Abstract
Background
The advent of tissue engineering and regenerative medicine has introduced innovative approaches to treating degenerative and traumatic injuries, particularly in cartilage, a tissue with limited self-repair capabilities. Among the various stem cell sources, umbilical cord-derived mesenchymal stromal cells (UC-MSCs) have garnered significant interest due to their non-invasive collection, minimal ethical concerns, and robust regenerative potential, particularly in cartilage regeneration.
Methods
A comprehensive literature review was conducted using multiple databases, including PubMed, Scopus, Web of Science, and Google Scholar. Search terms focused on "umbilical cordderived mesenchymal stromal cells," "chondrogenesis," "cartilage regeneration," and related topics. Studies published in the past two decades were included, with selection criteria emphasizing methodological rigor and relevance to UC-MSC chondrogenesis. The review synthesizes findings from various sources to provide a thorough analysis of the potential of UC-MSCs in cartilage tissue engineering.
Results
UC-MSCs exhibit significant chondrogenic potential, supported by their ability to differentiate into chondrocytes under specific conditions. Recent advancements include the development of biomaterial scaffolds and the application of genetic engineering techniques, such as CRISPR/Cas9, to enhance chondrogenic differentiation. Despite these advancements, challenges remain in standardizing cell isolation techniques, scaling up production for clinical use, and ensuring the long-term functionality of regenerated cartilage.
Conclusion
UC-MSCs offer a promising solution for cartilage regeneration in the field of regenerative medicine. Ongoing research is focused on overcoming current challenges through the use of advanced technologies, including bioreactors and gene editing. Collaborative efforts among researchers, clinicians, and bioengineers are essential to translating the potential of UC-MSCs into effective clinical therapies, which could significantly advance tissue regeneration and therapeutic innovation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of tissue engineering and regenerative medicine has heralded a new era in the treatment of degenerative and traumatic injuries to tissues, including cartilage, a tissue known for its limited self-repair capability. Stem cells can be categorized as embryonic stem cells (ESCs), which are pluripotent and derived from early stage embryos but ethically debated; adult stem cells, found in tissues like bone marrow and adipose, which are multipotent; umbilical cord-derived stem cells, collected non-invasively from umbilical-cord blood and Wharton's jelly; and induced pluripotent stem cells (iPSCs), reprogrammed from adult cells to a pluripotent state, avoiding ethical issues but involving complex procedures. Among the diverse sources of cells explored for regenerative purposes, mesenchymal stromal cells (MSCs) have emerged as a cornerstone due to their multipotent nature, with the ability to differentiate into various cell types, including chondrocytes—the cells integral to cartilage formation [1]. This unique characteristic positions MSCs as a prime candidate for pioneering approaches in cartilage regeneration. Within the spectrum of MSCs, those derived from the umbilical cord (UC-MSCs) have attracted considerable interest. Their appeal lies in several distinct advantages: the non-invasive nature of their collection, minimal ethical concerns compared to embryonic stem cells, reduced immunogenicity, and a prolific capacity for proliferation [2]. Recent studies underscore the potential of UC-MSCs in significantly reducing joint tissue damage and inflammation, highlighting their superiority over MSCs derived from other sources in terms of proliferation rates and regenerative capabilities [3]. This empirical evidence supports UC-MSCs as a uniquely advantageous resource for cartilage regeneration, offering non-invasive collection methods and minimal ethical concerns [3,4,5,6,7]. These features not only underscore the therapeutic potential of UC-MSCs but also address some of the limitations associated with MSCs from other sources, such as bone marrow or adipose tissue, including invasive collection procedures and lower proliferation rates [7, 8].
Despite the recognized potential of UC-MSCs in regenerating cartilage and the advancements in their application within tissue engineering, several knowledge gaps persist. The mechanisms underpinning the chondrogenic differentiation of UC-MSCs, the optimization of this differentiation process, and the translation of laboratory findings into clinical applications remain areas of ongoing research. Furthermore, challenges, such as ensuring the long-term functionality of regenerated cartilage, averting immune rejection, and scaling up UC-MSC production under Good Manufacturing Practice (GMP) conditions for clinical usage, need to be addressed [2, 7, 9,10,11]. Additionally, the exploration of innovative methodologies and technologies to enhance the chondrogenic differentiation and cartilage regeneration capabilities of UC-MSCs, including genetic modification techniques and the development of novel biomaterials, presents a fertile area for investigation [12,13,14,15].
