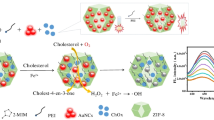
Abstract
When nanozymes are used in biological analysis, higher activity can improve the detection sensitivity, and better selectivity can eliminate other interference. To improve the specificity and sensitivity, we fabricated an innovative bioconjugated nanozyme with natural enzyme (BNNZ), in which natural ChOx was immobilized onto histidine-modified Fe3O4 (His–Fe3O4) with hydrophilic poly(ethylene glycol) (PEG) as a linker. ChOx could specifically catalyze the oxidation of cholesterol to generate H2O2 molecule, and then the newly formed H2O2 oxidized the colorless 3,3′,5,5′-tetramethylbenzidine (TMB) into blue ox-TMB by peroxidase-like His–Fe3O4. According to the above cascade reaction, the BNNZ-based colorimetric strategy was proposed for the detection of cholesterol. Wherein, natural enzymes specifically catalyzed substrates, which endowed BNNZ with excellent specificity for target molecules; meanwhile, the introduction of histidine on His–Fe3O4 effectively increased the peroxidase-like activity of BNNZ, which provided a guarantee for sensitivity. Furthermore, BNNZ after reaction could be rapidly separated by an external magnetic field without interfering with colorimetric quantitative detection. The proposed strategy exhibited excellent sensitivity with limit of detection of 0.446 μM and was successfully used for the detection of cholesterol in spiked human serum sample with recovery and relative standard deviation in the range of 97.9–103.5% and 2.5–4.0%, respectively. This work indicates that the bioconjugation of nanozyme and natural enzyme may be a universal strategy for synthesis of high-performance enzyme–nanozyme systems, and the new-type BNNZ will be widely used in biological detection and disease treatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanozymes are nanomaterials, which possess enzyme-mimicking activities and can catalyze the conversion of enzyme substrates to products [1]. Compared with natural enzymes, nanozymes show more excellent properties, including low cost, adjustable catalytic activity, high structural stability, unique surface chemistry, recyclability, and ease of large-scale preparation [2]. Additionally, they have been verified to mimic the catalytic activity of oxidase, peroxidase, catalase, haloperoxidase, uricase, hydrolase, glutathione peroxidase, methane monooxygenase, and superoxide dismutase [3]. Therefore, they have become potential substitutes and competitors of natural enzymes in recent years, and attracted extensive interest in the fields of bioanalysis, biomedicine, disease diagnosis, and climate and ecosystems management [4,5,6]. Since the pioneering work of Yan et al. reporting peroxidase-like activity of ferromagnetic (Fe3O4) nanoparticles in 2007 [7], many nanomaterials have been studied for their enzyme-mimicking activities, including metals [8], metal oxides [9], metal chalcogenides [10], metal–organic frameworks [11], nanocarbon materials [12], layered double hydroxides [13] and others [14].
Among them, magnetic iron oxide nanoparticles are of particular interest because of their appealing properties in terms of easy preparation, excellent biocompatibility, biodegradability, and rapid magnetic separation via a magnetic field [15]. So far, magnetic nanoparticles have been used in mimicking natural enzymes in three main different enzymatic reactions, namely peroxidases [16], oxidases [17] and catalases [18] for the oxidation of chromogenic substrates. Hence, magnetic nanoparticles have been widely employed as colorimetric sensors for the rapid detection of generous analytes [19]. However, they also have some inherent disadvantages, such as poor selectivity caused by multiple enzyme-mimicking activities and relatively weak enzyme-like activity.
In principle, better selectivity effectively eliminates internal and external interference, while higher activity reduces the limit of detection (LOD), which provides a solid guarantee for the good performance of the analytical method [20]. Inspired by the natural peroxidase, a heme cofactor-resembling Fe–N4 single-site-embedded graphene (Fe–N–rGO) has been fabricated and achieved a 700-fold improvement compared to the parent graphene [21]. Nevertheless, the synthesis undergoes high temperature (750 °C) in NH3 atmosphere [21], which is difficult to popularize on a large scale. Similarly, the introduction of functional groups similar to the active sites of natural enzymes can promote the contact between nanozymes and substrates, thus promoting the activity of nanozymes [22]. For example, histidine-modified Fe3O4 (His–Fe3O4) has been synthesized by a simple one-step solvothermal method [23]. The introduction of histidine improves the peroxidase-like activity of Fe3O4 nanozyme by enhancing the affinity between the imidazole group of histidine and H2O2 [23]. However, there are no reports on the integration of His–Fe3O4 and natural enzymes for bioanalysis.
Natural enzymes selectively catalyze substrates and produce specific products. For example, glucose oxidase (GOx) can catalyze the production of H2O2 from glucose. The H2O2 generated from oxidase would oxidize the colorless substrate into the colored oxidized substrate by peroxidase-like nanozymes. So, the integration of nanozyme and GOx endows the detection method with excellent specificity for target glucose molecules [24, 25]. Cholesterol has vital roles in immune system, brain synapses and protection against cancer [26,27,28], and serum cholesterol level plays important role in the diagnosis of various cardiovascular diseases, nephrosis, anemia and liver diseases [29, 30]. Previous studies have confirmed that cholesterol oxidase (ChOx) can selectively oxidize cholesterol to produce H2O2 [31]. Therefore, the colorimetric platform based on the coupling of ChOx and nanozyme with peroxidase property is expected to realize the colorimetric detection of cholesterol. Hitherto, various nanozymes have been developed for the colorimetric detection of cholesterol, including silver nanoparticles [32], Co-incorporated organic–inorganic hybrid nanoflowers [33], polypyrrole nanoparticles [34], CuO nanoparticles [35], Mo, S co-doped carbon quantum dot [36] and N, S-co-doped carbon/Co1-xS nanocomposite [37]. However, these methods have certain disadvantages, for example, the fabrication of nanozyme is tedious and time-consuming, and the nanozyme after reaction is difficult to separate and interferes with colorimetric quantification.
