Abstract
To improve bacterial photodynamic inactivation (PDI), this work analyzes the photodynamic effect caused by the combination of photosensitizers (PSs) on two bacterial models and different growth mode. Simultaneous administration of PSs from different families, zinc(II) 2,9,16,23-tetrakis[4-(N-methylpyridyloxy)]phthalocyanine (ZnPPc4+), 5,10,15,20-tetra(4-N,N,N-trimethylammonium phenyl)porphyrin (TMAP4+), meso-tetrakis(9-ethyl-9-methyl-3-carbazoyl)chlorin (TEMCC4+) and 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl] chlorin (TAPC) was investigated against Staphylococcus aureus and Escherichia coli, in planktonic form, biofilm and growth curve. Various PSs combinations showed greater inactivation compared to when used separately under the same conditions but at twice the concentration. However, differences were found in the effectiveness of the PSs combinations on Gram positive and negative bacteria, as well as in planktonic or biofilm form. Likewise, the combination of three PSs completely stopped E. coli growth under optimal nutritional conditions. PSs combination allows extending the range of light absorption by agents that absorb in different areas of the visible spectrum. Therefore, PDI with combined PSs increases its antimicrobial capacity using agents’ concentrations and light fluences lower than those necessary to cause the same effect as single PS. These advances represent a starting point for future research on the potentiation of PDI promoted by the combined use of PSs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bacterial infections pose a serious health problem that has drawn public attention worldwide due to the development and spread of antibiotic resistance [1, 2]. Antimicrobial resistance (AMR) makes treatments tedious and has adverse consequences, such as prolonged hospitalization, increased medical cost, overloading the public health system, and even increased mortality rates [3, 4]. Furthermore, microorganisms naturally have the ability to develop as biofilms. Their formation allows them to adapt and protect themselves from hostile environments, antimicrobials, and the defense mechanisms of the immune system of host organisms. Therefore, bacteria in biofilms are more resistant to antimicrobial treatments and host immune defense mechanisms than planktonic cell [5, 6]. The viscous matrix of biofilm can slow down diffusion and even act as a complete barrier against drug penetration, representing a significant therapeutic barrier for many antibiotics and a strategy for bacteria to develop host resistance [7]. This gives rise to biofilms generating infections that cause serious, persistent and/or chronic diseases, leading to prolonged hospital stays, with upper costs and high mortality. These global health threats have stimulated interest in the development of effective antimicrobial therapies with primary goals focused on this problem.
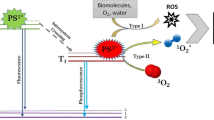
New approach to treat bacteria resistant to antibiotics and biofilms is photodynamic inactivation (PDI) [8, 9]. This alternative utilizes a photosensitizer (PS), visible light and oxygen to produce cytotoxic reactive oxygen species (ROS) [10, 11]. As a multiple target strategy, the generated ROS can not only oxidize several cellular components (e.g., lipids and DNA), that cause destruction of pathogenic microorganism, but can also attack extracellular polymeric substances molecules, causing the degradation of matrix structure [7, 12]. Therefore, this is an interesting therapy due to its noninvasiveness, flexibility and minimal risk of inducing microbial resistance [13, 14].Furthermore, photoinactivation is independent of its AMR pattern and generates a broad spectrum of antimicrobial activity; consequently it does not require identification of the bacteria and is very useful for infections caused by various microorganisms [11, 15].
As a general principle, PDI employs a single photosensitizing agent to induce inactivation of microbial pathogens [2, 8]. Most of the PSs investigated for PDI are porphyrinoid derivatives, with a heterocyclic ring structure, such as porphyrin, chlorins or phthalocyanine [14, 16,17,18]. Nowadays, drug combinations are increasingly used in the treatment of many conditions and diseases [19,20,21,22]. In particular, different combination strategies have been employed to attempt to increase bacterial photoinactivation: photodynamic treatment combined with potassium iodide [23, 24], antibiotics [25,26,27,28,29], antifungal [30, 31], or host defense mechanisms [32], PS incorporated with nanomaterials [33] or administration of PS covalently linked to an antimicrobial compound [34]. However, these approaches are based on the use of a merge of therapies with varied mechanisms of action. On the other hand, the simultaneous use of PSs for photodynamic therapy of cancer has been explored [35, 36]. Nevertheless, there is scarce literature on the effect of using light-activated PSs mixtures for bacterial inactivation [37, 38]. This combination could produce distinct impacts, such as potentiation or reduction of the photodynamic effect. Therefore, it is necessary to study the application of PDI using different combinations of PSs, to determine the consequence on bacteria.
The aim of this study was to explore the photodynamic effect of photosensitizing agents, derived from the main families of macrocycles, administrated simultaneously in bacterial cultures. The combinations of PSs that absorb in different areas of the spectrum allow extending the range of light absorption to gain PDI efficiency. Thus, according to the photochemical properties, the photodynamic action of the combined PSs was analyzed in two bacteria models (Gram positive and Gram negative), under different bacterial growth conditions (planktonic, biofilm) and optimal nutritional conditions (growth curve) to determine the interactions that can cause, using low PS concentrations and doses of light.
2 Materials and methods
2.1 Photosensitizers
Zinc(II) 2,9,16,23-tetrakis[4-(N-methylpyridyloxy)]phthalocyanine (ZnPPc4+), meso-tetrakis(9-ethyl-9-methyl-3-carbazoyl)chlorin (TEMCC4+) and 5,10,15,20-tetrakis[4-(3-N,N-dimethylamino propoxy)phenyl]chlorin (TAPC) were synthesized as previously described [39,40,41]. 5,10,15,20-Tetra(4-N,N,N-trimethylammoniumphenyl)porphyrin (TMAP4+) was purchased from Aldrich (Milwaukee, WI, USA). PS stock solutions (0.5 mM) were obtained by dissolution in 1 mL of N,N-dimethylformamide (DMF) (Merck, Darmstadt, Germany). The concentration was confirmed by spectroscopy. Bacterial cultures were irradiated with a Novamat 130 AF (Braun Photo Technik, Nürnberg, Germany) slide projector containing a 150 W lamp. Wavelength range between 350 and 800 nm was selected by optical filters with a fluence rate of 30 mW/cm2 at 650 nm. Emission spectrum of the light source is depicted in Fig. S1. Figure S2 shows the overlap of the emission spectra of the lamp and the absorption spectra of each PS. Furthermore, Fig. S3 shows the superimposed spectra of the different combinations of PSs with the emission spectrum of light used.
