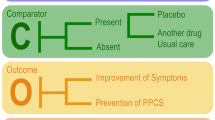
Abstract
Objective
This study investigates whether acute treatment with ibuprofen, acetaminophen, or both is associated with resolution of headache or reduction of headache pain at 7 days post-concussion in children and youth.
Methods
A secondary analysis of the Predicting and Preventing Post-concussive Problems in Pediatrics (5P) prospective cohort study was conducted. Individuals aged 5–18 years with acute concussion presenting to nine Canadian pediatric emergency departments (ED) were enrolled from August 2013 to June 2015. The primary outcome was the presence of headache at 7 days, measured using the Post-Concussion Symptom Inventory. The association between acute administration of ibuprofen, acetaminophen, or both and headache presence at 7 days was investigated with propensity scores and adjusted multivariate regression models.
Results
2277 (74.3%) of 3063 participants had headache upon ED presentation. Of these participants, 1543 (67.8%) received an analgesic medication before or during their ED visit [ibuprofen 754 (33.1%), acetaminophen 445 (19.5%), both 344 (15.1%); or no medication 734 (32.2%)]. Multivariate analysis pertained to 1707 participants with propensity scores based on personal characteristics and symptoms; 877 (51.4%) reported headache at 7 days post-concussion. No association emerged between treatment and presence of headache at 7 days [ibuprofen vs. untreated: (relative risk (RR) = 1.12 (95% CI 0.99,1.26); acetaminophen vs untreated RR = 1.02 (95% CI 0.87,1.22); both vs untreated RR = 1.02 (95% CI 0.86,1.18)].
Conclusions
Exposure to ibuprofen, acetaminophen, or both in the acute phase does not decrease the risk of headache at 7 days post-concussion. Non-opioid analgesics like ibuprofen or acetaminophen may be prescribed for short-term headache relief but clinicians need to be cautious with long-term medication overuse in those whose headache symptoms persist.
Résumé
Objectif
Cette étude vise à déterminer si un traitement aigu à l'ibuprofène, à l'acétaminophène ou aux deux est associé à la résolution des maux de tête ou à la réduction de la douleur des maux de tête 7 jours après la commotion cérébrale chez les enfants et les adolescents.
Méthodes
Une analyse secondaire de l'étude de cohorte prospective Predicting and Preventing Post-concussive Problems in Pediatrics (5P) a été réalisée. Des personnes âgées de 5 à 18 ans souffrant d'une commotion cérébrale aiguë se présentant dans neuf services d'urgence pédiatriques (SU) canadiens ont été inscrites d'août 2013 à juin 2015. Le résultat primaire était la présence de maux de tête à 7 jours, mesurée à l'aide du Post-Concussion Symptom Inventory. L'association entre l'administration aiguë d'ibuprofène, d'acétaminophène ou des deux et la présence de maux de tête à 7 jours a été étudiée à l'aide de scores de propension et de modèles de régression multivariés ajustés.
Résultats
2277 (74,3%) des 3063 participants avaient des maux de tête lors de la présentation aux urgences. Parmi ces participants, 1 543 (67,8%) ont reçu un médicament analgésique avant ou pendant leur visite aux urgences [ibuprofène 754 (33,1%), acétaminophène 445 (19,5%), les deux 344 (15,1%); ou aucun médicament 734 (32,2%)]. L'analyse multivariée a porté sur 1707 participants avec des scores de propension basés sur les caractéristiques personnelles et les symptômes; 877 (51,4%) ont signalé des maux de tête 7 jours après la commotion cérébrale. Aucune association n'est apparue entre le traitement et la présence de céphalées à 7 jours [ibuprofène vs non traité: (risque relatif (RR) = 1,12 (95%CI:0,99,1,26); acétaminophène vs non traité RR = 1,02 (95% IC: 0,87,1,22); les deux vs non traité RR = 1,02 (95% IC: 0,86,1,18)].