To overcome this shortcoming, we fabricated an innovative bioconjugated nanozyme with natural enzyme (BNNZ), in which His–Fe3O4 was prepared through a simple one-step solvothermal method, and then natural GhOx was immobilized onto His–Fe3O4 with hydrophilic poly(ethylene glycol) (PEG) as linker (Scheme 1A). To improve the performance of BNNZ, the functional group species and length of PEG linker were optimized in detail. Magnetic BNNZ not only possessed excellent peroxidase activity, but also could be rapidly separated without interfering with colorimetric quantitative detection after the reaction. Based on the cascade reaction, the BNNZ-based colorimetric strategy was proposed for ultrasensitive and specific detection of cholesterol. In brief, ChOx specifically catalyzed the oxidation of cholesterol to generate H2O2 molecules, and subsequently, the H2O2 oxidized the colorless TMB into blue ox-TMB by peroxidase-like His–Fe3O4 (Scheme 1B). Eventually, the BNNZ-based colorimetric strategy was successfully used in detection of cholesterol in human serum.
Experimental methods
Chemicals and materials
Cholesterol, cholesterol oxidase (ChOx), urate oxidase, 3,3′,5,5′-tetramethylbenzidine (TMB), uric acid, 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), o-phenylenediamine (OPD) and boric acid were supplied by Rhawn (Shanghai, China). Histidine, ethylene diamine tetraacetic acid (EDTA), N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were obtained from Macklin (Shanghai, China). Tritonx-100 was supplied by Elabscience Biotechnology Co., Ltd. Galactose, glucose, glycine, glutamic acid, tryptophan and serine were purchased from Bide Pharmatech Ltd. (Shanghai, China). Bovine serum albumin and 2-morpholinoethanesulfonic acid (MES) were obtained from Meryer (Shanghai, China). Amino- and carboxyl-containing homobifunctional PEG molecules, including NH2–PEG9, COOH–PEG9, COOH–PEG136 and COOH–PEG456, were supplied by Tianjin Welcome Science and Technology Development Co., Ltd. FeCl3·6H2O, glycol, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium acetate, dimethyl sulfoxide (DMSO), sodium hydroxide (NaOH), acetic acid, potassium chloride (KCl) and hydrogen peroxide (H2O2) were supplied by Tianjin Jiangtian chemical reagent Technology Co., Ltd.
Instrument
Transmission electron microscope (TEM) images were obtained from JEM-2100F TEM (JEOL) equipped with ESCALAB™ 250Xi + X-ray photoelectron spectroscopy (Thermo Fisher Scientific). Fourier transform infrared (FT-IR) spectrum analysis was carried out with a Nicolet 380 FT-IR spectrometer (Nicolet Instrument Co.). Ultraviolet and visible spectrophotometry (UV–vis) spectral analysis was performed on YU-1901 spectrophotometer (PERSEE). Particle size and zeta potential were obtained from Zetasizer Nano ZS90 (Malvern). Thermogravimetic analysis (TGA) was achieved on a TG209F3 thermogravimetric analyzer (NETZSCH). Magnetization behavior was characterized by a HH-15 Vibrating sample magnetometer (VSM, Nanjing NanDa Instrument Plant). Cyclic voltammetry assay was performed on an electrochemical workstation (CHI660E). Free radical was detected by an EMXplus ESR spectrometer (Bruker).
Fabrication of BNNZ
His–Fe3O4 nanoparticles were synthesized by the solvothermal method according to the previous report with minor modification [23]. In brief, 0.41 g FeCl3•6H2O, 1.8 g NaAc and 0.5 g histidine were dissolved in 20 mL ethylene glycol. The mixture was sealed in a 20 mL teflon-sealed autoclave and reacted at 200 °C for 12 h. After cooling to room temperature, the products were washed alternately with water and ethanol, and vacuum-dried at 50 °C overnight. Naked Fe3O4 nanoparticles were also synthesized following the same procedure in the absence of histidine.
Before covalent immobilization of ChOx, COOH–PEG9 was used for modification of His–Fe3O4. Typically, 5 mg COOH–PEG9 (molecular weight, 400) was dispersed in 5 mL MES buffer (pH = 6), and activated with 15 mg EDC and 30 mg NHS with gentle shaking at ambient temperature for 6.5 h. Subsequently, the activated PEG linker was reacted with 15 mg His–Fe3O4 under gentle shaking for 12 h. The PEG-functionalized His–Fe3O4 (His–Fe3O4@PEG) was washed with PBS (20 mM, pH = 7.4), and redistributed in 5 mL MES buffer (pH = 6). After adding 25 mg NHS for 1 h, the His–Fe3O4@PEG was mixed with 25 mg EDC and 5 mL ChOx PBS solution (1 mg mL−1) for 3 h. Finally, the His–Fe3O4@PEG@ChOx was washed with PBS and stored at – 20 °C.
Oxidase- and peroxidase-like activity of His–Fe3O4
The oxidase-like activity was evaluated by adding using 100 μg His–Fe3O4 into 0.7 mL Hac–NaAc buffer (0.1 M, pH 3.0) with 2.8 mM TMB. After incubating at 40 °C for 10 min, the His–Fe3O4 was separated by an external magnetic field. Then, the absorbance of supernatant at 652 nm was measured and used as an index to evaluate the oxidase-like activity of His–Fe3O4.
The peroxidase-like activity was further evaluated using 80 μg His–Fe3O4 with 2.0 mL reaction buffer (0.1 M Hac–NaAc buffer, pH 3.0) in the presence of H2O2 and TMB. The kinetic analysis of His–Fe3O4 with H2O2 as the substrate was carried out by varying the concentrations of H2O2 with 800 μM TMB, while the kinetic analysis with TMB as the substrate was performed by varying the concentrations of TMB with 250 μM H2O2. After the mixture solution was incubated at 40 °C for 10 min, the His–Fe3O4 was separated by an external magnetic field, and the absorbance of supernatant at 652 nm was measured by UV–vis absorption spectrophotometer. All the measurements were performed at least in triplicate, and the values were given as mean ± standard deviation (SD). The kinetic parameters were calculated based on the Michaelis–Menten equation as following:
where ν, Vmax, [S] and Km are initial velocity, maximal reaction velocity, concentration of substrate and Michaelis–Menten constant, respectively.
