Society, the department's management, is appreciated by the writers for supplying the facilities that are required.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murkute, A., Aher, H., Bhumkar, S. et al. Rapid spectrophotometric determination and extraction of platinum(IV) from pharmaceuticals assisted by 2-(2-(1-(thiophene-2-yl) ethylidene) hydrazinyl) benzoic acid (TEHBA). ANAL. SCI. 40, 1765–1777 (2024). https://doi.org/10.1007/s44211-024-00612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-024-00612-9