2.2 Photodynamic inactivation of planktonic bacteria
E. coli and S. aureus strains were used as representatives of Gram negative and Gram positive bacteria, respectively [42, 43]. Both bacteria were grown in tryptic soy (TS, Britania, Buenos Aires, Argentina) broth at 37 ºC overnight. S. aureus was incubated on a rotator shaker (100 rpm) and E. coli under static conditions. Aliquots (100 μL) were aseptically transferred to fresh medium (4 mL) and incubated at 37 ºC to the mid-log phase (absorbance ~ 0.4 for S. aureus and ~ 0.6 for E. coli at 660 nm). Bacteria were then centrifuged (3,000 rpm for 15 min) and re-suspended in an equal amount of 10 mM phosphate-buffered saline (PBS, pH 7.0) solution. Cells were diluted 1/1000 in PBS, corresponding to ~ 106 colony-forming units (CFU)/mL. In all experiments, 2 mL of this cell suspension were placed in tubes and the appropriate concentration of each PS alone or a combination was added. All samples were incubated in the dark for 15 min at 37 ºC. Then the cultures were exposed for different time intervals to visible light. Control and irradiated cell suspensions were tenfold serially diluted with PBS. Each solution was spread in triplicate on TS agar (Agar–agar, Britania, Buenos Aires, Argentina), the number of colonies formed after 18–24 h incubation at 37 ºC were counted and the mean determined.
2.3 Photodynamic inactivation of bacterial biofilm
Biofilm photoinactivation was developed as proposed by Reynoso et al. with modifications [43]. Bacteria were grown overnight as described above. S. aureus and E. coli fresh culture were standardized adjusting the absorbance (0.4 for S. aureus and 0.6 for E. coli at 660 nm). Then 100 μL of cell suspension was inoculated onto 24 well polystyrene microtiter plates (Deltalab, Barcelona, Spain) contained 1.9 mL of TS broth and a sterile acrylic disc (12 mm in diameter) per well. Plates were incubated for 24 h at 37 °C with agitation (100 rpm). Then the discs with the biofilm were aseptically washed twice with PBS (2 mL) to remove non-adherent cells. Subsequently, acrylic discs were incubated for 15 min with PS in dark, and then exposed to visible light for 30 min. Finally, the biofilm was disrupted mechanically with a vortex stirrer (IKA, Wilmington, USA) to take off the adhered bacteria. Cellular suspensions were tenfold serially diluted with PBS and each solution was quantified by the spread plate technique in triplicate. After 18–24 h incubation at 37 ºC viable S. aureus cells were counted and the number of CFU was determined. Additionally, wells containing sterile culture medium were included as sterility control.
2.4 Photodynamic inactivation of E. coli growth curve
E. coli cultures were grown overnight as described above. A portion (60 μL) of this culture was transferred to 20 mL of fresh TS broth. Aliquots (2 mL) of this suspension were incubated with the PS or with the mixture of PSs at 37 ºC and irradiated with visible light throughout the experiment. The culture grown was measured by absorbance at 550 nm using a Tuner SP-830 spectrophotometer [43].
2.5 Controls and statistical analysis
Control experiments were carried out in the presence of PSs without illumination and in the absence of PSs and irradiated. The experiments were repeated separately three times. The amount of DMF (< 1% v/v) used in each experiment was not toxic to microbial cells. Data were depicted as the mean ± standard deviation of each group. Variation between each experiment was calculated using the one-way ANOVA, with a confidence level of 95% (p < 0.05) considered statistically significant.
3 Results and discussion
3.1 Photosensitizers
The molecular structures of PSs used in this study are represented in Scheme 1. These agents belong to three large families of tetrapyrrolic macrocycles and have been proposed as active PSs for the PDI of microorganisms [39,40,41, 43, 44]. The spectroscopic and photodynamic properties and the spectral area of irradiation of each PSs used in this work are summarized in Table 1. The absorption spectra of TMAP4+, ZnPPc4+, TEMCC4+ and TAPC in DMF (Fig. 1) present sharp absorption bands indicating that PSs were mainly dissolved as monomer in this solvent. These PSs were chosen for their excellent individual PDI capabilities and for their complementary wavelengths absorption range. In particular, the TMAP4+ porphyrin-derived structure exhibits a high absorption coefficient at 412 nm (Soret band). In addition, chlorins derivatives (TAPC and TEMCC4+) present an important absorption band close to this wavelength (422 and 432 nm, respectively). Likewise, these PSs absorb at longer wavelengths, (Q bands, 650–670 nm) in the phototherapeutic window (600–800 nm) [40, 41]. On the other hand, ZnPPc4+ exhibits an intense absorption of red light, specifically at 678 nm [18, 39]. Thus, the light delivered by the irradiation system used allows all PSs to be excited simultaneously (350 and 800 nm, Fig. S1). Superposition of the spectral emission of the light source with the absorption spectra of each PS shows a broad excitation area of the PS, especially of phthalocyanine (Fig. S2). Moreover, the overlap of the absorption spectra of the PSs together and the lamp emission show that this capability increases when the chosen combinations are between phthalocyanine and porphyrin or chlorins. However, it is very similar when porphyrin is combined with chlorins (Fig. S3). This correlates with the spectral irradiated areas shown in Table 1. The largest irradiated area is exhibited by phthalocyanine. In addition, this PS is the only one that presents an appreciable absorbance in the red zone of the spectrum. Therefore, the incorporation of ZnPPc4+ with porphyrins and chlorines allows it to extend its absorption zone to the blue zone of the spectrum.
3.2 Photodynamic inactivation of planktonic bacteria
Photodynamic activity of each PS (TMAP4+, ZnPPc4+, TEMCC4+ and TAPC) was investigated to inactivate S. aureus and E. coli planktonic culture at different light doses, separately. Figure 2 shows the survival of these microorganisms after PDI. E. coli planktonic cells with TMAP4+ (1 μM) or ZnPPc4+ (2 μM), were completely inactivated after 30 min of irradiation (54 J/cm2) (p < 0.05) (Fig. 2A, bars 6 and 10). However, these concentrations were dark toxic for planktonic S. aureus (Fig. S4). Therefore, PSs concentration was reduced (0.25 μM) (Fig. 2B). Under these experimental conditions S. aureus planktonic cells were decreased ~ 3.5 log (99.97% of inactivation) for both PSs after 30 min irradiation (54 J/cm2) compared with control cultures without PS (p < 0.05) (Fig. 2B, bars 6 and 10). This significant difference in the photoinactivation capacity of PSs on Gram positive and negative bacteria is largely attributed to structural differences in their cell envelopes [18, 41]. On the other hand, E. coli survival was not modified by TEMCC4+-PDI, even at 10 μM and the longest exposure time to light (Fig. 2A, bar 14). Instead, this PS, at the same concentration, inactivates the Gram-positive bacteria (Fig. 2B, bars 13 and 14). This PDI depends on irradiation time, decreasing ~ 3 log (99.8%) of inactivation at the maximum light dose (p < 0.05). Besides, TAPC diminished the viability of both bacteria. The concentration required to induce 99.9% inactivation, after 30 min irradiation (54 J/cm2), was 5 and 1 μM for E. coli and S. aureus, respectively (Fig. 2A and B, bar 18). These results are in concordance with those previously found for these PSs and microorganisms [18, 39, 41, 44]. Nevertheless, some reports on the efficiency of cationic PSs on the PDI of planktonic bacteria are difficult to compare, mainly due to the use of different bacterial strains, densities and irradiation systems.