Mechanism investigation
The electrochemical behavior of His–Fe3O4 was studied by cyclic voltammetry. Briefly, 1 mg His–Fe3O4 was dispersed in 1 mL ethanol, and then 2 μL nafion solution was added. After ultrasonic treatment, 3 μL suspension was dropped on the surface of the glassy carbon electrode (GCE) and dried at room temperature. GCEs before and after treatment process were used as working electrodes. Platinum wire and Ag/AgCl electrodes were employed as auxiliary and reference electrodes, respectively. Hac–NaAc buffer (0.1 M, pH 3.5) deoxidized with N2 was selected as electrolyte solution. Finally, cyclic voltammetry assay was carried out on an electrochemical workstation.
5,5-Dimetheyl-1-pyrroline N-oxide (DMPO) was selected as a capture agent for the analysis of hydroxyl radicals (•OH) formed during following catalytic reaction. The Hac–NaAc buffer (pH 3, 0.2 M), including 20 μg mL−1 His–Fe3O4, 1.5 mM H2O2 and 100 mM DMPO, was incubated for 10 min at ambient temperature. The •OH in the mixture solution was detected by an EMXplus ESR spectrometer.
Colorimetric detection of cholesterol based on BNNZ
Key parameters of colorimetric method, including pH value of solution, reaction temperature, amount of BNNZ and incubation time, were optimized to improve analysis efficiency. Optimized cholesterol detection by BNNZ was shown as follows. The 200 μg mL−1 BNNZ, 1.0 mM TMB, and 1.0 mL cholesterol solution with different concentrations were mixed and fixed to 2.0 mL with Hac–NaAc buffer (0.1 M, pH 3.0). After incubating at 40 °C for 1 h, the His–Fe3O4 was separated, and the absorbance of supernatant at 652 nm was obtained by UV–vis absorption spectrophotometer.
Colorimetric detection of cholesterol based on free ChOx linked His–Fe3O4 strategy
The 400 μg His–Fe3O4, 1.0 mM TMB, 100 μL ChOx solution (50 μg mL−1) and 1.0 mL cholesterol (100 μM) were mixed and fixed to 2.0 mL with Hac–NaAc buffer (0.1 M, pH 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 or 5.0). After incubating at 20, 30, 40, 50 or 60 °C for 1 h, the His–Fe3O4 was separated, and the absorbance of supernatant at 652 nm was obtained by UV–vis absorption spectrophotometer.
Performance of BNNZ-based methods
Based on the procedure described in Sect. "Colorimetric detection of cholesterol based on BNNZ", the linear relationship between the absorbance at 652 nm and the cholesterol concentration ranging from 1 to 250 μM was evaluated. When the standard deviation of absorbance of HAc-NaAc buffer at 652 nm was defined as noise (σ), the limit of detection (LOD) and limit of quantity (LOQ) of the BNNZ-based method were set to 3σ/k and 10σ/k, respectively. k was the slope of the calibration curve. To evaluate the selectivity, the proposed BNNZ-based method was used to test the cholesterol and control substances, including bovine serum albumin (BSA), potassium chloride (KCl), galactose, glucose, glycine, glutamic acid, tryptophan and serine. To further investigate the specificity of the BNNZ-based method toward cholesterol, the interferences from the above control substances with molar ratios of 1:1, 10:1, and 100:1 were evaluated.
Practicality and universal applicability
To verify the applicability, the BNNZ-based method was used in the quantitative detection of cholesterol from spiked and non-spiked human serum samples. The study (KY2022K176) was conducted at the Second Hospital of Tianjin Medical University (Tianjin, China), and performed according to the ethical guidelines of the Declaration of Helsinki. Human serum samples were taken from healthy volunteers, which all signed informed consent. The serum sample was spiked with cholesterol (0, 100, 250 or 500 μM), and then diluted 100 times with Hac–NaAc buffer (pH 3, 0.1 M) including 0.4% Triton. Subsequently, 1.0 mL diluted serum sample was applied in the BNNZ-based method.
To verify the universal applicability of BNNZ-based method, the bioconjugated His–Fe3O4 with urate oxidase (BHUO) was also fabricated, in which the preparation process was consistent with that of Sect. "Fabrication of BNNZ", except that urate oxidase replaced ChOx. Subsequently, the performance of BHUO-based method for uric acid (UA) was also evaluated.
Results and discussion
Fabrication of BNNZ
In the natural active center of HRP, the terminal imidazole of His42 residue can assist H2O2 to enter the proper position through the H-bond interaction, facilitate heterolytic cleavage of O–O bond of H2O2 to form Fe4+=O, and further stabilize higher oxidation states of heme iron during the catalytic cycle [38]. Inspired by this, the histidine residues have been introduced onto the surface of Fe3O4 to improve the affinity of Fe3O4 nanozyme toward H2O2, and the histidine modification can significantly enhance the peroxidase-like activity of Fe3O4 nanozymes [23]. Unfortunately, the introduction of histidine may change the oxidase activity of His–Fe3O4, which determines the specificity of the BNNZ to the target bioactive molecules. To improve the performance of BNNZ, the correlation between activity and histidine content of nanozyme was investigated.
