Abstract
In this study, octanoic acid (OA) was used as an extraction solvent for the pre-concentration and determination of three antibiotic drugs (levofloxacin, metronidazole, and tinidazole) in urine samples. To extract the antibiotic drugs, a green solvent was used as the extraction solvent in the continuous sample drop flow microextraction method, followed by a high-performance liquid chromatography photodiode array detector. According to the findings, the present study offers an environmentally friendly analytical method with a high capacity for the microextraction of the antibiotic drugs at very low concentrations. The calculated detection limits were 6.0–10.0 µg/L and the linear range was found between 20 and 780 µg/L. The proposed method showed excellent repeatability with the RSD values ranging from 2.8 to 5.5%. The relative recoveries were between 79.0 and 92.0% in the urine samples with spiked levels of 40.0–100.0 µg/L for metronidazole and tinidazole, and 100.0–200.0 μg/L for levofloxacin.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of antibiotic drugs in the 1930s, a true medical revolution occurred, reducing the death rate caused by bacterial infections [1]. However, despite their valuable role in infection control and treatment [2], antibiotics can also be harmful to human and animals [3, 4]. Unfortunately, antibiotics are very stable chemicals and the majority of them will end up in wastewater without any major chemical change.
Looking at the literature, a number of different analytical techniques such as liquid chromatography (LC) [5], UV-spectrophotometry [6], capillary electrophoresis (CE) [7], gas chromatography (GC) [8], and high-performance liquid chromatography (HPLC) [9] have been used for the quantification of antibiotics in various samples. However, their application to environmental and biological samples is often very challenging, which is largely due to the complexity of the matrix as well as low concentrations. So, appropriate extraction and cleanup techniques are essentially required to remove the unwanted species and increase the antibiotics concentration before their quantification [10]. Solvent extraction methods are the most popular extraction methods due to their simplicity, ease of operation and low cost. However, they use a lot of organic solvents, which conflicts with the green chemistry goals. To reduce the solvent consumption, liquid-phase microextraction (LPME) techniques that require a significantly smaller volume of organic solvents were developed [11]. Different LPME methods have been used to extract antibiotic drugs in different samples, for example, deep eutectic solvent liquid–liquid microextraction (DES-LLME) for determination of fluoroquinolones in milk, honey and water samples [12], salting out-assisted dispersive liquid–liquid microextraction based on deep eutectic solvent for determination of levofloxacin in urine sample (SO-DLLME-DES-BE) [12]. However, these methods suffer from long extraction times and toxicity of organic solvents.
In this study, a liquid-phase microextraction method known as continuous sample drop microextraction (CSDF-ME) was used to extract three different antibiotic drugs. This technique was initially introduced by Moinfar and co-worker for the extraction of organic compounds in water sample in 2014 [13], and since then, it has been successfully applied to a variety of applications such as determination of organophosphorus compounds (OPPs) from different samples [14, 15] and the determination of metal ions in water samples [16, 17]. CSDF-ME is a semi-automated technique that offers high enrichment factors and great precision with a small amount of organic solvent. Octanoic acid is a medium-chain fatty acid found in palm oil, coconut oil, and human and animal milk. It is also part of medium-chain triglyceride (MCT) products. In this study, for the first time, we used octanoic acid which is a green solvent as the extraction solvent for extraction of three antibiotic drugs including levofloxacin, metronidazole, and tinidazole using CSDF-ME. The extracted drugs were subsequently determined by HPLC. Different experimental conditions such as pH, volume of extraction solvent, volume of sample, addition of salt, addition of acids, and flow rate were thoroughly investigated and the optimal conditions were determined. The proposed method was successfully applied for the extraction and quantification of the target antibiotics in urine samples and satisfactory results were achieved.
Experimental
Reagents and materials
Octanoic acid was sourced from Labpak (Helens, Liverpool, UK), the formic acid was supplied from Carl Roth (98.0%) (Karlsruhe, Germany) and glacial acetic acid (extra pure) was obtained from Scharlau (Barcelona, Spain). All HPLC solvents, including ultra-pure water, acetonitrile, dimethyl sulfoxide (DMSO), and methanol, were supplied from Merck (Darmstadt, Germany). Levofloxacin (98.0%), metronidazole (97.0%), and tinidazole (99.0%) antibiotic standards were bought from Sigma-Aldrich (Germany). Once a month, a standard solution of each antibiotic (1000 mg/L) was prepared in a mixture of water:acetonitrile (90:10 v/v) and stored at 4.0 °C. This solution was used as the stock solution and was diluted daily to make the working solutions.