Conclusions
L'exposition à l'ibuprofène, à l'acétaminophène ou aux deux dans la phase aiguë ne diminue pas le risque de céphalées 7 jours après la commotion. Les analgésiques non opioïdes comme l'ibuprofène ou l'acétaminophène peuvent être prescrits pour soulager les maux de tête à court terme, mais les cliniciens doivent faire attention à la surconsommation de médicaments à long terme chez les personnes dont les symptômes de maux de tête persistent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is known about the topic? |
Few studies have investigated pharmacological treatment strategies to prevent or treat pediatric post-concussion headache. |
What did this study ask? |
What is the association between acutely administered ibuprofen, acetaminophen, or both and presence of headache at 7 days post-injury? |
What did this study find? |
Exposure to ibuprofen, acetaminophen, or both in the acute phase does not decrease the risk of headache at 7 days post-concussion. |
Why does this study matter to clinicians? |
Non-opioid analgesics like ibuprofen or acetaminophen may be prescribed for short-term headache relief post-concussion. |
Introduction
Headache is the most commonly reported symptom in the acute phase after concussion and among children who develop persistent post-concussion symptoms [1]. About 70% of children who have sustained a concussion will report ongoing headaches at 1-week, 25–58% at 1-month, 5–7.8% at 3 months, and 1.2% at 1 year [1,2,3]. No evidence-based pharmacological treatments have been established to manage post-concussion headache in the pediatric population, and few studies have investigated pharmacological treatment strategies to prevent or treat post-concussion headache [2, 4, 5].
Ibuprofen and acetaminophen are widely recommended for post-traumatic headache [6], but evidence that they effectively reduce the risk of persistent headache is lacking. Poorly controlled acute pain is associated with many negative outcomes, including poorer psychological outcome, reduced quality of life, reduced productivity and academic performance, delayed recovery time, increased risk of development of chronic pain, increased hospitalization costs, and economic burden [7,8,9,10,11,12]. Early abortive therapy for pain has been hypothesized as a means of preventing neuroplastic changes that may occur in persistent pain [13,14,15]. Given the delayed nature of the neurobiological cascade that follows a concussion [16], the acute phase may offer a therapeutic window for abortive therapies for preventing persistent headache [17].
This study investigated whether ibuprofen, acetaminophen, or a combination of both to treat acute pain and administered before and/or during an emergency department (ED) visit was associated with headache presence in children and youth 7 days later. We also evaluated whether acutely administered pain medication was associated with the level of headache pain 7 days post-concussion. We hypothesized that participants receiving such treatment would have a lower risk of headache presence and reduced pain a week later.
Methods
Design and settings
This is an unplanned secondary analysis of data from the Predicting Persistent Post-concussive Problem in Pediatrics (5P) study [18, 19], a prospective, multi-centre cohort study that recruited participants from August 2013 to June 2015 in nine Pediatric Emergency Research Canada network tertiary pediatric EDs across Canada.
Study population
The study population consisted of individuals aged 5.00–17.99 years who presented to the ED with an acute (< 48 h) head injury and who reported an acute headache. Acute headache was defined as a positive change from retrospective pre-injury rating to the ED-collected post-injury rating (i.e., on the day before or current day) on the headache item of the Post-Concussive Symptom Inventory. The Post-Concussive Symptom Inventory is a set of symptom scales for parents (20-items, 7-point dimensional scale), and developmentally specific self-report forms for 5- to 7-year-olds (13-items, 3-point scale), 8- to 12-year-olds (17-items, 3-point scale), and 13- to 18-year-olds (21-items, 7-point scale).[20]. Exclusion criteria were Glasgow Coma Scale score ≤ 13; any abnormality on brain computed tomography or magnetic resonance imaging; neurosurgical intervention, intubation, or intensive care unit admission; multi-system injury requiring hospitalization; severe pre-existing neurological developmental delay resulting in communication difficulties; intoxication; absence of trauma as primary event; previously enrolled; language barrier; or inability to follow-up by telephone or electronic mail. The 5P study was approved by the ethics committee at each of the nine institutions. Participants provided written assent or informed consent. In cases where children provided assent, parents were required to consent to the study.