The oxidase-like activity of His–Fe3O4 was evaluated by direct oxidation of TMB. Obviously, the oxidase-like activity of His–Fe3O4 for TMB enhanced with the increase of histidine content (Fig. 1A). Therefore, His–Fe3O4 might be able to oxidize cholesterol like natural ChOx, thus interfering with the selectivity of natural ChOx for cholesterol. To further investigate the peroxidase-like activity of His–Fe3O4, the steady-state kinetics for H2O2 were investigated by fixing the TMB concentration while varying the H2O2 concentration, and vice versa. With the increase of histidine content, the Km values of H2O2 on His–Fe3O4 decreased first and then increased, and the Vmax values obviously enhanced (Fig. 1B). Yet, the Vmax values of TMB on His–Fe3O4 increased first and then decreased, and the Km values obviously decreased with the increase of histidine content (Fig. 1C). The abnormal change of the parameters might be attributed to that the oxidase-like activity of His–Fe3O4 changed with the addition of histidine. These results demonstrated that the introduction of histidine could both enhance the oxidase- and peroxidase-like activity of His–Fe3O4. To improve the sensitivity and selectivity of BNNZ, the His–Fe3O4 prepared with 50 mg histidine was used in the following experiment, because it possessed strong peroxidase-like activity and weak oxidase-like activity.
Due to the introduction of histidine, the surface of His–Fe3O4 contained carboxyl and amino groups, which could be used as active sites to conjugate His–Fe3O4 with natural enzymes (Scheme 1A). Hence, natural ChOx was immobilized on the surface of His–Fe3O4 through two kinds of linker, including amino- and carboxyl-containing homobifunctional PEG. Fortunately, there was no significant difference in the performance of BNNZs prepared by two strategies (Fig. 1D). The rapid transfer of H2O2 from natural ChOx to His–Fe3O4 may give the BNNZ excellent response-ability. Nevertheless, if the spacing between natural ChOx and His–Fe3O4 is too long, H2O2 needs a long time to reach the catalytic site of His–Fe3O4; on the contrary, if the spacing is too small, H2O2 may accumulate on the surface of His–Fe3O4 and cannot be catalyzed in time. Therefore, the spacing between natural ChOx and His–Fe3O4 was further optimized by different lengths of linker (Fig. 1D). As expected, the performance of BNNZs enhanced first and then decreased with increasing the length of linker, and the optimized BNNZ was obtained with COOH–PEG9 as linker.
The peroxidase-like activity of His–Fe3O4 was also evaluated using the model chromogenic reactions of ABTS, OPD, and TMB. As depicted in Fig. S1, after coincubation with His–Fe3O4, the colorless solutions of ABTS–H2O2, OPD–H2O2, and TMB–H2O2 changed to green, yellow, and blue with characteristic peaks at 415, 450, and 652 nm in the UV–vis absorption spectra, respectively. The stability of BNNZ was studied by investigating storage time and temperature. Not surprisingly, the activity of BNNZ decreased slightly with the extension of storage time. After one month at – 20 °C (Fig. S2A) and 4 °C (Fig. S2B), the activity retained more than 95.1 and 90.8% of the initial activity, respectively. This indicated that the BNNZ was more suitable for storage at – 20 °C.
To understand the mimetic enzyme activity, the electrochemical behavior of His–Fe3O4 was investigated. The electrocatalytic behavior of the bare electrode and His–Fe3O4 modified electrode before and after adding H2O2 was first determined by cyclic voltammetry (Fig. S3A). The current of the bare electrode didn’t change before and after the addition of H2O2, indicating that the bare electrode had no effect on the electrocatalysis system. The His–Fe3O4 modified electrode changed little, but the addition of H2O2 significantly enhanced the current. This indicated that H2O2 was reduced at the His–Fe3O4 modified electrode, and the electron transfer process was as follows: H2O2 + e− → H2O + O2 [39]. ESR spectroscopy was also used to investigate the effect of the generation of •OH on the peroxidase-like activity of His–Fe3O4 (Fig. S3B). When His–Fe3O4 and H2O2 existed alone, there was no obvious characteristic peak. Instead, when His–Fe3O4 and H2O2 coexisted, a quadruple characteristic peak with the relative signal intensity ratio of 1:2:2:1 was observed. This result indicated that •OH produced by the co-existence of His–Fe3O4 and H2O2 could be captured by DMPO to form spin adduct with characteristic signal of 1:2:2:1 quartet. According to the previous report [40], the redox of H2O2 on the His–Fe3O4 could be a single electron transfer process: Fe3+ + H2O2 → Fe2+ + •O2H− + H+, Fe2+ + H2O2 → Fe3+ + •OH + OH−. More narrowly, electron was transferred from H2O2 to Fe3+, resulting in Fe2+ and •O2H−; subsequently, Fe2+ further reacted with H2O2 to form •OH.
Characterization of BNNZ
The fabricated BNNZ was characterized by various techniques. According to TEM and SEM images, the His–Fe3O4 was composed of nanoparticles and had a rough surface (Figs. S4 and S5A), which provided generous catalytic sites. After bioconjugation, the morphology of BNNZ had no obvious change and the BNNZ exhibited a diameter of more than 100 nm (Figs. 2A and S5B). Elemental mapping showed that the BNNZ contained C, N, O, F, P, S and Fe elements (Fig. 2B). Fe element was derived from His–Fe3O4; P and S elements were originated from ChOx, suggesting the successful bioconjugation of nanozyme and natural enzyme. Elemental composition was further investigated by XPS. Before bioconjugation, the characteristic peaks of His–Fe3O4 were obtained at 724.3 eV for Fe2p1/2, 710.8 eV for Fe2p3/2, 533.1 eV for O1s, 399.4 eV for N1s and 286.1 eV for C1s (Fig. S6), respectively. For BNNZ, new characteristic peaks were obtained 161.1 eV for S2p and 132.3 eV for P2p (Figs. 2C and S7), which were derived from natural enzyme. In addition, the relative percentage of Fe element on BNNZ was obviously lower than that on His–Fe3O4 (Table S1). These results further proved the successful bioconjugation of nanozyme and natural enzyme.