Instrumentation
The selected antibiotics were all analyzed on a high-performance liquid chromatography photodiode array detector (HPLC–PDA) system (PerkinElmer, Clarus Flexar-15, USA) using a Thermo Scientific™ Hypersil GOLD™ C18 column (150.0 mm, 4.6 mm, 5.0 µm) conditioned at 37.0 °C. Methanol, ultra-pure water, and acetonitrile were used as mobile phase at a flow rate of 1.0 mL/min. The gradient mode was utilized to separate the analytes. The mobile phase was made up of two parts: part (A) water with 0.025% formic acid (v/v), and part (B) acetonitrile:methanol (60:40 v/v). The system was programmed to deliver the following linear gradient: start with 2.0 min of (92.0% A) and (8.0%B), then 2.5 min of (85.0%A) and (15.0%B), after that 3.5 min (80.0%A) and (20.0%B), next 3.0 min (75.0%A) and (25%B), then 3.0 min (70.0%A) and (30%B), finally 8.0 min (0%A) and (100%B). An autosampler system was used to inject a repeatable sample volume of 20.0 µL. The detector was a photodiode array and the separated analytes were detected at 320 nm. Peristaltic pump manufactured by Watson–Marlow in the UK was used in CSDF-ME.
Preparation of the sample
The urine samples were collected from several volunteers in Duhok City in Iraq. 3.2 mL of each sample was spiked with different amounts of standard solutions of the studied antibiotics. Then, the samples were diluted up to 15.0 mL using ultra-pure water and the pH of each solution was adjusted at 11 with 0.1 M sodium hydroxide. The prepared samples were filtered using paper filters before they were introduced into CSDF-ME.
Pre-concentration and extraction method
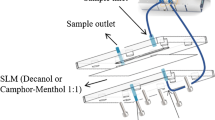
15.0 mL of urine samples prepared according to "Preparation of sample" was pumped at a flow rate of 0.35 mL/min using a peristaltic pump into a thin needle at the top of a conical vial that was placed with 65.0 µL of OA (Fig. 1). Because OA is lighter than water, it floats on the water surface in the conical part of the extraction vial. Urine sample droplets pass through it and exit through the waste part. The antibiotic drugs were extracted and concentrated in the green solvent (OA), while the urine sample passed through the organic solvent. After extraction, 45.0 μL of the remaining solvent was transferred into a micro-vial, diluted with 20.0 μL of methanol, and introduced into the autosampler of HPLC.
Result and discussion
Optimization of the microextraction condition
Different factors impacting the extraction efficiency such as pH, salt addition, volume of extraction solvent, flow rate of sample, and the volume of the sample were optimized.
The effect of salt addition
Different amounts of NaCl, ranging from 0 to 14% (w/v), were added to the sample solution to see the difference in ionic strength when it changed. The findings confirmed that the extraction efficiency of the proposed method was unaffected by the inclusion of NaCl in the process. As a result, no salt was added during antibiotics extraction.
The effect of sample pH
Different pH values ranging from 3 to 12 were tested to study the impact of sample pH on the extraction efficiency. Different volumes of 0.05 M HCl and NaOH were used to adjust the pH. It was revealed that the peak area increased by raising the sample pH [18,19,20,21]. However, the pH effect was more profound in levofloxacin extraction and the highest signal was observed at pH 11. For metronidazole and tinidazole, the highest impact was between pH 3 and 8, and afterward the signal did not change too much. So, the pH of 8 was considered as the optimal pH for these two antibiotics (Fig. 2).
The effect of sample flow rate
Sample flow rate has a significant effect on both extraction efficiency and extraction time. So, to find the optimal flow rate, different flow rates ranging from 0.35 to 0.55 mL/min were examined. As shown in Fig. 3, increasing the sample flow rate will decrease the analytical signal [22].This can be attributed to the short analyte exposure to the organic solvent at higher flow rates. Therefore, 0.35 mL/min was chosen as the optimal sample flow rate.
The effect of sample volume
The effect of sample volume on the extraction efficiency was investigated using different volumes ranging 5–20 mL. As indicated in Fig. 4, for all studied drugs, the peak area constantly increased by increasing the sample volume up to 15.0 mL. However, further increase in the sample volume, led to a considerable reduction in the peak area which can be attributed to the partial dissolution of the green solvent in water and hence reduced extractability. Therefore, the volume of 15 mL was selected to continue the rest of the study.
