Abstract
Purpose
The most widely used prehospital strategy for the management of hemorrhagic shock or trauma accompanied by hypotension is fluid resuscitation. Though current guidelines suggest early and aggressive fluid resuscitation, contemporary literature suggests a more restrictive approach. Our objective was to evaluate the effectiveness of low/ no IV fluids in comparison to standard resuscitation in reducing mortality for trauma patients in the prehospital setting.
Methods
Population—adults with blunt or penetrating trauma in the prehospital setting with severe injury (defined as SBP < 90 mm Hg and/or a shock index > (1). Intervention—low-dose/no IV fluids. Comparison—standard resuscitation. Outcome—mortality. A librarian-assisted search of five databases (Medline, Embase, Web of Science, and CINAHL, Cochrane trials) was completed in June 2021 (updated in November 2022). ROBINS-1 and ROB-2 tools were used to assess risk of bias in observational and randomized studies, respectively. An inverse variance method and random-effects model of statistical analysis were utilized, with data reported as risk ratios with related 95% confidence intervals. Heterogeneity of studies was assessed through analysis of the I2
Results
Seven studies (six observational and one randomized trial) were included, with three thousand and fifty study participants included for analysis. Four studies compared high- to low-dose fluids, and three compared fluids to no fluids. We found no difference in mortality when comparing standard resuscitation to restricted resuscitation (RR 0.99, 95% CI [0.80–1.22], I2 = 54%).
Conclusion
Weak, primarily observational evidence suggests that standard fluid resuscitation has no significant mortality benefit over restricting/withholding IV fluids in severe/hypotensive trauma. This review adds evidence to questioning the requirement for IV fluids in trauma given the lack of mortality benefit, in addition to demonstrating the need for more randomized studies in this area.
Résumé
Objectif
La stratégie préhospitalière la plus utilisée pour la prise en charge du choc hémorragique ou du traumatisme accompagné d'hypotension est la réanimation liquidienne. Bien que les directives actuelles suggèrent une réanimation liquidienne précoce et agressive, la littérature contemporaine suggère une approche plus restrictive. Notre objectif était d’évaluer l’efficacité des liquides intraveineux faibles ou inexistants par rapport à la réanimation standard pour réduire la mortalité des patients traumatisés en milieu préhospitalier.
Méthodes
Population - adultes ayant subi un traumatisme contondant ou pénétrant en milieu préhospitalier et présentant des lésions graves (définies par une PAS < 90 mm Hg et/ou un indice de choc > 1). Intervention - faible dose/absence de fluides IV. Comparaison - réanimation standard. Résultat - Mortalité. Une recherche assistée par un bibliothécaire dans 5 bases de données (Medline, Embase, Web of Science et CINAHL, essais Cochrane) a été effectuée en juin 2021 (mise à jour en novembre 2022). Les outils ROBINS-1 et ROB-2 ont été utilisés pour évaluer le risque de biais dans les études observationnelles et randomisées respectivement. Une méthode de variance inverse et un modèle d'analyse statistique à effets aléatoires ont été utilisés, les données étant présentées sous forme de rapports de risque avec les intervalles de confiance à 95 % correspondants. L'hétérogénéité des études a été évaluée par l'analyse de l'I2.
Résultats
Sept études (six études d'observation et un essai randomisé) ont été incluses, avec 3050 participants à l'analyse. Quatre études ont comparé des fluides à forte dose à des fluides à faible dose, et trois ont comparé des fluides à l'absence de fluides. Nous n’avons trouvé aucune différence dans la mortalité en comparant la réanimation standard à la réanimation restreinte (RR 0,99, IC à 95 % [0,80–1,22], I2 = 54 %).