A TEM image of BNNZ. B Elemental mapping of BNNZ. C XPS of BNNZ. D FT-IR spectra of His–Fe3O4 (a), His–Fe3O4@PEG (b) and BNNZ (c). E Magnetization curve of His–Fe3O4 and BNNZ; the photograph of the dispersion of BNNZ in the absence (a) and presence (b) of an external magnet (top left corner). F TGA of His–Fe3O4 (a), His–Fe3O4@PEG (b) and BNNZ (c)
The successful fabrication of BNNZ was further investigated by FT-IR analysis (Fig. 2D). For His–Fe3O4, the characteristic peak of Fe–O bond was obtained at 584 cm−1, and the characteristic peaks at 1634 cm−1 and 3433 cm−1 were attributed to the bending vibration of N–H bond and stretching vibration of C=O bond, and the stretching vibration of O–H and N–H bonds, respectively. After the introduction of PEG linker, the new peak at 1040 cm−1 was attributed to the stretching vibration of C–O–C of PEG. For BNNZ, the new characteristic peak at 1400 cm−1 was derived from the bending vibration of –CH3. To confirm the successful fabrication of BNNZ, the changes of surface charge and hydrodynamic size in the preparation process were also investigated (Fig. S8 and Table S2). The zeta potential of His–Fe3O4 was 38.4 mV because many positively charged histidine molecules were distributed on the surface. After the introduction of PEG, the zeta potential reduced obviously to 22.1 mV, because some positively charged amino groups of histidine were blocked by negatively charged carboxyl PEG. For BNNZ, the negatively charged carboxyl groups on PEG reacted with the negatively charged ChOx, so the zeta potential further reduced to 19.0 mV (Fig. S6). In addition, the average hydrodynamic sizes in the preparation process increased gradually, and were 199.0 nm for His–Fe3O4, 371.4 nm for His–Fe3O4@PEG and 404.1 nm for BNNZ (Table S2), respectively. The obvious change of surface charge and hydrodynamic size proved the successful fabrication of BNNZ.
Magnetization curves of His–Fe3O4 and BNNZ both passed through the origin and were symmetrical around the origin without hysteresis (Fig. 2E). Meanwhile, the saturation magnetization values of His–Fe3O4 and BNNZ were 71.4 emu g−1 and 64.1 emu g−1, respectively. Furthermore, the dispersed BNNZ could gather on the bottle wall within 15 s with the assistance of an external magnetic field (Fig. 2Ea–b). This result indicated that the paramagnetic BNNZ had fast magnetic responsiveness and could be quickly separated from the reaction liquid. The compositions of BNNZ and its precursors were investigated by TGA. With 200 °C as the cut-off point, the weight loss was divided into two stages (Fig. 2F). Within 200 °C, the dehydration and volatilization of unstable components were the principal consideration of weight loss; above 200 °C, the weight loss was mainly attributed to the decomposition of organic components, mainly including histidine, PEG and ChOx. At 800 °C, the maximum weight losses were 12.1% for His–Fe3O4, 12.8% for His–Fe3O4@PEG and 13.5% for BNNZ, respectively. This result indicated that ChOx accounted for about 0.8% of the total mass of BNNZ, which further proved the successful bioconjugation of nanozyme and natural enzyme.
Optimization of BNNZ-based cascade reaction
The colorimetric detection of cholesterol was performed by BNNZ-based cascade reaction, which included two steps (Scheme 1B). First, ChOx could specifically catalyze the oxidation of cholesterol to generate H2O2 molecule (Eq. 2). Subsequently, the H2O2 molecule was captured by histidine residue of His–Fe3O4 H-bond interaction, and immediately decomposed into hydroxyl radical, which could oxidize the colorless TMB into blue ox-TMB (Eq. 3).
Like natural enzyme, the activity of nanozyme is also affected by pH value and temperature [41]. To improve the efficiency of BNNZ-based cascade reaction, we first optimized the environmental pH value and reaction temperature. BNNZ-based colorimetric detection of cholesterol was compared with the detection method based on nanozyme alone, because the selected His–Fe3O4 had both peroxidase- and oxidase-like activity. As shown in Fig. 3A, the response signal enhanced first and then decreased with the increase of pH value, and the maximum value was obtained at pH 3.0. The optimal pH of His–Fe3O4 was very similar to that of natural horseradish peroxidase, which was consistent with previous report [7]. Meanwhile, the response signal based on BNNZ was significantly higher than that based on His–Fe3O4. This result demonstrated that the bioconjugation of ChOx and His–Fe3O4 endowed BNNZ with higher sensitivity to cholesterol.
The effect of reaction temperature was also investigated in the range of 20–60 °C (Fig. 3B). With increasing temperature, the response signal based on His–Fe3O4 gradually decreased, probably because the stability of His–Fe3O4 decreased as the temperature increased. As expected, the response signal based on BNNZ increased first and then decreased, and reached the maximum at 40 °C (Fig. 3B), which may be due to the optimal catalytic capacity of ChOx at this temperature. To improve analysis efficiency, the amount of BNNZ and incubation time was further optimized. Amount of BNNZ ranging from 50 to 400 μg was used to detect 100 μM cholesterol solution (1 mL). With the increase of amount, the response signal increased rapidly at first and then tended to be stable, and 400 μg BNNZ was sufficient to detect cholesterol in human serum (Fig. 3C). The effect of incubation time was also investigated by varying from 5 to 90 min. The optimal incubation time of His–Fe3O4-based method was within 5 min, and was obviously shorter than that of BNNZ-based method (Fig. 3D). This indicated that the His–Fe3O4 had excellent catalytic efficiency and the reaction speed of BNNZ-based method was limited by natural ChOx. The optimal incubation time of BNNZ-based method was 60 min and used in the following experiments.