The effect of extraction solvent volume
To find the optimal solvent volume, different volumes of OA ranging from 35.0 to 80.0 µL were examined. It is important to note that the autosampler vials need to have a minimum volume of (60.0–65.0) µL. After each extraction, the remaining solvent was diluted up to 65.0 μL. It was revealed that the peak area would increase by increasing the solvent volume for all three antibiotics (Fig. 5). On the other hand, the peak areas for all studied drugs remained relatively unchanged at volumes beyond 65.0 μL. So, volumes higher than 80.0 μL were not investigated.
The effect of acid addition
Three different acids were optimized [Formic acid, Acetic acid, and Dimethyl sulfoxide (DMSO)]. Each acid was added to the extraction solvent at various volumes ranging from 1.5 to 15.0 µL. The extraction efficiency was found to increase on increasing the acid amount up to 11.0 µL, and afterward it gradually decreased. On the other hand, it was revealed that DMSO addition had the biggest impact on the extraction efficiency of all studied drugs (Fig. 6A, B). So, 11 µL of DMSO was added to the extraction solvent to obtain the highest extraction efficiency in the following experiments.
Effect of acid addition on the extraction efficiency of levofloxacin, metronidazole, and tinidazole in diluted urine sample, A acid type, B volumes of DMSO. Extraction conditions: antibiotic drug concentration, 500.0 µg/L; volume of sample, 15.0 mL; flow rate of sample solution, 0.35 mL/min; extraction solvent 65.0 μL; pH 11.0
Validation of analytical method
The evaluation of the analytical performance of the CSDF-ME method has been summarized in Table 1. The limits of detection (LOD) and quantification (LOQ) were based on the signal-to-noise ratio (S/N = 3 and S/N = 10). The coefficient of determination (R2) was greater than 0.98 for all three antibiotic drugs; the linear range of the calibration curve is shown in Table 1. The relative standard deviations for intra- and inter-day were calculated at low, medium, and high concentrations. The enrichment factor for 15.0 mL and the relative recovery of antibiotics in urine were determined and are listed in Table 1. All experiments were repeated three times.
The enrichment factor (EF) was calculated using the following equation:
where CUrine and Corg. are the concentration of antibiotic drugs in aqueous phase and concentration of antibiotic drugs in the organic solvent, respectively.
The relative recovery (RR) was estimated using the following equation:
where Cfound, Creal, and Cadded are the concentrations of antibiotic drugs after addition of antibiotic drugs standard solution into samples, initial concentration of antibiotic drugs in real samples, and the concentration of standard solutions of antibiotic drugs added to the samples, respectively.
The matrix effect (ME%) was evaluated by analyzing water and urine samples under optimized conditions by the following equation:
where As, An, and Aw are the peak areas of the analyte in the spiked, non-spiked samples, and spiked ultra-pure water after extraction when CSDF-ME method was used at concentrations of 400.0 μg/L. The results are summarized in Table 1. As it can be seen, the matrix effect on the extraction of antibiotic drugs in the urine sample was low for levofloxacin and almost negligible for metronidazole and tinidazole [23] (Fig. 7).
Real sample analysis
The antibiotic drug levels were quantified in two different urine samples using the proposed method. One sample was collected from a volunteer who had not taken any antibiotics in the last 1 month (healthy sample) and the other sample was collected from a person who had taken the antibiotics at the prescribed dosage, 7 h after taking the drug (unhealthy sample). Prior to the extraction by CSDF-ME, the samples were treated according to the procedure described in "Preparation of sample". Then, each sample was analyzed using the proposed method at optimal condition. As it can be seen in Table 2, the resulting relative recoveries were between 79 and 99%. There is a small difference between the added and found analyte values using the present method. This indicates that the components in the urine sample and the density of the urine samples do not affect the performance of this developed method. Therefore, the OA-CSDF-ME-HPLC method is effective for the determination of target drugs in urine samples.
Comparison of CSDF-ME with other methods
To highlight the advantages of the presented CSDF-ME method, a comparison with other similar works for the determination of levofloxacin, metronidazole and tinidazole is summarized in Table 3. As it can be seen, the extraction time of the proposed method is comparable with the other methods, particularly, DES-LLME, LLE, SO-DLLME-DES-BE, DES-HLLME and LLME. Given the RSD values, the method precision is also superior to that of other methods. Besides, it provides acceptable recoveries for all studied drugs. In addition, the method complexity is much lower than the other techniques as many conventional devices such as vortex, centrifuge and shaker were not needed for the extraction process.