Conclusion
Des preuves faibles, essentiellement observationnelles, suggèrent que la réanimation liquidienne standard ne présente aucun avantage significatif en termes de mortalité par rapport à la restriction/rétention des liquides IV dans les cas de traumatismes graves/hypotensifs. Cette revue ajoute des preuves à la remise en question de la nécessité des fluides IV en traumatologie, étant donné l'absence de bénéfice en termes de mortalité, en plus de démontrer le besoin de plus d'études randomisées dans ce domaine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is known about the topic? |
Though current guidelines suggest early and aggressive fluid resuscitation in trauma, contemporary literature suggests a more restrictive approach. |
What did this study ask? |
What is the effect of restricted versus standard fluid resuscitation on mortality for trauma patients in the prehospital setting? |
What did this study find? |
There was no difference between strategies |
Why does this study matter to clinicians? |
This review allows emergency clinicians to incorporate contemporary evidence of fluid resuscitation into their practice. |
Introduction
For several decades, trauma has been a major cause of fatality in Canada [1] and globally. In 2020, unintentional injury and trauma was the fifth leading cause of death among all ages, and the leading cause of death among those under age 25 [2]. Despite advances in trauma systems, the mortality rate of major trauma remains up to 14% across Canada [2]. Severe trauma is often accompanied by hemorrhage, shock, and subsequent patient deterioration. Perhaps the most widely accepted strategy for the management of hemorrhagic shock or trauma accompanied by hypotension is through fluid resuscitation. By far, isotonic fluids such as crystalloids and colloids are the most commonly used fluid in resuscitation. As compared to blood and blood products, these fluids are more readily accessible, more easily managed, more cost effective, and less prone to leading to adverse reactions [3]. Despite the relative simplicity of these fluids, preferred dosage of fluid in the context of trauma remains unclear.
In the management of severe trauma and hypotension, some studies suggest that restrictive fluid resuscitation may reduce mortality [4, 5], while others find no association between the two [6, 7]. Some studies found benefits in mortality when comparing administering to withholding fluids [9] while others did not [10, 11]. The administration of IV fluids also carries some level of risk, including coagulopathy, compartment syndrome, electrolyte disturbances, and artificial inflation of the systolic blood pressure [3]. The controversy surrounding fluid resuscitation is further exacerbated by mounting evidence that increasing prehospital time to begin fluid resuscitation may drastically reduce positive outcomes and increase mortality [12,13,14]. Thus, clear evidence of the most appropriate resuscitation strategy in the context of trauma is warranted, especially in the prehospital context where fluid resuscitation most often takes place.
The objective of this review was to evaluate the effectiveness of non-blood product IV fluids (i.e., crystalloid/ isotonic fluids or colloids) in reducing mortality for severely injured adult trauma patients in the prehospital setting.
Methods
Eligibility criteria
This review sought to include randomized trials, non-randomized trials, and observational studies. Trauma was defined as any cause of blunt or penetrating injury and severity quantified to a corresponding prehospital systolic blood pressure (sBP) of 90 mm Hg or lower, or a shock index (SI) greater than 1. Certain patient populations including pregnancy, isolated head or spinal cord injury, burns, cardiac arrest, and non-hemorrhagic causes of shock were excluded to maximize homogeneity of results. The ideal primary intervention of interest was the complete withholding of any non-blood product IV fluid, although studies comparing high volume to low volume parenteral fluid administration were also considered. The primary outcome of interest was 30-day all-cause mortality. Any study with an outcome measure of all-cause mortality, regardless of timing or setting, was also included. Studies not reporting mortality as an outcome measure were excluded.
Information sources and search strategy
Between June 24 and June 28th, 2021, a systematic search of the electronic databases Ovid MEDLINE, Embase, CINAHL, and Web of Science was performed. The search strategy was developed in collaboration with an experienced health services librarian on the team, who completed each database search. A second health services librarian also peer-reviewed the search. For full search histories, please see Supplemental Digital Content 1. The search was repeated on November 9th 2022 in preparation for publication. The updated search included the original four databases identified above, in addition to a search of the Cochrane Database and Register of Clinical Trials.
Selection process
The literature search completed by the academic librarian was uploaded to RAYYAN [15], an online systematic review study screening and selection program, for review. Authors 1 and 2 reviewed the titles and abstracts of studies identified in the electronic search. A standardized form was used to determine eligibility of studies. Data extracted from all included studies can be found in Table 1.
Data collection process and data items
Data were extracted into an Excel spreadsheet, and reviewed independently by Authors 1 and 2. Included data items sought for retrieval can be found in Table 1. Raw and calculated numerical data included for meta-analysis were subsequently uploaded to Review Manager 5.4 [16].
Study risk of bias assessment
This review utilized two risk of bias assessment tools suggested by the Cochrane Database of Systematic Reviews; the ROB-2 [17] tool for randomized studies and the ROBINS-I [18] tool for non-randomized studies.