Performance of BNNZ-based strategy
To evaluate the feasibility of quantification of cholesterol, BNNZ-based strategy was used to detect cholesterol standard solutions of different concentrations. The BNNZ-based strategy exhibited a significant dependence on the target concentration, and the gradation of blue color and UV absorbance value obviously enhanced with increasing cholesterol concentration (Fig. 4A). Moreover, the absorbance values at 652 nm were positively correlated with the cholesterol concentrations within the ranges of 3–75 μM (y = 0.0095x + 0.2828, R2 = 0.9989) and 75–200 μM (y = 0.0042x + 0.6904, R2 = 0.9971) (Fig. 4B). This was similar to the previous results [42]. The LOD and LOQ values were as low as 0.446 and 1.488 μM, respectively, which were far lower than the cholesterol concentration in the blood of healthy people [43]. The excellent sensitivity of the BNNZ-based strategy could be attributed to the cascade reaction of natural enzyme and nanozyme.
A Visible absorption spectrum of BNNZ and TMB in the presence of different concentrations of cholesterol (3–200 μM, from bottom to top). B Plot of absorbance at 652 nm versus the concentration of cholesterol. C Absorbance of BNNZ-based method for cholesterol and control substances. D Absorbance of BNNZ-based method for 20 μM cholesterol with different ratios of interfering substances (CH, cholesterol;1, glucose; 2, serine; 3, galactose; 4, glutamic acid; 5, glycine; 6, KCl; 7, tryptophan; 8, BSA). E Effect of pH values on the performance of cholesterol detection method based on free enzyme and BNNZ. F Effect of temperature on the performance of cholesterol detection method based on free enzyme and BNNZ
To evaluate the selectivity, the BNNZ-based strategy was employed for the determination of cholesterol and analogues, including glucose, serine, galactose, glutamic acid, glycine, KCl, tryptophan and BSA. The UV absorbance values of cholesterol was obviously higher than those of analogues, and the latter were only 0.4–7.3% of the former (Fig. 4C). ChOx couldn’t catalyze analogues, but could only selectively catalyze the oxidation of cholesterol to generate H2O2 molecules. Therefore, the proposed BNNZ-based strategy exhibited excellent selectivity for detection of cholesterol. Specificity of BNNZ-based strategy was further investigated using the above analogues as interfering substances (Fig. 4D). Even though the concentrations of the interfering substances were 1-, 10- and 100-folds higher than that of the target cholesterol, the cross-reactivity of BNNZ-based strategy was still no more than 4.1%. This result demonstrated that the BNNZ-based strategy exhibited excellent specificity for target cholesterol and was very acceptable for the analysis of complex biological samples.
To prove the superiority of bioconjugation, the free ChOx was linked with His–Fe3O4, which was also used to detect cholesterol and compared with the BNNZ-based strategy. Although the two methods had the same performance under the optimal conditions (pH 3.0, 40 °C), the BNNZ-based strategy was obviously superior to free ChOx strategy in a wider range (Fig. 4E, F). For example, at pH 2.5, the response values of the BNNZ-based and free ChOx strategies were 79.2 and 38.0% of that under the optimal conditions, respectively (Fig. 4E); at 50 °C, the response values of two strategies were 85.5 and 58.7%, respectively (Fig. 4F). This result indicated that the bioconjugation might improve the stability of natural enzyme.
Universal applicability
To assess the universal applicability of BNNZ-based method, the His–Fe3O4 was linked with urate oxidase for fabricating BHUO, which could be used for ultrasensitive and specific detection of uric acid. Uric acid as a final product of purine metabolism is closely related to diseases such as urethritis, arthritis and chronic kidney disease [44, 45]. For BHUO, urate oxidase specifically catalyzed the oxidation of uric acid to generate H2O2 molecules, and subsequently, the newly generated H2O2 proportionally oxidized the colorless TMB into blue ox-TMB under the catalysis of His–Fe3O4. The absorbance values at 652 nm were positively correlated with the uric acid concentrations within the ranges of 15–150 μM (y = 0.0009x + 0.0073, R2 = 0.9977) and 150–600 μM (y = 0.0005x + 0.0809, R2 = 0.9947) (Fig. S9). The LOD and LOQ values were as low as 3.2 and 10.7 μM, respectively. Hence, these results proved that BNNZ-based method possessed universal applicability.
Detection of cholesterol in human serum
The BNNZ-based strategy was also used in the detection of cholesterol in human serum, and the original concentration of cholesterol was detected as 1025 μM. The precision and accuracy of the proposed BNNZ-based strategy were evaluated by using spiked serum samples with three levels of concentrations (100, 250 and 500 μM). As listed in Table 1, the recovery values ranged from 97.9 to 103.5%, and the relative standard deviation (RSD) was in the range of 2.5–4.0%. This result demonstrated that the proposed BNNZ-based strategy was selective and accurate for the determination of cholesterol in complex biological samples. As listed in Table S3, the performance of BNNZ-based strategy was comparable to or relatively better than other nanozyme-based colorimetric methods [32,33,34,35,36] and nanozyme-based other methods [46,47,48,49,50]. In addition, the BNNZ-based strategy possessed unique advantages, such as simple preparation of BNNZ, no influence on colorimetric quantification due to separation of BNNZ after reaction, and no expensive equipment involved in quantitative detection. Therefore, the BNNZ-based strategy had the potential to be further used for cholesterol detection in the clinic.
Conclusion
In conclusion, an innovative bioconjugation of nanozyme and natural enzyme, named BNNZ, was proposed for ultrasensitive and specific detection of bioactive molecules. The integration of natural GhOx and His–Fe3O4 was performed with hydrophilic PEG as a linker. The advantages of BNNZ include the following points: (1) the introduction of histidine effectively increases the peroxidase-like activity of His–Fe3O4; (2) the rapid magnetic separation of BNNZ after reaction does not interfere with colorimetric quantitative detection; (3) the cascade reaction of natural enzyme and nanozyme endows the BNNZ with superior specificity and sensitivity. To further prove the excellent performance, the BNNZ was successfully used for the determination of cholesterol in human serum. Therefore, we believe that the bioconjugation of nanozyme and natural enzyme has great potential to become a universal strategy for fabrication of intelligent enzyme-nanozyme systems for numerous other bioactive molecules, and will be used in various biomedical applications such as injury, cancer, infectious disease and neurodegenerative disease therapies.