We plan to investigate the chondrogenic potential of UC-MSCs in relation to cartilage tissue engineering. Our goals involve clarifying the complex mechanisms involved in the differentiation of UC-MSCs into chondrocytes, as well as examining the internal and external factors that influence this process. Additionally, we strive to evaluate recent developments aimed at improving the effectiveness of cartilage regeneration using UC-MSCs. Our approach entails a detailed analysis of recent studies, both pre-clinical and clinical, that utilize UC-MSCs for repairing cartilage. Through this in-depth examination, we seek to determine their potential for clinical application, while also identifying the challenges faced and potential areas for further research in this rapidly advancing field.
Methodology
A multi-database approach was adopted, encompassing PubMed, Scopus, Web of Science, and Google Scholar to capture a wide array of studies. The search terms employed included combinations of "umbilical cord-derived mesenchymal stromal cells," "chondrogenesis," "cartilage regeneration," "tissue engineering," "growth factors," "scaffold design," and "genetic engineering." Inclusion criteria focused on peer-reviewed articles published in English over the past two decades. Studies were selected based on their methodological rigor, the relevance of findings to UC-MSC chondrogenesis, and their contributions to advancing understanding in the field. Reviews, meta-analyses, and original research articles were included to provide a balanced and comprehensive overview. The gathered literature was then critically appraised and synthesized, with a narrative review approach adopted to integrate findings from diverse sources.
Characterization of UC-MSCs
UC-MSCs represent a promising cellular resource in the field of regenerative medicine, offering a non-controversial, readily available, and potent cell source for tissue engineering applications [16, 17]. This segment delves into the nuanced methodologies for the isolation, detailed phenotypic characterization, and the multifaceted functional properties of UC-MSCs, with a particular emphasis on their chondrogenic differentiation potential, drawing from a wealth of recent scientific inquiries.
Isolation Techniques
The procurement of MSCs from the umbilical cord is executed through meticulously designed protocols that significantly influence the cells' purity, viability, and therapeutic applicability as described below.
-
A)
Enzymatic Digestion: This technique involves the application of collagenase, hyaluronidase, and dispase to the Wharton's jelly component of the umbilical cord, efficiently releasing MSCs into a suspension [18, 19]. This process, documented in various studies [20, 21], is praised for its effectiveness in maximizing cell yield and viability, crucial for subsequent therapeutic uses [22, 23].
-
B)
Explant Culture Method: An alternative approach that entails culturing umbilical-cord tissue segments, allowing MSCs to naturally migrate out of the tissue and proliferate in the culture medium. Celebrated for its simplicity and minimal cell manipulation—preserving the native cellular characteristics—this method is advantageous for applications requiring the most natural cell state [24, 25].
-
C)
Density Gradient Centrifugation: Occasionally employed to isolate MSCs from umbilical-cord blood, a technique that, while efficient, is gradually becoming secondary to the direct extraction from Wharton's jelly due to the latter's richer MSC content [8, 26, 27] as illustrated in Table 1.
Phenotypic Characterization
UC-MSCs are distinguished by a specific set of surface markers crucial for their identification and subsequent application by flow cytometry as listed in Table 2.
-
Surface Markers: These cells robustly express CD73, CD90, and CD105, markers indicative of their mesenchymal lineage, while lacking expression of hematopoietic lineage markers such as CD34 and CD45. This consistent immunophenotypic profile across numerous studies confirms the mesenchymal identity and purity of UC-MSCs [15, 28].
-
Morphological Characteristics: In culture, UC-MSCs adhere to plastic surfaces, presenting a fibroblast-like morphology. This trait, combined with their characteristic growth patterns, is essential for their classification as MSCs and suggests their potential functional behavior in vitro and in vivo [8, 29].
Functional Properties and Chondrogenic Differentiation Potential
The application of UC-MSCs in cartilage regeneration is underpinned by their remarkable functional properties, specifically their proliferation and differentiation capabilities.