PDI of A E. coli planktonic cells incubated with different PS for 30 min at 37 ºC in dark and exposed to different light doses. (1) Control cultures without PS and irradiated 30 min (54 J/cm2), (2) Control cultures without PS in dark, (3–6) 1 µM TMAP4+, (7–10) 2 µM ZnPPc4+, (11–14) 10 μM TEMCC4+ and (15–18) 5 µM TAPC, in dark, irradiated 5 min (9 J/cm2), 15 min (27 J/cm2) and 30 min (54 J/cm2), respectively and B S. aureus planktonic cells incubated with different PS for 30 min at 37 ºC in dark and exposed to different light doses. (1) Control cultures without PS and irradiated 30 min (54 J/cm2), (2) Control cultures without PS in the dark, (3–6) 0.25 µM TMAP4+, (7–10) 0.25 µM ZnPPc4+, (11–14) 10 μM TEMCC4+ and (15–18) 1 µM TAPC, in dark, irradiated 5 min (9 J/cm2), 15 min (27 J/cm2) and 30 min (54 J/cm2), respectively. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with control cultures cells
Figure 3 shows the results of testing the photodynamic action of simultaneous administration of PSs against E. coli (Fig. 3A) and S. aureus (Fig. 3B) planktonic cells. The concentration of each PS was half of that tested separately, except for TEMCC4+ on E. coli cell. In this case, the same concentration was used due to the low photoinactivation obtained. The use of lower concentrations of the PSs is intended to investigate whether similar results can be obtained or their activity enhanced by using half the concentration as when the PSs are used separately. This would be doubly beneficial, being more environmentally friendly and economical. Thus, this experiment was compared with the photoinactivation produced with the same PSs separately (Fig. 2) to determine the effect caused by the combinations of PSs. Complete inactivation of planktonic E. coli with TMAP4+ (0.5 μM) + ZnPPc4+ (1 μM) was accomplished after 30 min irradiation (54 J/cm2) (p < 0.05) (Fig. 3A, bar 5). This inactivation was similar to that found with these PSs separately (Fig. 2A, bars 6 and 10). Total bacterial eradication did not allow distinguishing the enhancement of the PDI effect at the same light dose (54 J/cm2). However, at shorter light doses, a greater death can be observed when both PSs were administered simultaneously, compared to the effect shown by the PSs separately, at double concentration. Likewise, the combination of TMAP4+ (0.5 μM) with TAPC (2.5 μM) induced a complete decrease in E. coli viability after 30 min of illumination (54 J/cm2). In this case, an increase in the inactivation of Gram-negative bacteria is observed relative to TAPC alone (3 log, 5 μM, Fig. 2A, bar 18). Furthermore, after 15 min of irradiation (27 J/cm2), the mutual use of TMAP4+-TAPC inactivated 99.999% of planktonic E. coli in contrast to the effectiveness of these agents separately, (99.97% and 99.77%), respectively, under the same irradiation conditions. Therefore, this combination presents an enhancer effect with respect to the effect caused by TAPC alone. On the other hand, the administration of phthalocyanine with chlorins, TEMCC4+ or TAPC (Fig. 3A, bar 17 or 21), drastically reduced the photodynamic effect of ZnPPc4+ (Fig. 2A, bar 10), but maintained the photodynamic action of TAPC or TEMCC4+ against E. coli (Fig. 2A, bar 18 or 14). Similarly, when TEMCC4+ and TMAP4+ were combined (Fig. 3A, bar 9), chlorin nullifies the effect of TMAP4+. Therefore, the combined use of TEMCC4+ with porphyrin or phthalocyanine has a lower photodynamic effect against E. coli planktonic cultures with respect to the action of these PSs separately. Nevertheless, TAPC only decreases the photodynamic action of ZnPPc4+, without affecting the action of TMAP4+.
PDI of A E. coli planktonic cells incubated with different PS combinations for 30 min at 37 ºC in dark and exposed to different light doses. (1) Control cultures without PS and irradiated 30 min (54 J/cm2), (2–5) 0.5 µM TMAP4+ + 1 µM ZnPPc4+, (6–9) 0.5 µM TMAP4+ + 10 µM TEMCC4+, (10–13) 0.5 µM TMAP4+ + 2.5 µM TAPC, (14–17) 1 µM ZnPPc4+ + 10 µM TEMCC4+ and (18–21) 1 µM ZnPPc4+ + 2.5 µM TAPC, and B S. aureus planktonic cells incubated with different PS combinations for 30 min at 37 ºC in dark and exposed to different light doses. (1) Control cultures without PS and irradiated 30 min (54 J/cm2), (2–5) 0.125 µM TMAP4+ + 0.125 µM ZnPPc4+ (6–9) 0.125 µM TMAP4+ + 5 µM TEMCC4+ (10–13) 0.125 µM TMAP4+ + 0.5 µM TAPC, (14–17) 0.125 µM ZnPPc4+ + 5 µM TEMCC4+ and (18–21) 0.125 µM ZnPPc4+ + 0.5 µM TAPC in dark, irradiated 5 min (9 J/cm2), 15 min (27 J/cm2) and 30 min (54 J/cm2), respectively. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with control cultures
Moreover, dual use of TMAP4+-ZnPPc4+ on planktonic cultures of S. aureus showed an enhancement of photodynamic action, after 15 and 30 min irradiation (27 and 54 J/cm2, respectively) (Fig. 3B, bars 4 and 5), compared with these PSs separately (Fig. 2B, bars 5, 6, 9 and 10), at the same light doses but at twice the concentration (p < 0.05). The combination of both PSs allowed reducing the concentration used in the treatments with respect to the compounds separately to obtain effective photoinactivation [39, 41, 43]. This effect is due to the small overlap of the spectra of both PSs. This minor superposition allows both PSs to excite and produce ROS. In addition, these PSs have the highest quantum yield of singlet oxygen production. Thus, both molecules can absorb light, be photoactivated and yield more ROS than when PSs are alone. Otherwise, less inactivation was observed when phthalocyanine was combined with chlorins (TEMCC4+ or TAPC) (Fig. 3B, bars 17 or 21) or when porphyrin was added with TEMCC4+ (Fig. 3B, bar 9) than with these PSs alone, as on E. coli planktonic cultures. The addition of chlorin reduces the amount of light that the other PS (porphyrin or phthalocyanine) can absorb (Fig. S3) and consequently decreases their subsequent ROS production. However, the simultaneous administration of TAPC-TMAP+ does not produce significant differences regarding its separate photodynamic action on S. aureus. This could be because both PSs are very effective separately and, at such low concentrations, the potentiation effect cannot be distinguished.