Effect measures
Extracted elements were uploaded into RevMan [16], where dichotomous mortality outcome data were expressed as risk ratios (RR) with related 95% confidence intervals (CI).
Synthesis methods
This review undertook the meta-analysis techniques outlined by the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 [19]. An inverse variance method was utilized in accordance with the Cochrane Handbook [19]. Mortality data were uploaded into RevMan [16], where calculated risk ratios with related 95% CI were reported in a forest plot. A specified set of subgroup analyses were identified prior to study inclusion with the intention of being implemented if significant heterogeneity was present. The primary characteristic of interest for subgroup analysis was fluid dosage. After completion of data collection and synthesis, a subgroup analysis of blunt vs. penetrating trauma was also considered if significant heterogeneity was identified.
Reporting bias assessment
Reporting bias was assessed by visual inspection of the funnel plot of compiled risk ratio data, with a plan for analysis as to possible causes of asymmetry other than reporting bias if marked funnel plot asymmetry was observed.
Certainty assessment
Certainty of the evidence was assessed utilizing the BMJ GRADE [20] system.
Results
Study selection
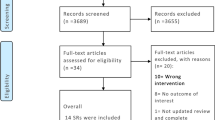
A total of 5,625 records were identified through the 4 primary databases searched. A summary of the results of the search can be found in the PRISMA [21] flow diagram (Fig. 1). An updated search in November 2022 included the Cochrane Database and Register of Clinical Trials, yielding an additional 221 records total and 167 duplicates from the previous search, for a result of 54 new records screened. No new studies were identified. A total of 641 new records from the primary 4 databases searched were also screened in the November 2022 update, yielding no additional studies. The final count of records included in this review was seven primary studies.
Study characteristics
A summary of the characteristics of excluded studies, including reasons for exclusion, can be found in Supplemental Digital Content 2. A summary of the characteristics of included studies can be found in the characteristics of included studies table (Table 1). Six non-randomized observational studies [5, 8, 10, 11, 22, 23] and one randomized pilot trial [7] were included. A total of 10,073 patients were included across the 7 studies, with the mean age ranging from 26 to 47. All studies included adults only, with the inclusion age ranging from > 15 to > 18. A mean Injury Severity Score (ISS) was reported in all seven studies, ranging from 10 to > 50. In terms of injury mechanism, two studies [7, 8] reported exclusively on penetrating trauma, two [22, 23] exclusively on blunt trauma, and the remaining three [7, 8, 11] on both penetrating and blunt mechanisms. The exact type of fluid utilized was noted for all studies, with three [5, 11, 22] including exclusively crystalloid, one [7] including a mixture of crystalloid and colloid, and three [8, 10, 23] studies which did not specify the fluid used. Of the seven studies, four [5, 7, 8, 22] compared high-dose fluid to low-dose fluid and three [10, 11, 23] compared receiving fluids to not receiving fluids. The exact level of fluid defined as high or low dose, as well as other defining parameters of each group, varied between studies (see Table 1). The reported outcome of interest was mortality, with the authors’ conclusions of the effect of fluids on mortality reported for all seven studies. Additional relevant outcomes such as fluid level in each arm, transport time, and proportion of blunt vs. penetrating trauma, were also included in the table (see Table 1).
Risk of bias in studies
Of the seven studies included, one [7] had low risk of bias, three [4, 11, 22] had moderate risk of bias, and three [8, 10, 23] had serious risk of bias. The risk of bias of the included studies is summarized in the risk of bias of included studies table (see Table 2).
Results of individual studies, results of syntheses, and reporting biases
A funnel plot of the included studies is shown in Fig. 2. A forest plot of the included studies is shown in Fig. 3. Moderate heterogeneity of the included studies was confirmed statistically (Chi2 = 13.01, I2 = 54%, p = 0.04). As expected, the low number of included studies limited the ability of undertaking subgroup analyses. Tests for overall effect showed no significant difference between groups (RR 0.99, 95% CI [0.80–1.22], p = 0.90).
Certainty of the evidence
The overall grade of evidence in this review was determined to be low in accordance with the BMJ GRADE [20] recommendations, signifying significant uncertainty of the estimated effect size. The GRADE [20] table can be found in Supplemental Digital Content 3.