-
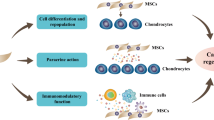
Proliferation and Multipotency: UC-MSCs exhibit significant proliferative abilities, essential for generating the requisite cell numbers for therapeutic purposes. Their capacity to differentiate into various cell lineages, particularly into chondrocytes under defined conditions, underscores their versatility for applications such as bone and cartilage repair as shown in Fig. 1 [12, 13, 30].
-
Immunomodulatory Functions: The immunomodulatory effects of UC-MSCs, capable of modulating immune responses and fostering an anti-inflammatory environment, are crucial for their integration into host tissues and success in clinical applications [28, 31]. Recent advancements have illuminated methods to enhance UC-MSCs' chondrogenic differentiation, with studies demonstrating the efficacy of pulsed electromagnetic fields (PEMF) and specific pharmacological agents in augmenting this process. These findings open new avenues for optimizing UC-MSCs' therapeutic application in cartilage regeneration [32].
-
Enhancement of Chondrogenic Differentiation: Recent research has illuminated methods to enhance the chondrogenic potential of UC-MSCs further. Techniques such as the application of PEMF and the use of specific pharmacological agents have been shown to significantly augment chondrogenic differentiation, opening new avenues for optimizing their therapeutic application in cartilage regeneration [8, 14].

Adapted from Zhang et al.[12]
Proliferative potential of UC-MSCs to bone and cartilage tissue. A Empty sponge implanted in mice. B Undifferentiated UCB-MSCs cultured in collagen sponges for 24 h before implantation. C UCB-MSCs cultured in collagen sponges in the absence of growth factors for 14 days before implantation. D UCB-MSCs cultured in the presence of BMP-2 and TGF-β1 for 14 days before implantation. (Control) Healthy human articular cartilage and human bone. The scale bar corresponds to 100 μm. The global aspect of the sponge construct, at lower magnification, is presented in the inset of the left images (A–D).
Harvesting and Delivery Methods of UC-MSCs
Harvesting Techniques
The efficiency of harvesting UC-MSCs is pivotal for leveraging their therapeutic potential in regenerative medicine, particularly for cartilage repair. These cells are extracted primarily from umbilical-cord tissue or blood through less invasive and ethically favorable methods compared to other stem cell sources. The enzymatic digestion of the Wharton's Jelly or the umbilical-cord blood (UCB) using a combination of collagenase and hyaluronidase represents a significant advancement in this domain, yielding higher success rates of MSC isolation [8, 19, 33]. This method marks a crucial improvement over traditional isolation techniques, aiming to enhance cell yield and viability. However, achieving consistent outcomes requires overcoming the challenges of optimizing enzyme concentrations and incubation times, as well as standardizing the isolation process to minimize cell damage and ensure high viability of the isolated MSCs. Various storage and retrieval techniques for UC-MSCs have been developed to maintain cell viability and functionality (Table 3).
Preparation for Transplantation
Following isolation, UC-MSCs undergo a critical preparation phase involving cell culture expansion and scaffold integration, essential for their successful application in tissue engineering. The proliferation of UC-MSCs in vitro is a requisite step to amass cells in quantities sufficient for therapeutic use. A notable strategy in this context is the employment of bioreactors which simulate physiological conditions to foster cell expansion while maintaining their stemness [28]. Moreover, the integration of UC-MSCs into fibrin scaffolds has been demonstrated to support their chondrogenic differentiation significantly [12]. Innovations in scaffold design, incorporating growth factors like transforming growth factor-beta (TGF-β), further enhance this differentiation, crucial for cartilage regeneration applications [13]. These advancements underscore the importance of both the expansion techniques and the scaffold materials in preparing UC-MSCs for clinical use as illustrated in Fig. 2.
Delivery Methods
The methodologies for delivering UC-MSCs to damaged tissues are integral to their effectiveness in regenerative therapies. Direct injection of these cells into the target sites is a common approach, complemented by innovative strategies to augment their retention and differentiation. For instance, the adjunctive use of PEMF has been shown to significantly enhance chondrogenic differentiation [8]. Moreover, magnetic nanoparticles have been explored for their potential to guide and maintain injected MSCs at the target sites, addressing the challenges of cell dispersion [20]. Scaffold-based delivery offers a promising alternative, providing a three-dimensional matrix for UC-MSCs that supports their attachment, proliferation, and differentiation. This method benefits from the use of hydrogels that can solidify upon injection, creating an optimal environment for cell growth and integration into host tissues [21, 34, 35]. Recent advancements also include the development of injectable hydrogels that encapsulate UC-MSCs for sustained release at the injury site, merging the benefits of direct injection and scaffold-based delivery [28, 36, 37]. Innovations in these delivery methods, including the co-delivery of UC-MSCs with chondroprotective agents, aim to not only repair damaged cartilage but also modulate the local environment to support comprehensive tissue regeneration [29].