According to the results obtained, the combinations of PSs that possess their maximum absorption peak in different areas of the spectrum maintain their effectiveness when they are photoactivated together. Furthermore, in most cases, its activity is enhanced, even at one-half the concentration used when PS is alone. However, this effect depends on the combination of PSs used and the microorganism to be inactivated. Previous investigation observed that the simultaneous use of curcumin (λmax 428 nm) and methylene blue (λmax 665 nm), which absorb in different spectral areas but do not belong to the group of tetrapyrrolic macrocycles, did not result in an additive action against S. aureus, since the same inactivation as when the PSs were applied separately was found [38]. The authors reported that the combined use of the nitrogen-based-PS and curcumin mainly produced a competition between them for the formation of ROS and 1O2. This is because curcumin acts as an oxygen trap.
On the other hand, the use of PSs that absorb in the same area of the spectrum shows light attenuation by another PS (Fig. S3). This effect allows inducing the activation of a single PS and, therefore, only generates ROS that cause similar damage to that produced when it is the only agent. Furthermore, PSs with reduced photokilling capacity, such as TEMCC4+, decreases the effectiveness of PSs that are photoactive when alone. Nevertheless, the literature on combining PS molecules to photoinactivate microorganisms is limited to a few reports. One of these studies investigated the photoinactivation of planktonic S. aureus induced by the combined use of PSs to improve the PDI, but linking them covalently to form dyads [45]. The photodynamic activity of the cationic porphyrin-fullerene C60 dyad on S. aureus was greater than that obtained with the separate dyad moieties. However, the dyad concentration was much higher than that of the PSs used in this work. Moreover, it is easier to add them separately, to avoid the many synthesis steps [46].
3.3 Photodynamic inactivation of bacterial biofilm
Microbial biofilms are known to be difficult to inactivate due to the anionic hydrophilic polymeric matrix that coats them, which decreases the penetration and availability of PSs and light in the deeper layers and therefore reduces the photosensitizing process [7, 43]. Consequently, the concentration of PSs was doubled with respect to that used on planktonic cultures. Control cultures displayed that the viability of the bacteria was unaffected by increasing the concentration of these PSs in the dark or by light, separately (Fig. S5). Figure 4 shows that all PSs and their combinations significantly reduced the survival of E. coli biofilm compared to the control (Fig. 4, line 1) (p < 0.05). The photoinactivation of PSs against E. coli biofilm, after 30 min irradiation, followed the increasing order: TEMCC4+ < TAPC < ZnPPc4+ < TMAP4+. This inactivation sequence was the same as that found on E. coli planktonic cultures, but employing twice the concentration of each PS. Besides, PSs were used at very different concentrations between them: 20, 10, 4 and 2 μM, respectively. Although the highest concentrations of PSs used were those of chlorins, they were the least efficient on Gram-negative bacteria.
PDI of E. coli biofilm incubated with different PSs or combinations of them for 15 min and irradiated with visible light for 30 min (54 J/cm2). (1) Control culture without PS in dark, (2) 2 µM TMAP4+, (3) 4 µM ZnPPc4+, (4) 20 µM TEMCC4+, (5) 10 µM TAPC, 6) 1 µM TMAP4+ + 2 µM ZnPPc4+, (7) 1 µM TMAP4+ + 10 µM TEMCC4+, (8) 1 µM TMAP4+ + 5 µM TAPC, (9) 2 µM ZnPPc4+ + 10 µM TEMCC4+ and (10) 2 µM ZnPPc4+ + 5 µM TAPC. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with biofilm without PS
Moreover, the simultaneous administration of PSs was studied. The combination use of TMAP4+-ZnPPc4+ (1 and 2 μM, respectively) (Fig. 4, line 6) produced an indifference result since PDI of E. coli biofilm was similar to the death caused by TMAP4+ (2 μM) (Fig. 4, line 2) and ZnPPc4+ (4 μM) (Fig. 4, line 3), individually. Besides, the simultaneous administration of porphyrin with TEMCC4+ (1 and 10 μM, respectively) (Fig. 4, line 7) induced the same photodynamic effect as 2 μM TMAP4+ (~ 3.7 log decrease). Likewise, the combination TMAP4+-TAPC (Fig. 4, line 8) or ZnPPc4+-TAPC (Fig. 4, line 10) provokes a similar impact to TAPC (Fig. 4, line 5). Nevertheless, the combined use of ZnPPc4+-TEMCC4+ (2 and 10 μM, respectively) (Fig. 4, line 9) generated a significant improvement in the photodynamic effect against E. coli biofilm. Surprisingly, this combination results in a ~ 4.6 log decrease from control (p < 0.05), and a 1 log and 3.5 log improvement in ZnPPc4+ and TEMCC4+, respectively.
Furthermore, the photodynamic effect of PSs against S. aureus biofilm was studied (Fig. 5). Control cultures showed that the biofilm was unaffected by the higher dose of light or by PSs in the dark (Figure S6). This indicated that the decrease in the number of CFUs obtained after irradiation was induced by the photosensitizing activity of the agents. Thereby, illumination for 30 min with 0.25 µM of porphyrin derivative performs a ~ 3.2 log reduction in biofilm survival (Fig. 5, bar 2), while photodynamic activity induced by the same concentration of ZnPPc4+ was ~ 1 log (Fig. 5, bar 3), compared to control (Fig. 5, bar 1) (p < 0.05). However, the PDI of S. aureus biofilm was enhanced by simultaneous administration of these PSs, achieving the highest kill of ~ 4.4 log (Fig. 5, bar 6) (p < 0.05). Moreover, this destruction was achieved at half the concentration used when PS were tested alone. On the other hand, administration of TEMCC4+ (10 µM) yielded a ~ 2.7 log decrease in S. aureus biofilm viability (Fig. 5, bar 4) (p < 0.05). Nevertheless, the combined use of this PS with TMAP4+ generated a reduction in photokilling activity, causing a decrease of ~ 1.7 log (Fig. 5, bar 7) (p < 0.05). Moreover, the administration of TEMCC4+ with ZnPPc4+ produces an intermediate inactivation respect to the effect produced by these PSs separately (~ 2 log) (Fig. 5, bar 9) (p < 0.05). S. aureus biofilm inactivation with TAPC (2 µM) reduces viability by 2 log compared to control (Fig. 5, line 5) (p < 0.05). The combination of TAPC-ZnPPc4+ (Fig. 5, bar 10) did not result in significant differences with respect to the photoinactivation produced by these agents separately. However, the addition of TAPC to porphyirin reduced the death caused by TMAP4+ (Fig. 5, bar 8).