Exposure of interest
Participants who received ibuprofen, acetaminophen, or both in the acute phase (within 48-h of the injury, before or during ED visit) comprised the treated groups. Participants who presented with headache but did not receive either drug before or during their ED visit comprised the untreated group.
Procedure
The study protocol was published previously [19]. Trained research assistants (RAs) conducted acute post-concussion ED evaluations. Participants rated headache and other symptom severity on the Post-Concussive Symptom Inventory [20]. Information was collected about the traumatic event and previous health history. Participants were asked if they had received ibuprofen or acetaminophen since injury. Information about ED treatment was provided by emergency physicians to RAs or derived by RAs based on chart abstraction and was documented electronically via Research Electronic Data Capture (REDCap) [21]. After their ED visit, participants completed the 7-day follow-up Post-Concussive Symptom Inventory questionnaires via REDCap or by telephone interview.
Outcomes
The primary outcome was the presence of headache at 7 days, a dichotomous variable defined as the difference between self-reported 7-day headache rating and retrospective pre-injury headache rating. A positive difference (increase on Post-Concussive Symptom Inventory scale of 1-point or more) was considered headache presence [18]. Headache presence at 7 days implies new or worse headache, compared with the pre-injury baseline. The 7-day outcome was chosen as this is the time period with the highest rate of recovery [22].
The secondary outcome was the level of headache pain at 7 days, a continuous variable representing the difference between self-reported 7-day headache rating and retrospective pre-injury rating. To ensure a common metric for the headache Post-Concussive Symptom Inventory item (delta score) for all participants, child rating delta scores [5- to 12-year-olds (original item range 0–2 [2 = A lot of symptoms])] were multiplied by 3 to align with the possible delta score range for adolescents [13- to 18-year-olds (original item range 0–6 [6 = Severe problem])]. All negative delta scores were truncated to zero.
Statistical analysis
Descriptive statistics
Frequencies and descriptive statistics were used to summarize participant baseline characteristics for the overall sample and by study group.
Propensity score analysis
A propensity score approach was applied to examine the association between treatment (ibuprofen, acetaminophen, combination, none) and headache presence at 7 days, where estimated propensity scores for each individual were regarded as a “summary confounder” and used for covariate adjustment in subsequent multivariable regression modelling. A propensity represents the probability of being assigned to a particular treatment group based on the participant’s values on a set of pre-determined variables (e.g., baseline characteristics) [23]. Participants with missing data on the outcome (headache at 7 days) or any of the necessary covariates were not included in the analysis. Baseline characteristics of those included and excluded from the analysis were compared.
Propensity score estimation
Given our four treatment groups, a multivariable multinomial logistic regression model was initially fitted to estimate four sets of propensity scores for each participant, corresponding to the probability of receiving ibuprofen, acetaminophen, both, or neither. To estimate propensity scores, 25 baseline measures were identified: age; sex; personal history of migraine headache; family history of migraine headache; headache severity in the ED (baseline Post-Concussive Symptom Inventory item delta score); duration of prior concussion (no prior concussion/concussion lasting < 1 week; prior concussion lasting ≥ 1 week); history of depression; history of anxiety; hospital site; early signs of concussion (appears dazed and confused, confused about events, answers questions slowly, repeats questions, forgets recent information); sensitivity to light (baseline Post-Concussive Symptom Inventory item delta score); sensitivity to noise (baseline Post-Concussive Symptom Inventory item delta score); number of concomitant pain/nausea medications received in ED (oral [morphine, dimenhydrinate, or ondansetron] and intravenous [metoclopramide, ondansetron, ketorolac, morphine, fluids]). In the propensity score model, flexible restricted cubic splines were applied to all continuous covariates to allow for nonlinearity in their relationship to the outcome. No interaction terms between covariates were specified in the model.