Chondrogenicity of UC-MSCs
In Vitro Studies
The exploration of UC-MSCs in vitro has provided foundational insights into their chondrogenic differentiation capabilities. High success rates in isolating MSCs from human umbilical-cord blood (HUCB) underscore the feasibility of utilizing these cells for cartilage tissue engineering. The ability of these isolated cells to undergo differentiation into chondrocytes under specific culture conditions highlights their intrinsic chondrogenic potential [8].
Further advancing the field, the application of PEMF has emerged as a potent enhancer of chondrogenic differentiation. The PEMF treatment notably augments cell proliferation and density and stimulates the expression of chondrocyte-specific markers, thereby fostering an environment conducive to chondrogenesis [12]. This finding posits PEMF as a beneficial adjunctive therapy for chondrogenic differentiation, offering a non-invasive method to augment the chondrogenic capacity of UC-MSCs.
The utility of chondrogenic differentiation mediums in inducing MSC differentiation into chondrocytes has been well documented. These methods have facilitated the expression of critical chondrogenic markers and the formation of cartilage-like tissue structures in vitro, substantiating the potential of UC-MSCs for cartilage regeneration [28]. Coculture systems, especially those incorporating UC-MSCs with rabbit chondrocytes, have proven particularly efficacious. These systems significantly enhance chondrogenic differentiation, evidenced by the upregulation of chondrogenic markers such as aggrecan and collagen type II. Such coculture approaches provide a symbiotic environment that mimics physiological conditions, further optimizing the chondrogenic differentiation process [13].
In Vivo Applications and Clinical Perspectives
Translating in vitro achievements into in vivo applications has been crucial in demonstrating the chondrogenic and therapeutic efficacy of UC-MSCs. One landmark study detailed how UC-MSCs, when incorporated into a collagen hydrogel and implanted into a rabbit model of cartilage defect, not only successfully underwent chondrogenic differentiation but also effectively integrated with the surrounding native cartilage. This application exemplifies the potential of UC-MSCs for direct clinical applications in cartilage repair, showcasing their ability to regenerate cartilage-like tissue and meld seamlessly with the existing cartilage structures, thereby underscoring their practical utility in repairing cartilage defects [20].
Clinical trials provide concrete evidence of UC-MSCs' regenerative capabilities. For instance, a phase I/II clinical trial assessing the safety and efficacy of injecting autologous UC-MSCs into patients with knee osteoarthritis reported marked improvements in patient pain and function. Additionally, MRI scans post-treatment indicated signs of cartilage regeneration, offering compelling evidence of the chondrogenic and therapeutic efficacy of UC-MSCs in a clinical setting [21, 38].
Beyond cartilage regeneration, UC-MSCs exhibit a profound capability in modulating immune responses and enhancing tissue healing. In vivo applications in a murine model of osteoarthritis have not only shown UC-MSCs' ability to promote cartilage repair but also their role in reducing inflammatory cytokines within the joint environment. This dual action suggests a multifaceted therapeutic potential of UC-MSCs, encompassing both regenerative and anti-inflammatory effects [29]. Moreover, the combination of UC-MSCs with innovative scaffolding materials has opened new avenues for enhancing chondrogenic differentiation and cartilage repair. A study exploring a novel scaffold made of hyaluronic acid and gelatin for delivering UC-MSCs into cartilage defect sites observed significant enhancements in cartilage regeneration and integration with native tissue. This research highlights the critical role of scaffold materials in supporting cell differentiation and the repair process, pointing towards the importance of biomaterials in augmenting the therapeutic efficacy of UC-MSCs in cartilage regeneration [14].