PDI of S. aureus biofilm incubated with different PSs or combinations of them for 15 min and irradiated with visible light for 30 min (54 J/cm2). (1) Control culture without PS in dark, (2) 0.25 µM TMAP4+, (3) 0.25 µM ZnPPc4+, 4) 10 µM TEMCC4+, (5) 2 µM TAPC, (6) 0.125 µM TMAP4+ + 0.125 µM ZnPPc4+, (7) 0.125 µM TMAP4+ + 5 µM TEMCC4+, (8) 0.125 µM TMAP4+ + TAPC 1 µM, (9) 0.125 µM ZnPPc4+ + 5 µM TEMCC4+ and (10) 0.125 µM ZnPPc4+ + 1 µM TAPC. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with biofilm without PS
The results obtained after S. aureus biofilm photoactivation with combined PSs have the same sequence as those found on planktonic cultures. In contrast, the photodynamic effects observed between E. coli planktonic and biofilm forms with a combination of PSs were not the same, especially in the case of the simultaneous administration of TEMCC4+ with ZnPPc4+. This combination did not decrease the viability of planktonic cultures compared to the untreated culture, while on biofilms it produced the highest PDI. This may be because the combination of these PSs provides a higher absorption of light in the red region, which could allow better penetration into the biofilm matrix structure [16]. The sum of the absorbance in the phototherapeutic window increases the penetration of light in the biofilm and activates the molecules that are found deeper in it. As stated above, the disintegration and elimination of bacterial biofilm is more difficult than the inactivation of bacteria in a planktonic state [43]. For this reason, the possibility of combining PSs with other compounds, such as inorganic salts [23, 24] or antimicrobials (antibiotics [25, 26, 28] or antifungals [30]) to optimize or enhance the photoantibiofilm action, has been investigated. However, there is no bibliography that analyses the simultaneous addition of two PSs in solution for the PDI of bacterial biofilms.
3.4 Photodynamic inactivation of E. coli growth curve
The consistency of the bacterial suspending medium can strongly influence the efficacy of the antimicrobial PDI [43, 47, 48]. For this reason, to investigate the photodynamic effect of the PSs combination on bacteria under optimal nutrition conditions, growth curves were analyzed. Microbial growth curves are performed in a nutrient-rich culture medium (TS broth). The high concentration of nutrients present in this culture medium allows the bacteria to replicate freely, in contrast to the starvation they suffer from being in a medium without the necessary nutrients, such as PBS. Consequently, it is more difficult to stop their development and inactivate them [43, 49]. On the other hand, these nutrients are mostly proteins, which bind to PS, inhibiting its photodynamic action [48, 50]. For these reasons, the PS concentration and light doses required to obtain inactivation results comparable to those found in PBS must be higher. In addition, the growth curve assay is intended to mimic the conditions under which PDI might be performed in a food or nutrient-dense area (such as the food industry or hospitals). First, the photodynamic consequences produced on the E. coli growth curve by each PS separately were studied. The results found allow observing that E. coli cultures with PSs without illumination or without PS and irradiated did not show growth delay compared to cultures without PS in dark (p > 0.05) (Fig. 6). However, E. coli growth curves with ZnPPc4+ (10 µM) or TEMCC4+ (20 µM), separately, showed an absorbance decrease at 550 nm reached in the stationary phase with respect to control cultures (p < 0.05), displaying a similar behavior between them. In contrast, growth curves with TMAP4+ (2 µM) or TAPC (10 µM) presented an elongation of the lag phase, reaching the stationary phase after 8 h of treatment. This represents a significant decrease in the growth rate compared to the growth curve without PS in the dark (p < 0.05).
PDI of the growth curve of E. coli with 2 µM TMAP4+ (●), 10 µM ZnPPc4+ (▲), 20 µM TEMCC4+ (▼) and 10 µM TAPC4+ (◆) and irradiated with visible light in TS broth at 37 °C. Control cultures without PS and irradiated (■), without PS in dark (□) and cultures with 2 µM TMAP4+ (○), 10 µM ZnPPc4+ (△), 20 µM TEMCC4+ (∇) and 10 µM TAPC4+ (◇) in dark. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with growth curve without PS in dark
Subsequently, the photodynamic action of combined PSs against E. coli growth curve was studied. First, the effect of mixing non-irradiated PSs on the growth curve of E. coli was analyzed. The growth curves treated with a combination of PSs without illumination or without PS and irradiation exhibited the same behavior as the control curve (without PS and without irradiation) (p > 0.05). As can be observed in Fig. 7, E. coli growth curve in the presence of the TMAP4+ + ZnPPc4+ combination showed an elongation of the lag phase and reached the stationary phase with lower absorbance than the control curve (p < 0.05). However, this combination caused a lesser antibacterial effect than that obtained by TMAP4+ alone. As is known, ZnPPc4+ and TMAP4+ produce 1O2 efficiently (Table 1) [39, 44]. Therefore, it is possible that the production of these reactive species generated by one PS may negatively affect the other, decreasing its antibacterial action [38]. Furthermore, the curves with TMAP4+ + TEMCC4+ or ZnPPc4+ + TEMCC4+ combinations displayed a notable decrease in the exponential phase, reaching the stationary phase with lower absorbance than their respective curves in the dark (p < 0.05). On the other hand, cultures with the combinations containing TAPC (TMAP4+ + TAPC and ZnPPc4+ + TAPC) caused the curves to not reach the exponential phase until after more than 6 h of irradiation. Finally, E. coli growth curve was analyzed when the combination of three PSs (TMAP4+ + ZnPPc4+ + TEMCC4+ or TMAP4+ + ZnPPc4+ + TAPC) was added. Both combinations cause a reduction in the rate of bacterial duplication in the dark. However, the cytotoxic activity of both mixtures increases considerably when the cultures were irradiated with visible light, accomplishing a total photoinactivation (p < 0.05). The latter effect can be attributed to the photodynamic action produced by the combination of PSs, which enhances the inactivation of E. coli cells, demonstrating a significant increase in the PDI effect. Therefore, these findings indicate that the combination of three PSs belonging to different families (porphyrin, phthalocyanine and chlorin) is highly efficient to arrest E. coli cells, even under optimal growth conditions cultures. Previous studies have shown that the photodegradation lifetime of porphyrin is approximately 1.5 h in homogeneous media [51]. However, the photobleaching time is nearly doubled in a biological medium in the presence of bacterial cells [52]. In the present work, despite the fact that this inactivation is carried out with long periods of irradiation, most of the combinations of PSs manage to reduce the bacterial doubling time in less than 3 h, preventing E. coli from reaching the exponential phase or lengthening the lag phase.