Approach to outcomes analysis
To avoid excessive extrapolation bias in our final outcomes analysis, we restricted our analysis to observations whose propensity scores (all four sets) were within regions of common support. An observation was considered outside this region and excluded if the propensity score for any given treatment was smaller than the largest minimum propensity score or greater than the smallest maximum across all four sets of propensity score estimates. For example, in Fig. 1 (online), the propensity score range for being in the acetaminophen group was from 0.02 (largest min) to 0.61 (smallest max). A participant was included if all four of their propensity scores were within this common region of interest. Restricting the propensity score range ensures that treatment effects are estimated predominately from relatively high information areas [24].
Once eligible observations were selected, multivariable regression models were fitted to estimate the relative risk for the primary outcome (headache at 7 days) associated with comparisons between treatment groups. We applied the modified Diaz-Quijano method [25], which transforms estimates from a logistic regression to a relative risk estimate. As a sensitivity analysis, we also applied a modified (robust) Poisson regression to directly estimate the relative risk. For covariate adjustment, to avoid multicollinearity, we included three of the four possible sets of propensity scores (after transforming them to logit scale), as well as 20 of the 25 variables originally used for propensity score estimation (owing to sparse cells counts, five variables on specific concomitant medications were excluded: oral administration of morphine and dimenhydrinate, and intravenous administration of morphine, ondansetron, and fluids). The purpose of reapplying these variables as covariates in the final outcomes model was to capture outcome heterogeneity (they also partly contribute to outcome explanation), which increased precision in our final treatment estimates. Flexible restricted cubic splines were applied to all continuous covariates in the outcomes model. A Holm–Bonferroni correction [26] was applied as multiplicity adjustment when estimating the treatment effects for all possible combinations of treatment arm pairs (6 total hypotheses).
To estimate treatment effect on our secondary outcome, headache pain level at 7 days, we conducted a linear regression using the same overall methodology as above.
All analyses were performed using R version 3.5.2 [27]. This was an unplanned sub-study; sample size was calculated for the original 5P study [18].
Results
A total of 2277 (74.3%) of 3063 participants, median age 12.5, had a headache upon ED presentation (flow diagram, Fig. 1). Of these participants, 1543 (67.8%) received an analgesic medication before or during their ED visit: ibuprofen 754 (33.1%), acetaminophen 445 (19.5%), or both 344 (15.1%); the remaining 734 (32.2%) received neither. Of those who received medication, 673 (43.6%) did so only before their ED visit; 277 (18.0%), only in the ED; and 593 (38.4%), both before and during the ED visit [eTable1 online]. Median (IQR) time from injury to triage was 3.00 (1.45, 15.52) hours.
Of the 2277 participants, 281 were lost to follow-up, and 60 were missing covariates. The remaining 1886 had complete data and were considered for the propensity score analysis. A total of 1707/1886 (90.5%) had a propensity score in the range of common support (non-outliers) and were included in the multivariate analysis [Fig. 1 online]. Of these, 877 (51.4%) had a positive headache score on the Post-Concussive Symptom Inventory at 7 days.
Table 1 presents characteristics of eligible participants and those included in the study. eTable 2 (online) presents the concomitant pain/nausea medications received during the ED visit. eTable 3 (online) compares baseline variables of those who contributed to the statistical analysis versus those who did not.
Effect of treatment on presence of headache at 7 days
Neither analysis showed a significant statistical association between administration of ibuprofen, acetaminophen or both, and headache presence at 7 days: ibuprofen vs. untreated: relative risk (RR) = 1.12 (95% CI 0.99, 1.26); acetaminophen vs untreated: RR = 1.02 (95% CI 0.87, 1.22); both vs. untreated: RR = 1.02 (95% CI 0.86, 1.18) (Tables 2 and 3). In addition, no evidence suggested that one treatment was more effective than any other in reducing the presence of headache at 7 days (Tables 2 and 3). However, confidence intervals for the comparison of both medications combined vs. ibuprofen alone indicated a 9% decrease in risk of headache at 7 days, with the interval ranging from a 21% decreased risk to a 4% increased risk.