Engineered Chondrogenesis by UC-MSCs
Tissue Engineering Strategies
Tissue engineering strategies aim to recreate a conducive microenvironment that closely mimics the natural cartilage tissue niche, thereby promoting the chondrogenic differentiation of UC-MSCs. This involves a comprehensive approach encompassing the development of biomaterial scaffolds and the strategic administration of growth factors to guide differentiation.
Biomaterial Scaffolds
Central to the concept of engineered chondrogenesis is the deployment of biomaterial scaffolds, designed to offer a three-dimensional (3D) matrix that not only supports cell attachment, proliferation, and differentiation but also emulates the extracellular matrix (ECM) of native cartilage [39, 40]. Among various biomaterials, collagen hydrogels have emerged as a frontrunner due to their biocompatibility and their structural and functional resemblance to the cartilage ECM. Research has demonstrated that UC-MSCs cultured within collagen hydrogels show elevated levels of chondrogenic markers, such as collagen type II and aggrecan, indicative of successful chondrogenic differentiation [13].
Further innovation is seen in the creation of composite scaffolds, which integrate the desirable properties of natural polymers with the mechanical robustness of synthetic materials like poly(lactic-co-glycolic acid) (PLGA) [41,42,43,44]. These composite scaffolds are engineered to fine-tune mechanical properties and degradation rates to match the requirements of the chondrogenic environment, further enhancing UC-MSC differentiation [20]. Moreover, the advent of electrospun nanofibrous scaffolds marks a significant advance, providing a microenvironment with nanoscale features akin to the native cartilage matrix, thus offering a conducive setting for chondrogenic differentiation [29] as listed in Table 4.
Growth Factor Supplementation
The role of growth factors in modulating the chondrogenic differentiation of UC-MSCs is indispensable. TGF-β3 and BMP-6, in particular, have been identified as pivotal in orchestrating chondrogenesis when supplemented in culture media [45]. Their synergistic action significantly enhances the expression of SOX9 and collagen type II, critical markers of chondrogenic differentiation, highlighting the nuanced interplay of growth factors in chondrogenesis protocols [12]. Furthermore, the impact of fibroblast growth factor (FGF-2) on UC-MSC proliferation and chondrogenic potential has been recognized, emphasizing the importance of growth factor selection in optimizing chondrogenic outcomes [21].
Genetic Engineering Approaches
The genetic engineering of UC-MSCs presents a frontier for enhancing their intrinsic chondrogenic capabilities, employing strategies to modulate gene expression directly involved in chondrogenesis.
Gene Editing
The advent of CRISPR/Cas9 gene editing technology has opened new avenues for chondrogenesis by allowing precise modification of genes central to the chondrogenic differentiation pathway [46, 47]. Editing genes such as SOX9 to augment its expression has shown promise in boosting chondrogenic differentiation efficiency, underscoring the potential of gene editing in enhancing the chondrogenic phenotype of UC-MSCs [28].
Transfection with Chondrogenic Transcription Factors
Transfection of UC-MSCs with vectors carrying chondrogenic transcription factors, including SOX9, RUNX2, and AGGRECAN, offers a strategic approach to drive cells toward chondrogenesis [48, 49]. This strategy has been validated by research showing that UC-MSCs transfected with SOX9 exhibit increased chondrogenic marker expression and enhanced synthesis of cartilage-specific ECM components, marking a significant step towards effective chondrogenic differentiation [8].
Silencing of Inhibitory Molecules
The innovative use of RNA interference (RNAi) technology to knock down genes that act as inhibitors of chondrogenesis represents a critical strategy in genetic engineering. By silencing the expression of molecules within inhibitory pathways, such as those belonging to the WNT signaling cascade, a more favorable environment is created for chondrogenic differentiation of UC-MSCs [14].
The exploration of engineered chondrogenesis employing UC-MSCs encapsulates a dynamic and promising field within regenerative medicine, underscored by significant strides in both tissue engineering and genetic engineering strategies. Through the development of sophisticated biomaterial scaffolds and precise growth factor supplementation, a conducive microenvironment for chondrogenic differentiation has been established. Concurrently, genetic engineering techniques offer unprecedented control over the cellular and molecular mechanisms underpinning chondrogenesis, promising to overcome the existing limitations and pave the way for innovative cartilage repair and regeneration therapies. As research progresses, these advanced strategies are poised to transform the landscape of regenerative medicine, heralding a new era of therapeutic interventions for cartilage-related conditions.