PDI of the growth curve of E. coli with 2 µM TMAP4+ + 10 µM ZnPPc4+ (●), 2 µM TMAP4+ + 20 µM TEMCC4+ (▲), 2 µM TMAP4+ + 10 µM TAPC (◆), 10 µM ZnPPc4+ + 20 µM TEMCC4+ (▼), 10 µM ZnPPc4+ + 10 µM TAPC (★), 2 µM TMAP4+ + 10 µM ZnPPc4+ + 20 µM TAPC (◄) and 2 µM TMAP4+ + 10 µM ZnPPc4+ + 10 µM TEMCC4+ (►) and irradiated with visible light in TS broth at 37 °C. Control cultures without PSs and irradiated (■), without PSs in dark (□) and cultures with TMAP4+ + ZnPPc4+ (○), TMAP4+ + TEMCC4+ (△), TMAP4+ + TAPC (◇), ZnPPc4+ + TEMCC4+ (∇), ZnPPc4+ + TAPC (☆), TMAP4+ + ZnPPc4+ + TAPC (◃) and TMAP4+ + ZnPPc4+ + TEMCC4+ (▹) in dark. Values represent mean ± standard deviation of three separate experiments. *p < 0.05, compared with growth curve without PS in dark
On the other hand, it is known that exposure to visible light has a phototoxic effect on some bacteria since these microorganisms are capable to produce endogenous porphyrins [53, 54]. For this reason, the S. aureus growth curve without PS and irradiated was first determined. The results demonstrated that the in vitro growth curve exposed to radiation could not reach the exponential phase (Figure S5). This is because S. aureus, through the photo-stimulation of its endogenous intracellular porphyrins, induce cell death [53]. Therefore, it was not possible to perform S. aureus growth curves with PSs and PS combinations.
The combination of different PSs for the photoinactivation of bacteria is not a widely studied methodology and even less under optimal growth conditions. Only a research group studied the effect of combining different PSs (hypericin, Photofrin II and meso-tetrahydroxyphenylchlorin (mTHPC)) on a strain of S. aureus growing in TS broth culture medium. The results obtained did not show complete inhibition of bacterial growth, and on the contrary, the use of hypericin presented growth stimulation, causing an antagonistic effect compared to the photodynamic action exerted by these PSs separately [37]. On the other hand, another investigation evaluated the use of two PSs (the cationic hydrophilic pyridinium zinc phthalocyanine and 14C-labeled protoporphyrin IX) activated by light, but with a different methodology. The methodology used consisted first of pre-treating the E. coli cells with a PS, washing, adding the other PS and finally irradiating. Although greater incorporation of PSs was observed with this methodology, it did not result in an increase in PDI [55]. However, the combination of the PSs used in this work allows a complete inactivation of E. coli under optimal replication and growth conditions and with very low PSs concentrations. Therefore, it is interesting to continue investigating the combination of PS under different experimental conditions, to determine the combination of PSs that is required depending on the microorganism to be inactivated, its mode of growth and the culture medium in which it develops.
4 Conclusions
The combination of PSs allows the simultaneous use of agents that absorb in different regions of the visible spectrum, extending the range of light absorption. This permits both PSs to be excited simultaneously, producing a greater amount of ROS and, consequently, a greater death of microorganisms. However, the antimicrobial photoactivity of the combined PSs depends on the macrocycle, the microorganism, its mode of growth, and the culture medium in which it is found. In most cases, the combined use of PSs increases its antimicrobial capacity even with lower concentrations of the agents and shorter irradiation periods than those necessary to cause the same effect as a single PS. Consequently, this highly efficient combined strategy can provide higher photodynamic effects than conventional photodynamic treatments. This could result in an additional benefit to be provided by this therapy since it would cover a wide range of wavelengths capable of absorbing without the need to use large concentrations or doses of light. Therefore, a combination of different PSs is a simple and effective way to improve PDI.
References
Gaoa, L., Wanga, H., Zhenga, B., & Huang, F. (2021). Combating antibiotic resistance: Current strategies for the discovery of novel antibacterial materials based on macrocycle supramolecular chemistry. Giant, 7, 100066. https://doi.org/10.1016/j.giant.2021.100066
Vinagreiro, C. S., Zangirolami, A., Schaberle, F. A., Nunes, S. C. C., Blanco, K. C., Inada, N. M., da Silva, G. J., Pais, A. A. C. C., Bagnato, V. S., Arnaut, L. G., & Pereira, M. M. (2020). Antibacterial photodynamic inactivation of antibiotic-resistant bacteria and biofilms with nanomolar photosensitizer concentrations. ACS Infectious Diseases., 6, 1517–1526.
Cieplik, F., Tabenski, L., Buchalla, W., & Maisch, T. (2014). Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Frontiers in Microbiology, 5, 405.
Andersson, D. I., & Hughes, D. (2010). Antibiotic resistance and its cost: Is it possible to reverse resistance? Nature Reviews Microbiology, 8, 261–271.
Kranjec, C., Morales Angeles, D., Torrissen Mårli, M., Fernández, L., García, P., Kjos, M., & Diep, D. B. (2021). Staphylococcal biofilms: Challenges and novel therapeutic perspectives. Antibiotics, 10, 131. https://doi.org/10.3390/antibiotics
Koo, H., Allan, R. N., Howlin, R. P., Hall-Stoodley, L., & Stoodley, P. (2017). Targeting microbial biofilms: Current and prospective therapeutic strategies. Nature Reviews Microbiology, 15(12), 740–755.
Pinto, R. M., Soares, F. A., Reis, S., Nunes, C., & Van Dijck, P. (2020). Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Frontiers in Microbiology, 11, 952.