Discussion
Interpretation
The findings of this review show no significant effect of the administration of IV fluids in the prehospital setting for severely injured adult trauma patients. Five studies [7, 10, 11, 22, 23] found no significant difference between high/low and high/no fluids, one [4] found a significant decrease in mortality with delayed resuscitation, while another [8] found an increase in unadjusted mortality in the low/no fluid group. The calculated effect estimate suggests that both high and low-dose fluids have no mortality benefit for severe trauma in the prehospital context.
Previous studies
Recent evidence suggests the use of smaller doses (< 1L) of IV fluids in trauma, allowing for permissive hypotension until hospitalization or surgical intervention can take place [4,5,6, 12, 13, 25]. This lower level of IV fluid administration may also partly be due to evidence suggesting the overall benefit of prioritizing rapid transport as opposed to advanced life support at the scene, where the resulting decreased level of fluids was found to lead to decreased mortality [6, 12,13,14, 24]. Though the evidence of high versus low levels of fluid has been somewhat well established, the implications of fluids versus no fluids has not. The effect estimate in this review suggests no benefit in the reduction of mortality in administering IV fluids in the prehospital setting versus not administering fluids. Only three studies [10, 11, 23] actually measured fluids versus no fluids, though the possibility of no net benefit is supported by the overall effect estimate showing no significant benefit in fluid administration. To the knowledge of the authors, virtually no consensus has ever declared that the complete withholding of fluids would be the best approach in terms of fluid resuscitation for severely injured trauma patients.
Strengths and limitations
This review has various strengths. First, internal validity was a high priority. Various sources of evidence in systematic review methodology were utilized, including the Cochrane [19], PRISMA [21], and GRADE [20]. Further, the involvement of two experienced health system librarians strengthens the quality of the literature search of this review, with a high likelihood that all potentially relevant studies were identified. This review also has certain limitations. First, though a preemptive protocol was developed by the authors, this review was not registered. Further, the small number of included studies limited the ability for a more robust meta-analysis and reduced confidence in the estimated effect size. This small number of studies also limited the ability to perform a subgroup analysis, which may have found a difference in outcome if performed (for example, analysis of blunt vs. penetrating trauma). In addition, the observational nature and high risk of bias of the included studies limited the strength of the evidence. Lastly, combination of randomized and observational data may further limit accuracy of the effect estimate.
Clinical implications
This review holds important implications for practice. As mentioned, the effect estimate of this review suggests that both high- and low-dose fluids have no mortality benefit for severely injured adult trauma patients in the prehospital setting. IV fluid administration also carries the potential inherent risks of coagulopathy, electrolyte disturbance, and artificial sBP inflation [3]. Though these findings may appear somewhat significant at face value, clinicians should be reminded of the low quality of currently available evidence, with only one randomized trial and several poor to moderate quality observational studies to guide practice.
At best, this review adds evidence to questioning the requirement for IV fluids in trauma given the lack of mortality benefit, and certainly demonstrates the need for more randomized studies in this area.
Research implications
This review holds important implications for research by identifying the need for randomized trials evaluating the effect of prehospital IV fluids in trauma patients. If undertaken, these trials should compare the prehospital administration of standard dose IV fluids to administering no fluid in patients with severe trauma (similarly to this review, defined as a SI > 1 or sBP < 90 mmHg).
Conclusion
Weak, primarily observational evidence suggests that standard fluid resuscitation has no significant mortality benefit over restricting/withholding IV fluids in the context of severe/hypotensive trauma. This review adds evidence to questioning the requirement for IV fluids in trauma given the lack of mortality benefit, in addition to demonstrating the need for more randomized studies in this area.
References
Moore L, Evans D, Hameed SM, et al. Mortality in Canadian trauma systems: a multicenter cohort study. Ann Surg. 2017;265(1):212–7. https://doi.org/10.1097/SLA.0000000000001614.
Statistics Canada. Leading causes of death, total population, by age group. https://doi.org/10.25318/1310039401-eng. Accessed 28 Oct 2022.
Greaves I, Porter KM, Revell MP. Fluid resuscitation in pre-hospital trauma care: a consensus view. J R Coll Surg Edinb. 2022;47(2):451–7. https://www.pubmed.ncbi.nlm.nih.gov/12018688/. Accessed 13 Aug 2021.
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. https://doi.org/10.1056/NEJM199410273311701.