Conclusion
In this study, octanoic acid, which is a known green solvent, was used as an extraction solvent for pre-concentration and extraction of three common antibiotic drugs from urine samples. The antibiotic drugs were extracted using continuous sample drop flow microextraction, followed by HPLC–PDA analysis. The use of OA as the extraction solvent had several advantages which makes the proposed method very interesting for routine analysis of antibiotics. Firstly, the toxicity of the extraction process remarkedly decreased as only a few microliters of a green solvent (OA) were required to perform the extraction. Secondly, the extraction efficiency significantly increased for some of the studied drugs, enabling their detection at very low concentrations. Besides, the method precision was very high, the extraction time was very short and the target analytes were recovered at acceptable rates. The authors believe that the proposed method has a great potential for trace analysis of toxic chemicals not only in clinical samples, but also in the environmental samples with complex matrices such as wastewater.
Data availability
Data available based on the request.
References
C. Kirchhelle, Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun. 4(1), 1–13 (2018)
A. Bryce, A.D. Hay, I.F. Lane, H.V. Thornton, M. Wootton, C. Costelloe, Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. Bmj, 352 (2016)
J. Bonnedahl, P. Drobni, A. Johansson, J. Hernandez, Å. Melhus, J. Stedt, B. Olsen, M. Drobni, Characterization, and comparison, of human clinical and black-headed gull (Larus ridibundus) extended-spectrum β-lactamase-producing bacterial isolates from Kalmar, on the Southeast Coast of Sweden. J. Antimicrob. Chemother. 65(9), 1939–1944 (2010)
A.M. Radwan, N.E. Ahmed, L.M. Elakabawy, M.Y. Ramadan, R. Elmadawy, Prevalence and pathogenesis of some filarial nematodes infecting donkeys in Egypt. Vet. World 9(8), 888 (2016)
R. Pascale, G. Bianco, D. Coviello, M. Cristina Lafiosca, S. Masi, I.M. Mancini, S.A. Bufo, L. Scrano, D. Caniani, Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 43(5), 886–895 (2020)
S. Jaskaran, U. Himanshi, S.K. Shukla, S. Madhulika, K. Suraj, UV spectrophotometric detection of carbapenem antibiotics in forensic samples. J. Indian Acad. Forensic Med. 40(2), 179–183 (2018)
T.N.M. Pham, T.B. Le, D.D. Le, T.H. Ha, N.S. Nguyen, T.D. Pham, P.C. Hauser, T.A.H. Nguyen, T.D. Mai, Determination of carbapenem antibiotics using a purpose-made capillary electrophoresis instrument with contactless conductivity detection. J. Pharm. Biomed. Anal. 178, 112906 (2020)
B. Chiavarino, M.E. Crestoni, A. Di Marzio, S. Fornarini, Determination of sulfonamide antibiotics by gas chromatography coupled with atomic emission detection. J. Chromatogr. B Biomed. Sci. Appl. 706(2), 269–277 (1998)
S. Yudthavorasit, C. Chiaochan, N. Leepipatpiboon, Simultaneous determination of multi-class antibiotic residues in water using carrier-mediated hollow-fiber liquid-phase microextraction coupled with ultra-high performance liquid chromatography tandem mass spectrometry. Microchim. Acta 172(1), 39–49 (2011)
S.A. Khatibi, S. Hamidi, M.R. Siahi-Shadbad, Application of liquid-liquid extraction for the determination of antibiotics in the foodstuff: recent trends and developments. Crit. Rev. Anal. Chem. 52(2), 327–342 (2022)
A. Sarafraz-Yazdi, A. Amiri, Liquid-phase microextraction. TrAC Trends Anal. Chem. 29(1), 1–14 (2010)
K. Yu, M.-E. Yue, J. Xu, T.-F. Jiang, Determination of fluoroquinolones in milk, honey and water samples by salting out-assisted dispersive liquid-liquid microextraction based on deep eutectic solvent combined with MECC. Food Chem. 332, 127371 (2020)
S. Moinfar, G. Khayatian, M.-R. Milani-Hosseini, Continuous sample drop flow-based microextraction method as a microextraction technique for determination of organic compounds in water sample. Talanta 129, 309–314 (2014)