References
R. Zhang, X. Yan, K. Fan, Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2(7), 534–547 (2021)
Y. Huang, J. Ren, X. Qu, Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119(6), 4357–4412 (2019)
M. Liang, X. Yan, Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52(8), 2190–2200 (2019)
H. Jin, D. Ye, L. Shen, R. Fu, Y. Tang, J.C.Y. Jung, H. Zhao, J. Zhang, Perspective for single atom nanozymes based sensors: advanced materials, sensing mechanism, selectivity regulation, and applications. Anal. Chem. 94(3), 1499–1509 (2022)
B. Das, J.L. Franco, N. Logan, P. Balasubramanian, M.I. Kim, C. Cao, Nanozymes in point-of-care diagnosis: an emerging futuristic approach for biosensing. Nano-Micro Lett. 13(1), 193 (2021)
M. Wei, J. Lee, F. Xia, P. Lin, X. Hu, F. Li, D. Ling, Chemical design of nanozymes for biomedical applications. Acta Biomater. 126, 15–30 (2021)
L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett, X. Yan, Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2(9), 577–583 (2007)
Y. Hu, H. Cheng, X. Zhao, J. Wu, F. Muhammad, S. Lin, J. He, L. Zhou, C. Zhang, Y. Deng, P. Wang, Z. Zhou, S. Nie, H. Wei, Surface-enhanced Raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 11(6), 5558–5566 (2017)
X. Wang, F. Wen, L. He, J. Su, P. Jiang, D. He, Engineering porous Co–Mn oxide nanosheets with abundant oxygen vacancy as an efficient oxidase-like mimic for heparin colorimetric sensing. Anal. Chim. Acta 1198, 339564 (2022)
X. Meng, D. Li, L. Chen, H. He, Q. Wang, C. Hong, J. He, X. Gao, Y. Yang, B. Jiang, G. Nie, X. Yan, L. Gao, K. Fan, High-performance self-cascade pyrite nanozymes for apoptosis–ferroptosis synergistic tumor therapy. ACS Nano 15(3), 5735–5751 (2021)
N. Wang, J. Shi, Y. Liu, W. Sun, X. Su, Constructing bifunctional metal–organic framework based nanozymes with fluorescence and oxidase activity for the dual-channel detection of butyrylcholinesterase. Anal. Chim. Acta 1205, 339717 (2022)
H. Sun, A. Zhao, N. Gao, K. Li, J. Ren, X. Qu, Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew. Chem. Int. Ed. 54(24), 7176–7180 (2015)
T. Zhan, J. Kang, X. Li, L. Pan, G. Li, W. Hou, NiFe layered double hydroxide nanosheets as an efficiently mimic enzyme for colorimetric determination of glucose and H2O2. Sens. Actuators B Chem. 255, 2635–2642 (2018)
R. Cai, D. Yang, S. Peng, X. Chen, Y. Huang, Y. Liu, W. Hou, S. Yang, Z. Liu, W. Tan, Single nanoparticle to 3D supercage: framing for an artificial enzyme system. J. Am. Chem. Soc. 137(43), 13957–13963 (2015)
R. Cai, D. Yang, K. Lin, T.S. Cao, Y. Lyv, K. Chen, Y. Yang, J. Ge, L. Xia, G. Christou, Y. Zhao, Z. Chen, W. Tan, 3D halos assembled from Fe3O4/Au NPs with enhanced catalytic and optical properties. Nanoscale 11(43), 20968–20976 (2019)
H. Wei, E. Wang, Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 80(6), 2250–2254 (2008)
A.A. Vernekar, T. Das, S. Ghosh, G. Mugesh, A remarkably efficient MnFe2O4-based oxidase nanozyme. Chem. Asian J. 11(1), 72–76 (2016)
Z. Chen, J.J. Yin, Y.T. Zhou, Y. Zhang, L. Song, M. Song, S. Hu, N. Gu, Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 6(5), 4001–4012 (2012)
M.L. Ye, Y. Zhu, Y. Lu, L. Gan, Y. Zhang, Y.G. Zhao, Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: a review. Talanta 230, 122299 (2021)
S. Li, Y. Zhang, Q. Wang, A. Lin, H. Wei, Nanozyme-enabled analytical chemistry. Anal. Chem. 94(1), 312–323 (2022)
M.S. Kim, J. Lee, H.S. Kim, A. Cho, K.H. Shim, T.N. Le, S.S.A. An, J.W. Han, M.I. Kim, J. Lee, Heme cofactor-resembling Fe–N single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 30(1), 1905410 (2020)
W. Wu, Q. Wang, J. Chen, L. Huang, H. Zhang, K. Rong, S. Dong, Biomimetic design for enhancing the peroxidase mimicking activity of hemin. Nanoscale 11(26), 12603–12609 (2019)
K. Fan, H. Wang, J. Xi, Q. Liu, X. Meng, D. Duan, L. Gao, X. Yan, Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 53(2), 424–427 (2017)
L. Dewangan, J. Korram, I. Karbhal, R. Nagwanshi, M.L. Satnami, N-doped carbon quantum dot-MnO2 nanowire FRET pairs: detection of cholesterol, glutathione, acetylcholinesterase, and chlorpyrifos. ACS Appl. Nano Mater. 4(12), 13612–13624 (2021)
C. Hong, L. Chen, C. Wu, D. Yang, J. Dai, Z. Huang, R. Cai, W. Tan, Green synthesis of Au@WSe2 hybrid nanostructures with the enhanced peroxidase-like activity for sensitive colorimetric detection of glucose. Nano Res. 15(2), 1587–1592 (2022)
T.L. Steck, Y. Lange, Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 20(11), 680–687 (2010)
J. Zhang, Q. Liu, Cholesterol metabolism and homeostasis in the brain. Protein Cell 6(4), 254–264 (2015)
G. Llaverias, C. Danilo, I. Mercier, K. Daumer, F. Capozza, T.M. Williams, F. Sotgia, M.P. Lisanti, P.G. Frank, Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 178(1), 402–412 (2011)