Duke MD, Guidry C, Guice J, Stuke L, Marr AB, Hunt JP, Maeade P, McSwain NE Jr, Duschesne JC. Restrictive fluid resuscitation in combination with damage control resuscitation: time for adaptation. J Trauma Acute Care Surg. 2012;73(3):674–8. https://doi.org/10.1097/TA.0b013e318265ce1f.
Stiell IG, Nesbitt LP, Pickett W, et al. The OPALS major trauma study: impact of advanced life-support on survival and morbidity. CMAJ. 2008;178(9):1141–52. https://doi.org/10.1503/cmaj.071154.
Schreiber MA, Meier EN, Tisherman SA, Kerby JD, Newgard CD, Brasel K, Egan D, Witham W, Williams C, Daya M, et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–97. https://doi.org/10.1097/TA.0000000000000600.
Yaghoubian A, Lewis RJ, Putnam B, De Virgilio C. Reanalysis of prehospital intravenous fluid administration in patients with penetrating truncal injury and field hypotension. Am Surg. 2007;73(10):1027–30. https://doi.org/10.1177/000313480707301023.
Hampton DA, Fabricant LJ, Differding J, Diggs B, Underwood S, De La Cruz D, Holcomb JB, Brasel KJ, Cohen MJ, Fox EE, et al. Prehospital intravenous fluid is associated with increased survival in trauma patients. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S9–15. https://doi.org/10.1097/TA.0b013e318290cd52.
Kaweski SM, Sise MJ, Virgilio RW. The effect of prehospital fluids on survival in trauma patients. J Trauma. 1990;30(10):1215–9. https://doi.org/10.1097/00005373-199010000-00005.
Zitek T, Ataya R, Farino L, Mohammed S, Miller G. Is the use of greater than 1 L of intravenous crystalloids associated with worse outcomes in trauma patients? Am J Emerg Med. 2021;40:32–6. https://doi.org/10.1016/j.ajem.2020.12.013.
Eckstein M, Chan L, Schneir A, Palmer R. Effect of prehospital advanced life support on outcomes of major trauma patients. J Trauma. 2000;48(4):643–8. https://doi.org/10.1097/00005373-200004000-00010.
Liberman M, Roudsari BS. Prehospital trauma care: what do we really know? Curr Opin Crit Care. 2007;13(6):691–6. https://doi.org/10.1097/MCC.0b013e3282f1e77e.
Sampalis JS, Tamim H, Denis R, Boukas S, Ruest SA, Nikolis A, Lavoie A, Fleiszer D, Brown R, Mulder D, et al. Ineffectiveness of on-site intravenous lines: is prehospital time the culprit? J Trauma. 1997;43(4):608–17. https://doi.org/10.1097/00005373-199710000-00008.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration. 2020. https://www.training.cochrane.org/system/files/uploads/protected_file/RevMan5.4_user_guide.pdf. Accessed 3 Aug 2021.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldrige SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Deeks J, Higgins J, Altman D (2021) Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane. https://training.cochrane.org/handbook. Accessed 18 Aug 2021
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. https://doi.org/10.1136/bmj.n71.
Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cuschieri J, Sperry JL, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74(5):1207–14. https://doi.org/10.1097/TA.0b013e31828c44fd.
Dula DJ, Wood GC, Rejmer AR, Starr M, Leicht M. Use of prehospital fluids in hypotensive blunt trauma patients. Prehosp Emerg Care. 2002;6(4):417–20. https://doi.org/10.1080/10903120290938058.
Talving P, Pålstedt J, Riddez L. Prehospital management and fluid resuscitation in hypotensive trauma patients admitted to Karolinska University Hospital in Stockholm. Prehosp Disast Med. 2005;20(4):228–34. https://doi.org/10.1017/s1049023x00002582.
Ramesh GH, Uma JC, Farhath S. Fluid resuscitation in trauma: what are the best strategies and fluids? Int J Emerg Med. 2019;12(1):38. https://doi.org/10.1186/s12245-019-0253-8. (Published 2019 Dec 4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SH reports no conflict of interest, financial or otherwise. EK reports no conflict of interest, financial or otherwise. AC reports no conflict of interest, financial or otherwise. RO reports no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hébert, S., Kohtakangas, E., Campbell, A. et al. The efficacy of prehospital IV fluid management in severely injured adult trauma patients: a systematic review and meta-analysis. Can J Emerg Med 25, 200–208 (2023). https://doi.org/10.1007/s43678-023-00447-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43678-023-00447-9