Table 5 provides a detailed overview of the tissue engineering strategies employed for engineered chondrogenesis using UC-MSCs, highlighting the deployment of biomaterial scaffolds, growth factor supplementation, and genetic engineering approaches, along with their specific benefits and relevant references.
Challenges and Future Perspectives
Challenges
The efficient isolation and differentiation of UC-MSCs into chondrocytes are pivotal for their application in cartilage regeneration. However, this process is fraught with variability. The success rate of isolating viable MSCs from umbilical-cord blood is notably inconsistent, with reports of success as low as 63%, emphasizing the critical need for standardization in isolation techniques to improve efficiency and cell viability [8, 26, 50, 51]. The differentiation process is equally complex, influenced by a plethora of factors including the origin of the cells and the microenvironmental conditions such as specific growth factors, underscoring the need for a nuanced approach to enhance differentiation outcomes [12, 13].
Translating the success of chondrogenic differentiation protocols from bench to bedside introduces significant scalability and quality control challenges. Laboratory-scale experiments that demonstrate promise face obstacles when scaled up for clinical applications, including increased risks of contamination, cell heterogeneity, and compromised differentiation potential. These challenges necessitate the development of sophisticated bioprocessing techniques and rigorous quality control measures to ensure the production of high-quality chondrocytes at a scale that is clinically relevant [20, 21].
The ultimate objective of cartilage regeneration is to achieve functional integration and longevity of the regenerated tissue. However, current methodologies struggle to replicate the intricate architecture and biomechanical properties of native cartilage, leading to regenerative outcomes that may fail under long-term physiological conditions [29]. Furthermore, the potential immunogenic responses to allogeneic UC-MSC transplants highlight the importance of advancing research in immune modulation and compatibility to mitigate the risk of rejection or adverse reactions [14].
Prospective Innovations and Future Directions
The application of advanced bioreactor technologies and three-dimensional culture systems holds promise for enhancing the efficiency and scalability of cartilage regeneration. Furthermore, the advent of gene editing technologies, particularly CRISPR/Cas9, offers an innovative approach to modulate the expression of genes critical to chondrogenesis, potentially revolutionizing the differentiation capacity of UC-MSCs [15, 31]. Innovations in scaffold design, aimed at emulating the extracellular matrix of cartilage, alongside bioprinting techniques for crafting patient-specific implants, present groundbreaking opportunities for improving regenerative outcomes [24].
The utilization of gene editing tools such as CRISPR/Cas9 to target and modulate the expression of genes involved in chondrocyte differentiation and matrix synthesis represents a cutting-edge strategy. By fine-tuning the genetic controls of chondrogenesis, researchers can significantly enhance the efficiency and efficacy of cartilage regeneration, paving the way for more effective therapeutic interventions [31, 52]. Addressing the challenges of scalability and standardization remains pivotal for the clinical application of UC-MSCs. Innovative solutions, such as the development of advanced bioreactors and three-dimensional culture systems, are being explored to overcome these hurdles, highlighting the crucial role of interdisciplinary collaboration in advancing regenerative therapies [53, 54]. The completed clinical trials utilizing UC-MSCs are listed in Table 6 along with their domain where it is being experimented.
Beyond cartilage regeneration, the pluripotent and immunomodulatory properties of UC-MSCs offer a wide range of applications in regenerative medicine [55]. From treating autoimmune diseases to reducing transplant rejection and generating diverse tissue types, UC-MSCs embody a versatile tool with the potential to transform the landscape of regenerative therapies [30]. Navigating the utilization of UC-MSCs for chondrogenesis encompasses confronting numerous challenges, from the intricacies of cell isolation and differentiation to the complexities of scalability and functional integration. However, the horizon of biotechnology and bioengineering is replete with innovative solutions and approaches that promise to surmount these obstacles. By delving into the molecular underpinnings of chondrogenesis, embracing gene editing technologies, and exploring novel scaffold designs, the field of regenerative medicine stands on the cusp of groundbreaking advancements. The collaborative synergy between researchers, clinicians, and bioengineers is indispensable in translating the vast potential of UC-MSCs into efficacious, reliable regenerative therapies, marking a new chapter in the saga of tissue regeneration and therapeutic innovation. UC-MSCs present a promising therapeutic modality with encouraging results in various domains of tissue engineering and regenerative medicine, as illustrated in Table 6. They have the potential for matrix biogenesis along with trophic and reparative functional capabilities despite the adverse local milieu in which they are transplanted [22, 56]. Hence, these cells warrant further research for their potential regenerative properties in various applications.