Effect of treatment on reduction of headache pain level at 7 days
Median (IQR) delta headache scores in the ED for the different treatment groups are displayed in Table 1. Patients who did not receive pain medication had a lower median headache score in the ED [3.0 (IQR: 3.0,6.0)] and the lowest delta score for headache pain at 7 days [0.0 (IQR: 0.0,3.0)]. Those who received both medications had the highest median ED headache pain score [5.0 (IQR: 3.0,6.0)], and a 7-day median Post-Concussive Symptom Inventory score [1.0 (IQR: 0.0,5.0)]. All groups had a decreased median Post-Concussive Symptom Inventory headache score (within the range of 3–4 points) at 7 days. No associations emerged between acute treatment and reduced headache pain at 7 days: ibuprofen vs. untreated: estimate difference = 0.18 (− 0.03, 0.39); acetaminophen vs. untreated: estimate difference = 0.05 (95% CI − 0.19, 0.30); both vs. untreated: estimate difference = − 0.01 (95% CI − 0.28, 0.26) (Table 4).
Discussion
We found that analgesic medications were frequently used to relieve pain in children and youth with acute concussion, either just before or during their ED visit. More than half reported headache presence at 7 days post-concussion, but in all groups, pain had decreased considerably. No significant statistical association was found between the acute use of common analgesic medications and headache presence at 7 days. Clinically, the findings suggest a potential risk associated with the acute administration of ibuprofen. After adjusting for numerous personal characteristics such as ED headache pain, participants exposed to ibuprofen in the acute phase appeared to be more likely than the untreated group to report the presence of headache at 7 days. None of the treatments increased the level of headache pain level at 7 days.
Data on the use of these drugs and their acute efficacy and role in reducing the presence of headache post-concussion are limited. A feasibility pilot RCT (N = 79;20 per group) with controlled standard dosing over a 3-day period found that routine administration of ibuprofen (10 mg/kg every 8 h, max dose of 3200 mg/day), acetaminophen (10–15 mg/kg every 4 h, max dose 4 g/day), or a combination (alternation) of both, compared with standard clinical care for 72 h, decreased the intensity and number of headaches per day and facilitated return to school 7 days post-concussion [28]. Similar to our results, the most pronounced decreases were in the group that combined both medications. Contrary to our results, Petrelli et al. found that ibuprofen also reduced intensity of headache symptoms. The reason why children in our study who took ibuprofen had increased risk of having headaches at 7 days is unclear. Given that Petrelli et al. [28] was a feasibility trial, a large comparative effectiveness study designed to examine pharmacotherapy is required to investigate the effect of these drugs in the acute and post-acute phases.
Strengths and limitations
The current study was based on a large prospective pan-Canadian observational study with good generalizability to both an ED pediatric sample and to the different possible mechanisms of injury. However, this study was not a randomized clinical trial; confounding variables may be unequally distributed, which might explain differences between treatments and propensity scores. The results were based on the administration of drugs in the acute phase (before and during the ED visit); the study was not designed to control for treatment after ED discharge. Thus, we cannot conclude that pain management in the acute phase is not linked to a lower headache risk at 7 days. Because we did not control for post-ED treatment or dosage, we do not know if the presence of headache at 7 days was related to overuse of medication [29] or whether the untreated group took these medications post-ED. Information about the time of administration, dose frequency, or dosages of pain medication patients received before or during their ED visit was not part of the original 5P design. We did not collect data on headache immediately after ED treatment; we cannot conclude that these treatments are associated with reduced headache pain in the immediate post-ED period. We did not adjust for any other types of analgesia medication taken before the ED visit.
Clinical implications
Guidelines recommend ibuprofen and acetaminophen to treat acute post-concussion headache pain [30] and recently were identified as the most widely used analgesia in the ED to control for moderate post-concussion headache pain [2, 4]. These routine analgesics have been reported to be safe in the general pediatric population [31]. Non-opioid analgesics like ibuprofen or acetaminophen may be prescribed for short-term headache relief but caution must be taken not to overuse these medications given that they can trigger and exacerbate headache.