Delcanale, P., Abbruzzetti, S., & Viappiani, C. (2022). Photodynamic treatment of pathogens. La Rivista del Nuovo Cimento, 45, 407–459. https://doi.org/10.1007/s40766-022-00031-4
Kashef, N., & Hamblin, M. R. (2017). Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resistance Updates, 31, 31–42.
Baptista, M. S., Cadet, J., Greer, A., & Thomas, A. H. (2021). Photosensitization reactions of biomolecules: Definition targets and mechanisms. Photochemistry and Photobiology., 97(6), 1456–1483.
Aroso, R. T., Schaberle, F. A., Arnaut, L. G., & Pereira, M. M. (2021). Photodynamic disinfection and its role in controlling infectious diseases. Photochemical & Photobiological Sciences, 20, 1497–1545.
Cieplik, F., Deng, D., Crielaard, W., Buchalla, W., Hellwig, E., Al-Ahmad, A., & Maisch, T. (2018). Antimicrobial photodynamic therapy—what we know and what we don’t. Critical Reviews in Microbiology, 44(5), 571–589. https://doi.org/10.1080/1040841X.2018.1467876
Wainwright, M., Maisch, T., Nonell, S., Plaetzer, K., Almeida, A., Tegos, G. P., & Hamblin, M. R. (2017). Photoantimicrobials—Are we afraid of the light? The Lancet Infectious Diseases, 17, 49–55.
Sobotta, L., Skupin-Mrugalska, P., Piskorz, J., & Mielcarek, J. (2019). Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. European Journal of Medicinal Chemistry, 175, 72–106.
Sabino, C. P., Wainwright, M., Simoes Ribeiro, M., Parra Sellera, F., dos Anjos, C., da Silva Baptista, M., & Lincopan, N. (2020). Global priority multidrug-resistant pathogens do not resist photodynamic therapy. Journal of Photochemistry and Photobiology, B: Biology, 208, 111893.
Santos, I., Gamelas, S. R. D., Vieira, C., Faustino, M. A. F., Tomé, J. P. C., Almeida, A., Gomes, A. T. P. C., & Lourenço, L. M. O. (2021). Pyrazole-pyridinium porphyrins and chlorins as powerful photosensitizers for photoinactivation of planktonic and biofilm forms of E. coli. Dyes and Pigments., 193, 109557.
da Silveira, C. H., Vieceli, V., Clerici, D. J., Santos, R. C. V., & Iglesias, B. A. (2020). Investigation of isomeric tetra-cationic porphyrin activity with peripheral [Pd(bpy)Cl]+ units by antimicrobial photodynamic therapy. Photodiagnosis and Photodynamic Therapy, 31, 101920.
Spesia, M. B., & Durantini, E. N. (2022). Evolution of phthalocyanine structures as photodynamic agents for bacteria inactivation. Chemical Record, 22, e202100292.
Hu, X., Huang, Y.-Y., Wang, Y., Wang, X., & Hamblin, M. R. (2018). Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Frontiers in Microbiology, 9, 1299.
Youf, R., Müller, M., Balasini, A., Thétiot, F., Müller, M., Hascoët, A., Jonas, U., Schönherr, H., Lemercier, G., Montier, T., & Le Gall, T. (2021). Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics, 13, 1995.
Postiglione, I., Chiaviello, A., & Palumbo, G. (2011). Enhancing photodynamic therapy efficacy by combination therapy: Dated, current and oncoming strategies. Cancers, 3, 2598–2629.
Tyers, M., & Wright, G. D. (2019). Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nature Reviews Microbiology, 17, 141–155.
Yuan, L., Lyu, P., Huang, Y.-Y., Du, N., Qi, W., Hamblin, M. R., & Wang, Y. (2020). Potassium iodide enhances the photobactericidal effect of methylene blue on Enterococcus faecalis as planktonic cells and as biofilm infection in teeth. Journal of Photochemistry and Photobiology, B: Biology, 203, 111730.
Vieira, C., Santos, A., Mesquita, M. Q., Gomes, A. T. P. C., Neves, M. G. P. M. S., Faustino, M. A. F., & Almeida, A. (2019). Advances in aPDT based on the combination of a porphyrinic formulation with potassium iodide: Effectiveness on bacteria and fungi planktonic/biofilm forms and viruses. J. Porphyr. Phthalocyanines, 23, 1–12.
Feng, Y., Coradi Tonon, C., Ashraf, S., & Hasan, T. (2021). Photodynamic and antibiotic therapy in combination against bacterial infections: Efficacy, determinants, mechanisms, and future perspectives. Advanced Drug Delivery Reviews, 177, 113941.
Wozniak, A., & Grinholc, M. (2018). Combined antimicrobial activity of photodynamic inactivation and antimicrobials–state of the art. Frontiers in Microbiology, 9, 930.
Dastgheyb, S. S., Eckmann, D. M., Composto, R. J., & Hickok, N. J. (2013). Photo-activated porphyrin in combination with antibiotics: Therapies against Staphylococci. Journal of Photochemistry and Photobiology, B: Biology, 129, 27–35.
Cassidy, C. M., Donnelly, R. F., Elborn, J. S., Magee, N. D., & Tunney, M. M. (2012). Photodynamic antimicrobial chemotherapy (PACT) in combination with antibiotics for treatment of Burkholderia cepacia complex infection. Journal of Photochemistry and Photobiology, B: Biology, 106, 95–100.
Cahan, R., Swissa, N., Gellerman, G., & Nitzan, Y. (2010). Photosensitizer–antibiotic conjugates: A novel class of antibacterial molecules. Photochemistry and Photobiology, 86, 418–425.
Pérez-Laguna, V., García-Luque, I., Ballesta, S., Rezusta, A., & Gilaberte, Y. (2021). Photodynamic therapy combined with antibiotics or antifungals against microorganisms that cause skin and soft tissue infections: A planktonic and biofilm approach to overcome resistances. Pharmaceuticals, 14, 603.
Pérez-Laguna, V., Gilaberte, Y., Millán-Lou, M. I., Agut, M., Nonell, S., Rezusta, A., & Hamblin, M. R. (2019). Combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochemical & Photobiological Sciences, 18(5), 1020–1029.
Di Poto, A., Sbarra, M. S., Provenza, G., Visai, L., & Speziale, P. (2009). The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials, 30, 3158–3166.
Ghorbani, J., Rahban, D., Aghamiri, S., Teymouri, A., & Bahador, A. (2018). Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Therapy., 27, 293–302.
Mora, S. J., Cormick, M. P., Milanesio, M. E., & Durantini, E. N. (2010). The photodynamic activity of a novel porphyrin derivative bearing a fluconazole structure in different media and against Candida albicans. Dyes and Pigments, 87, 234–240.