Conclusion
UC-MSCs offer a promising avenue for cartilage regeneration in regenerative medicine. Despite facing challenges such as standardized cell isolation and scalable production, ongoing research is leveraging advanced technologies like bioreactors and gene editing to overcome limitations. This interdisciplinary approach promises not only more efficient cartilage regeneration but also broader applications in regenerative medicine. Collaboration among scientists, clinicians, and bioengineers is essential to translate UC-MSC potential into impactful therapies, marking a significant advancement in tissue regeneration and therapeutic innovation.
Data availability
Data is contained within the manuscript.
References
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., & Rybak, Z. (2019). Stem cells: Past, present, and future. Stem Cell Research & Therapy, 10, 68. https://doi.org/10.1186/s13287-019-1165-5
Mebarki, M., Abadie, C., Larghero, J., & Cras, A. (2021). Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Research & Therapy, 12(1), 152. https://doi.org/10.1186/s13287-021-02222-y
Pan, X., Li, X., Zhang, L., Wu, F., Zhang, Q., Xu, S., et al. (2023). Umbilical cord mesenchymal stem cells relieve osteoarthritis in rats through immunoregulation and inhibition of chondrocyte apoptosis. Scientific Reports, 13(1), 14975. https://doi.org/10.1038/s41598-023-42349-x
Muthu, S., Jeyaraman, M., Jain, R., Gulati, A., Jeyaraman, N., Prajwal, G. S., et al. (2021). Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees—a panoramic review. Stem Cell Investigation. https://doi.org/10.21037/sci-2020-055
Russo, E., Caprnda, M., Kruzliak, P., Conaldi, P. G., Borlongan, C. V., & La Rocca, G. (2022). Umbilical cord mesenchymal stromal cells for cartilage regeneration applications. Stem Cells International, 2022, 2454168. https://doi.org/10.1155/2022/2454168
Lee, D. H., Kim, S. A., Song, J.-S., Shetty, A. A., Kim, B.-H., & Kim, S. J. (2022). Cartilage regeneration using human umbilical cord blood derived mesenchymal stem cells: A systematic review and meta-analysis. Medicina (Kaunas, Lithuania), 58(12), 1801. https://doi.org/10.3390/medicina58121801
Zhang, P., Dong, B., Yuan, P., & Li, X. (2023). Human umbilical cord mesenchymal stem cells promoting knee joint chondrogenesis for the treatment of knee osteoarthritis: A systematic review. Journal of Orthopaedic Surgery and Research, 18(1), 639. https://doi.org/10.1186/s13018-023-04131-7
Mostafa, I. A., Mohamed, E. N., Mohamed, M. M., & Yahia, I. O. (2015). Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in vitro. Microscopy Research and Technique, 78(8), 667–675. https://doi.org/10.1002/jemt.22520
Menaa, F., Shahrokhi, S., & Shastri, V. P. (2018). Impact and challenges of mesenchymal stem cells in medicine: an overview of the current knowledge. Stem Cells International. https://doi.org/10.1155/2018/5023925
Van Pham, P., & Phan, N. K. (2015). Production of good manufacturing practice-grade human umbilical cord blood-derived mesenchymal stem cells for therapeutic use (pp. 73–85). NY: Springer.
Aydoğdu, N., Öztel, O. N., & Karaöz, E. (2021). Isolation, Culture, Cryopreservation, and Preparation of Umbilical Cord-Derived Mesenchymal Stem Cells as a Final Cellular Product Under Good Manufacturing Practices-Compliant Conditions. In K. Turksen (Ed.), stem cells and good manufacturing practices: methods, protocols, and regulations (pp. 73–84). NY: Springer US.
Esposito, M., Lucariello, A., Costanzo, C., Fiumarella, A., Giannini, A., Riccardi, G., et al. (2013). Differentiation of human umbilical cord-derived mesenchymal stem cells, wj-mscs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo, 27(4), 495–500.