S. Moinfar, M.-R.M. Hosseini, Development of dispersive liquid-liquid microextraction method for the analysis of organophosphorus pesticides in tea. J. Hazard. Mater. 169(1–3), 907–911 (2009)
S. Moinfar, A. Khodayari, S. Sohrabnezhad, A. Aghaei, L.A. Jamil, MIL-53 (Al)/Fe2O3 nanocomposite for solid-phase microextraction of organophosphorus pesticides followed by GC-MS analysis. Microchim. Acta 187(12), 1–10 (2020)
S. Moinfar, G. Khayatian, Continuous sample drop flow-based microextraction combined with graphite furnace atomic absorption spectrometry for determination of cadmium. Microchem. J. 132, 293–298 (2017)
M. Ezati, S. Moinfar, S. Mohammadi, G. Khayatian, A continuous sample drop flow-based microextraction method for spectrophotometric determination of cobalt with 1-(2-pyridylazo)-2-naphthol in water samples. J. Anal. Chem. 76(2), 172–179 (2021)
W. Ma, K.H. Row, PH-induced deep eutectic solvents based homogeneous liquid-liquid microextraction for the extraction of two antibiotics from environmental water. Microchem. J. 160, 105642 (2021)
H. Zhao, S. Hou, X. Zhao, D. Liu, Adsorption and PH-responsive release of tinidazole on metal-organic framework CAU-1. J. Chem. Eng. Data 64(4), 1851–1858 (2019)
K. Alagumalai, R. Shanmugam, S.-M. Chen, S.M. Babulal, A. Periyalagan, Novel electrochemical method for detection of cytotoxic tinidazole in aqueous media. Process Saf. Environ. Prot. 148, 992–1005 (2021)
R.R. Jha, N. Singh, R. Kumari, D.K. Patel, Ultrasound-assisted emulsification microextraction based on a solidified floating organic droplet for the rapid determination of 19 antibiotics as environmental pollutants in hospital drainage and Gomti river water. J. Sep. Sci. 40(13), 2694–2702 (2017)
S. Moinfar, L.A. Jamil, H.Z. Sami, Determination of organophosphorus pesticides in juice and water by modified continuous sample drop flow microextraction combined with gas chromatography-mass spectrometry. Food Anal. Methods 13(5), 1050–1059 (2020)
L.A. Jamil, H.Z. Sami, A. Aghaei, S. Moinfar, S. Ataei, Combination of modified ultrasound-assisted extraction with continuous sample drop flow microextraction for determination of pesticides in vegetables and fruits. Microchem. J. 160, 105692 (2021)
W. Tang, Y. Dai, K.H. Row, Evaluation of fatty acid/alcohol-based hydrophobic deep eutectic solvents as media for extracting antibiotics from environmental water. Anal. Bioanal. Chem. 410(28), 7325–7336 (2018)
J. Emami, M. Rezazadeh, Rapid, sensitive, and validated HPLC method for analysis of metronidazole and tinidazole under identical chromatographic conditions with UV detection and liquid-liquid extraction: application in bioequivalence studies. Acta Chromatogr. 25(1), 111–125 (2013)
L.Y. Klimenko, G.L. Shkarlat, Z.V. Shovkova, O.V. Kolisnyk, Development and validation of HPLC/UV-procedures for quantification of metronidazole in the blood and urine. J. Org. Pharm. Chem. 17(66), 38–51 (2019)
S.A. Helmy, Simultaneous quantification of linezolid, tinidazole, norfloxacin, moxifloxacin, levofloxacin, and gatifloxacin in human plasma for therapeutic drug monitoring and pharmacokinetic studies in human volunteers. Ther. Drug Monit. 35(6), 770–777 (2013)
Acknowledgements
The authors would like to express their gratitude to the University of Zakho for their assistance with the project.
Author information
Authors and Affiliations
Contributions
ARY: methodology, validation, formal analysis, writing—original draft, writing the final draft. LAJ: supervision, investigation, funding acquisition, resources, writing—review and editing.
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yaqoub, A.R., Jamil, L.A. A new application of continuous sample drop flow microextraction using octanoic acid as a green extraction solvent for the determination of antibiotic drugs in urine samples. ANAL. SCI. 39, 893–900 (2023). https://doi.org/10.1007/s44211-023-00292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00292-x