F.J. Alenghat, A.M. Davis, Management of blood cholesterol. JAMA 321(8), 800–801 (2019)
F.R. Maxfield, I. Tabas, Role of cholesterol and lipid organization in disease. Nature 438(7068), 612–621 (2005)
V. Narwal, R. Deswal, B. Batra, V. Kalra, R. Hooda, M. Sharma, J.S. Rana, Cholesterol biosensors: a review. Steroids 143, 6–17 (2019)
L. Dewangan, J. Korram, I. Karbhal, R. Nagwanshi, V.K. Jena, M.L. Satnami, A colorimetric nanoprobe based on enzyme-immobilized silver nanoparticles for the efficient detection of cholesterol. RSC Adv. 9(72), 42085–42095 (2019)
M. Chung, Y. Jang, M. Kim, Convenient colorimetric detection of cholesterol using multi-enzyme Co-incorporated organic-inorganic hybrid nanoflowers. J. Nanosci. Nanotechnol. 18(9), 6555–6561 (2018)
C. Hong, X. Zhang, C. Wu, Q. Chen, H. Yang, D. Yang, Z. Huang, R. Cai, W. Tan, On-site colorimetric detection of cholesterol based on polypyrrole nanoparticles. ACS Appl. Mater. Interfaces 12(49), 54426–54432 (2020)
Q. Wu, L. He, Z.W. Jiang, Y. Li, Z.M. Cao, C.Z. Huang, Y.F. Li, CuO nanoparticles derived from metal-organic gel with excellent electrocatalytic and peroxidase-mimicking activities for glucose and cholesterol detection. Biosens. Bioelectron. 145, 111704 (2019)
L. Zhao, Z. Wu, G. Liu, H. Lu, Y. Gao, F. Liu, C. Wang, J. Cui, G. Lu, High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J. Mater. Chem. B 7(44), 7042–7051 (2019)
J. Li, T. Liu, R.A. Dahlgren, H. Ye, Q. Wang, Y. Ding, M. Gao, X. Wang, H. Wang, N, S-co-doped carbon/Co1-xS nanocomposite with dual-enzyme activities for a smartphone-based colorimetric assay of total cholesterol in human serum. Anal. Chim. Acta 1204, 339703 (2022)
G.I. Berglund, G.H. Carlsson, A.T. Smith, H. Szöke, A. Henriksen, J. Hajdu, The catalytic pathway of horseradish peroxidase at high resolution. Nature 417(6887), 463–468 (2002)
Z. Mu, S. Wu, J. Guo, M. Zhao, Y. Wang, Dual mechanism enhanced peroxidase-like activity of iron–nickel bimetal–organic framework nanozyme and its application for biosensing. ACS Sustain. Chem. Eng. 10(9), 2984–2993 (2022)
M.A. Voinov, J.O.S. Pagán, E. Morrison, T.I. Smirnova, A.I. Smirnov, Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Am. Chem. Soc. 133(1), 35–41 (2011)
Q. Liu, A. Zhang, R. Wang, Q. Zhang, D. Cui, A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 13(1), 154 (2021)
C. Zhang, C. Chen, D. Zhao, G. Kang, F. Liu, F. Yang, Y. Lu, J. Sun, Multienzyme cascades based on highly efficient metal–nitrogen–carbon nanozymes for construction of versatile bioassays. Anal. Chem. 94(8), 3485–3493 (2022)
A. Menotti, M. Lanti, A. Zanchetti, G. Botta, M. Laurenzi, O. Terradura-Vagnarelli, M. Mancini, The role of HDL cholesterol in metabolic syndrome predicting cardiovascular events. The Gubbio population study. Nutr. Metab. Cardiovas. 21(5), 315–322 (2011)
S. Qu, Z. Li, Q. Jia, Detection of purine metabolite uric acid with picolinic-acid-functionalized metal–organic frameworks. ACS Appl. Mater. Interfaces 11(37), 34196–34202 (2019)
I. Kim, Y.I. Kim, S.W. Lee, H.G. Jung, G. Lee, D.S. Yoon, Highly permselective uric acid detection using kidney cell membrane-functionalized enzymatic biosensors. Biosens. Bioelectron. 190, 113411 (2021)
Y. Li, S. Li, M. Bao, L. Zhang, C. Carraro, R. Maboudian, A. Liu, W. Wei, Y. Zhang, S. Liu, Pd nanoclusters confined in ZIF-8 matrixes for fluorescent detection of glucose and cholesterol. ACS Appl. Nano Mater. 4(9), 9132–9142 (2021)
Y. Huang, Y. Gu, X. Liu, T. Deng, S. Dai, J. Qu, G. Yang, L. Qu, Reusable ring-like Fe3O4/Au nanozymes with enhanced peroxidase-like activities for colorimetric-SERS dual-mode sensing of biomolecules in human blood. Biosens. Bioelectron. 209, 114253 (2022)
J. Hassanzadeh, A. Khataee, Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta 178, 992–1000 (2018)
V. Román-Pizarro, M. Ramírez-Gutiérrez, A. Gómez-Hens, J.M. Fernández-Romero, Usefulness of magnetically-controlled MNPs-enzymes microreactors for the fluorimetric determination of total cholesterol in serum. Talanta 208, 120426 (2020)
H.C. Chang, J.A. Ho, Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal. Chem. 87(20), 10362–10367 (2015)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82172829, 21605114).
Funding
National Natural Science Foundation of China, 82172829, Hong-Tao Zhao, 21605114, Xian-Hua Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, HT., Lang, JY., Wang, Z. et al. Bioconjugation of nanozyme and natural enzyme for ultrasensitive detection of cholesterol. ANAL. SCI. 39, 503–515 (2023). https://doi.org/10.1007/s44211-022-00258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-022-00258-5