Villanueva, A., Stockert, J. C., Cañete, M., & Acedo, P. (2010). A new protocol in photodynamic therapy: Enhanced tumour cell death by combining two different photosensitizers. Photochemical & Photobiological Sciences, 9, 295–297.
Acedo, P., Stockert, J. C., Cañete, M., & Villanueva, A. (2014). Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death & Disease, 5, 1122.
Kubin, A., Wierrani, F., Jindra, R. H., Loew, H. G., Grünberger, W., Ebermann, R., & Alth, G. (1999). Antagonistic effects of combination photosensitization by hypericin, meso-tetrahydroxyphenylchlorin (mTHPC) and photofrin II on Staphylococcus aureus. Drugs Under Experimental and Clinical Research, 25, 13–21.
Dias, L. D., Correa, T. Q., & Bagnato, V. S. (2021). Cooperative and competitive antimicrobial photodynamic effects induced by a combination of methylene blue and curcumin. Laser Physics Letters, 18, 075601.
Scalise, I., & Durantini, E. N. (2005). Synthesis, properties and photodynamic inactivation of Escherichia coli using a cationic and a noncharged Zn(II) pyridyloxyphthalocyanine derivatives. Bioorganic & Medicinal Chemistry, 13, 3037–3045.
Ferreyra, D. D., Spesia, M. B., Milanesio, M. E., & Durantini, E. N. (2014). Synthesis and photodynamic properties of 5,10,15,20-tetrakis[3-(N-ethyl-N-methylcarbazoyl)]chlorin and its analogous porphyrin in solution and in human blood cells. Journal of Photochemistry and Photobiology, A: Chemistry, 282, 16–24.
Ferreyra, D. D., Reynoso, E., Cordero, P., Spesia, M. B., Alvarez, M. G., Milanesio, M. E., & Durantini, E. N. (2016). Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N, N-dimethylaminopropoxy)phenyl]chlorin as potential broad-spectrum antimicrobial photosensitizers. Journal of Photochemistry and Photobiology, B: Biology, 158, 243–251.
Spesia, M. B., Milanesio, M. E., & Durantini, E. N. (2008). Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. European Journal of Medicinal Chemistry, 43, 853–861.
Reynoso, E., Ferreyra, D. D., Durantini, E. N., & Spesia, M. B. (2019). Photodynamic inactivation to prevent and disrupt Staphylococcus aureus biofilm under different media conditions. Photodermatology, Photoimmunology and Photomedicine, 35, 322–331.
Gsponer, N. S., Spesia, M. B., & Durantini, E. N. (2015). Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli mediated by cationic and anionic porphyrins. Photodiagnosis and Photodynamic Therapy, 12, 67–75.
Ballatore, M. B., Spesia, M. B., Milanesio, M. E., & Durantini, E. N. (2014). Synthesis, spectroscopic properties and photodynamic activity of porphyrinefullerene C60 dyads with application in the photodynamic inactivation of Staphylococcus aureus. European Journal of Medicinal Chemistry, 83, 685–694.
Quiroga, E. D., Mora, S. J., Alvarez, M. G., & Durantini, E. N. (2016). Photodynamic inactivation of Candida albicans by a tetracationic tentacle porphyrin and its analogue without intrinsic charges in presence of fluconazole. Photodiagnosis and Photodynamic Therapy., 13, 334–340.
Lambrechts, S. A. G., Aalders, M. C. G., Verbraak, F. D., Lagerberg, J. W. M., Dankert, J. B., & Schuitmaker, J. J. (2005). Effect of albumin on the photodynamic inactivation of microorganisms by a cationic porphyrin. Journal of Photochemistry and Photobiology, B: Biology, 79, 51–57.
Spesia, M. B., Rovera, M., & Durantini, E. N. (2010). Photodynamic inactivation of Escherichia coli and Streptococcus mitis by cationic zinc(II) phthalocyanines in media with blood derivatives. European Journal of Medicinal Chemistry, 45, 2198–2205.
Seneviratne, C. J., Yip, J. W. Y., Chang, J. W. W., Zhang, C. F., & Samaranayake, L. P. (2013). Effect of culture media and nutrients on biofilm growth kinetics of laboratory and clinical strains of Enterococcus faecalis. Archives of Oral Biology, 58, 1327–1334.
Caminos, D. A., Spesia, M. B., Pons, P., & Durantini, E. N. (2008). Mechanisms of Escherichia coli photodynamic inactivation by an amphiphilic tricationic porphyrin and 5,10,15,20-tetra(4-N, N, Ntrimethylammoniumphenyl)porphyrin. Photochemical & Photobiological Sciences, 7, 1071–1078.
Caminos, D. A., & Durantini, E. N. (2006). Photodynamic inactivation of Escherichia coli immobilized on agar surfaces by a tricationic porphyrin. Bioorganic & Medicinal Chemistry, 14, 4253–4259.
Heredia, D. A., Durantini, J. E., Ferreyra, D. D., Reynoso, E., Gonzalez Lopez, E. J., Durantin, A. M., Milanesio, M. E., & Durantini, E. N. (2021). Charge density distribution effect in pyrrolidine-fused chlorins on microbial uptake and antimicrobial photoinactivation of microbial pathogens. Journal of Photochemistry and Photobiology, B: Biology, 225, 112321.
Kossakowska, M., Nakonieczna, J., Kawiak, A., Kurlenda, J., Bielawski, K. P., & Grinholc, M. (2013). Discovering the mechanisms of strain-dependent response of Staphylococcus aureus to photoinactivation: Oxidative stress toleration, endogenous porphyrin level and strain’s virulence. Photodiagnosis and Photodynamic Therapy., 10, 348–355.
Lipovsky, A., Nitzan, Y., Friedmann, H., & Lubart, R. (2009). Sensitivity of Staphylococcus aureus strains to broadband visible light. Photochemistry and Photobiology, 85(1), 255–260.
Minnock, A., Vernon, D. I., Schofield, J., Griffiths, J., Howard Parish, J., & Brown, S. B. (2000). Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrobial Agents and Chemotherapy, 44, 522–527.
Acknowledgements
This work was supported by ANPCYT (PICT N°1482/19 and PICT N°2391/19). E.N.D. and M.B.S. are Scientific Members of CONICET. M.B.S. would like to thank J.A.T for their support in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spesia, M.B., Durantini, E.N. Photosensitizers combination approach to enhance photodynamic inactivation of planktonic and biofilm bacteria. Photochem Photobiol Sci 22, 2433–2444 (2023). https://doi.org/10.1007/s43630-023-00461-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00461-x