Research implications
Evidence supporting the use of these treatments post-injury is lacking [4]. In light of our results, more research and careful planning of future studies are necessary to evaluate whether treatment of post-traumatic headache with ibuprofen and/or acetaminophen is beneficial and whether these treatments can reduce persistent post-traumatic headache when administered acutely. A large comparative effectiveness study designed to prospectively track dosage, frequency, and duration of use of common analgesics post-injury is required to determine the benefit of these drugs in the acute and post-acute phases of concussion.
Conclusions
For 5- to 18-year-olds with concussion-related headache, treatment with ibuprofen, acetaminophen or both just before or during an ED visit did not have a statistically significant association with headache presence 7 days later. Non-opioid analgesics like ibuprofen or acetaminophen may be prescribed for short-term headache relief, but clinicians need to be cautious with long-term medication overuse in those whose headache symptoms persist.
References
Eisenberg M, Meehan WP, Mannix R. Duration and course of post-concussive symptoms. Pediatrics. 2014;133(6):999–1006.
Blume HK. Posttraumatic headache in pediatrics. Curr Opin Pediatr. 2018;30(6):755–63.
Kuczynski A, Crawford S, Bodell L, Dewey D, Barlow KM. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: A prospective cohort. Dev Med Child Neurol. 2013;55(7):636–41.
Lambrinakos-Raymond K, Dubrovsky AS, Gagnon I, Zemek R, Burstein B. Management of pediatric post-concussion headaches: national survey of abortive therapies used in the emergency department. J Neurotrauma. 2022;39(1–2):144–50.
Kamins J, Richards R, Barney BJ, Locandro C, Pacchia CF, Charles AC, et al. Evaluation of posttraumatic headache phenotype and recovery time after youth concussion. JAMA Netw Open. 2021;4(3):1–12.
Halstead ME. Pharmacologic therapies for pediatric concussions. Sports Health. 2016;8(1):50–2.
Glowacki D. Effective pain management and improvements in patients’ outcomes and satisfaction. Crit Care Nurse. 2015;35(3):33–41.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25.
Lin RJ, Reid MC, Liu LL, Chused AE, Evans AT. The barriers to high-quality inpatient pain management: a qualitative study. Am J Hosp Palliat Care. 2015;32(6):594–9.
Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO’s classification of functioning, disability and health (ICF). J Headache Pain. 2005;6(6):429–40.
Bellini B, Arruda M, Cescut A, Saulle C, Persico A, Carotenuto M, et al. Headache and comorbidity in children and adolescents. J Headache Pain. 2013;14(1):79.
Bruijn J, Locher H, Passchier J, Dijkstra N, Arts W-F. Psychopathology in children and adolescents with migraine in clinical studies: a systematic review. Pediatrics. 2010;126(2):323–32.
Rome HP, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med. 2000;1(1):7–23.
Walker WC, Marwitz JH, Wilk AR, Ketchum JM, Hoffman JM, Brown AW, et al. Prediction of headache severity (density and functional impact) after traumatic brain injury: A longitudinal multicenter study. Cephalalgia. 2013;33(12):998–1008.
Post RM, Susan R, Weiss B. Sensitization, kindling, and carbamazepine: an update on their implications for the course of affective illness. Pharmacopsychiatry. 1992;25(1):41–3.
Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2015;75(4):S24-33.
Kamins J, Charles A. Posttraumatic headache: basic mechanisms and therapeutic targets. Headache. 2018;58(6):811–26.
Zemek R, Barrowman N, Freedman SB, Gravel J, Gagnon I, Mcgahern C, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA J Am Med Assoc. 2016;315(10):1014–25.
Zemek R, Osmond MH, Barrowman N. Team PERC (PERC) C Predicting and preventing postconcussive problems in paediatrics (5P) study: protocol for a prospective multicentre clinical prediction rule derivation study in children with concussion. BMJ Open. 2013;3:8.
Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol. 2014;29(4):348–63.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Ledoux A-A, Tang K, Yeates KO, Pusic MV, Boutis K, Craig WR, et al. Natural progression of symptom change and recovery from concussion in a pediatric population. JAMA Pediatr. 2019;173:1.
Haukoos JS, Lewis RJ. The propensity score. JAMA J Am Med Assoc. 2015;314(15):1637–8.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Diaz-Quijano FA. A simple method for estimating relative risk using logistic regression. BMC Med Res Methodol. 2012;12(1):14.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70.
R_Core_Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna; 2018.
Petrelli NP TM, Farrokhyar MPhil F, McGrath P, Sulowski FRCPC C, Sobhi PHM GB, DeMatteo DipP C, et al. The use of ibuprofen and acetaminophen for acute headache in the postconcussive youth: A pilot study. 2017;2–6.
Heyer GL, Idris SA. Does analgesic overuse contribute to chronic post-traumatic headaches in adolescent concussion patients? Pediatr Neurol. 2014;50(5):464–8.
Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, et al. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172(11): e182853.
Southey ER, Soares-Weiser K, Kleijnen J. Systematic review and meta-analysis of the clinical safety and tolerability of ibuprofen compared with paracetamol in paediatric pain and fever. Curr Med Res Opin. 2009;25(9):2207–22.
Acknowledgements
We would like to acknowledge all research coordinators and research assistants across the nine sites responsible for patient recruitment, enrollment and follow-up. Student volunteers at CHEO, ACH, CHUSJ and HSC provided invaluable assistance in patient screening at the Emergency Department. We would also like to acknowledge the Predicting Persistent Post-concussive Problems in Pediatrics (5P) team: Peter Anderson, PhD; Miriam H. Beauchamp, PhD; Darcy Beer, MD; Kathy Boutis, MD; Brian L. Brooks, PhD; Emma Burns, MD; William Craig, MDCM ; Carol DeMatteo, MSc; Ken J. Farion, MD; Isabelle Gagnon, PhD; Gerard Gioia, PhD; Blaine Hoshizaki, PhD; Yael Kamil, BSc; Michelle Keightley, PhD; Terry Klassen, MD; Candice McGahern, BA; William P. Meehan III, MD; Willem Meeuwisse, MD, PhD; Angelo Mikrogianakis, MD; Gurinder Sangha, MD; Martin H. Osmond, MDCM; Michael Vassilyadi, MDCM, MSc; Keith Owen Yeates, PhD.
Funding
Dr. Stephen Freedman is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness. Roger Zemek holds a Clinical Research Chair in Pediatric Concussion from University of Ottawa, has received competitively funded research grants from the National Football League (NFL) Scientific Advisory Board, is on the concussion advisory board for Parachute Canada (a non-profit injury prevention charity), and is the co-founder, Scientific Director and a minority shareholder in 360 Concussion Care, an interdisciplinary concussion clinic. Dr. Rebekah Mannix is supported by the Children’s Hospital Physicians’ Association Research Award. Dr. Yeates is supported by the Ronald and Irene Ward Chair in Pediatric Brain Injury from the Alberta Children’s Hospital Foundation and has received competitively funded research grants from the National Football League (NFL) Scientific Advisory Board. This study was supported by the Canadian Institutes of Health Research (CIHR) operating grant (MOP 126197); the CIHR-Ontario Neurotrauma Foundation Mild Traumatic Brain Injury team grant (TM1 127047); and CIHR planning grant (MRP 119829).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors AAL, KT, SBF, JG, KB, KY, RM, LR, MB, and RZ have no conflict of interest to disclose. No honorarium, grant or other form of payment was given to anyone to produce the manuscript. All authors have no financial disclosures relevant to this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ledoux, A.A., Tang, K., Freedman, S.B. et al. Early analgesic administration and headache presence 7 days post-concussion in children. Can J Emerg Med 24, 876–884 (2022). https://doi.org/10.1007/s43678-022-00367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43678-022-00367-0