Zheng, P., Ju, L., Jiang, B., Chen, L., Dong, Z., Jiang, L., et al. (2013). Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells by co-culture with rabbit chondrocytes. Molecular Medicine Reports, 8(4), 1169–1174. https://doi.org/10.3892/mmr.2013.1637
Tanthaisong, P., Imsoonthornruksa, S., Ngernsoungnern, A., Ngernsoungnern, P., Ketudat-Cairns, M., & Parnpai, R. (2017). Enhanced Chondrogenic Differentiation of Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stem Cells by GSK-3 Inhibitors. PLoS ONE, 12(1), e0168059. https://doi.org/10.1371/journal.pone.0168059
Wu, H., Yin, Z., Wang, L., Li, F., & Qiu, Y. (2017). Honokiol improved chondrogenesis and suppressed inflammation in human umbilical cord derived mesenchymal stem cells via blocking nuclear factor-κB pathway. BMC Cell Biology, 18(1), 29. https://doi.org/10.1186/s12860-017-0145-9
Alatyyat, S. M., Alasmari, H. M., Aleid, O. A., Abdel-maksoud, M. S., & Elsherbiny, N. (2020). Umbilical cord stem cells: Background, processing and applications. Tissue and Cell, 65, 101351. https://doi.org/10.1016/j.tice.2020.101351
Forraz, N., & McGuckin, C. P. (2011). The umbilical cord: A rich and ethical stem cell source to advance regenerative medicine. Cell Proliferation, 44(Suppl 1), 60–69. https://doi.org/10.1111/j.1365-2184.2010.00729.x
Han, Y.-F., Tao, R., Sun, T.-J., Chai, J.-K., Xu, G., & Liu, J. (2013). Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology, 65(5), 819–827. https://doi.org/10.1007/s10616-012-9528-0
Skiles, M. L., Marzan, A. J., Brown, K. S., & Shamonki, J. M. (2020). Comparison of umbilical cord tissue-derived mesenchymal stromal cells isolated from cryopreserved material and extracted by explantation and digestion methods utilizing a split manufacturing model. Cytotherapy, 22(10), 581–591. https://doi.org/10.1016/j.jcyt.2020.06.002
Chen, X., Zhang, F., He, X., Xu, Y., Yang, Z., Chen, L., et al. (2013). Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury, 44(4), 540–549. https://doi.org/10.1016/j.injury.2012.09.024
Wang, L., Tran, I., Seshareddy, K., Weiss, M. L., & Detamore, M. S. (2009). A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Engineering Part A, 15(8), 2259–2266. https://doi.org/10.1089/ten.tea.2008.0393
Russo, E., Caprnda, M., Kruzliak, P., Conaldi, P. G., Borlongan, C. V., & La Rocca, G. (2022). Umbilical cord mesenchymal stromal cells for cartilage regeneration applications. Stem Cells International, 2022, 1–23. https://doi.org/10.1155/2022/2454168
Lee, T. J., Jeong, C. D., & Lee, T. H. (2023). Dry arthroscopic cartilage repair of the knee joint using umbilical cord mesenchymal stem cells: Kelly clamp technique. Arthroscopy Techniques, 12(8), e1355–e1359. https://doi.org/10.1016/j.eats.2023.04.004
Hildner, F., Wolbank, S., Redl, H., Van Griensven, M., & Peterbauer, A. (2010). How chondrogenic are human umbilical cord matrix cells? A comparison to adipose-derived stem cells. Journal of Tissue Engineering and Regenerative Medicine, 4(3), 242–245. https://doi.org/10.1002/term.236
Hassan, G., Kasem, I., Antaki, R., Mohammad, M. B., AlKadry, R., & Aljamali, M. (2019). Isolation of umbilical cord mesenchymal stem cells using human blood derivatives accompanied with explant method. Stem Cell Investigation, 6, 27. https://doi.org/10.21037/sci.2019.08.06
Nguyen, L. T., Tran, N. T., Than, U. T. T., Nguyen, M. Q., Tran, A. M., Do, P. T. X., et al. (2022). Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum- and xeno-free conditions. Stem Cell Research & Therapy, 13(1), 15. https://doi.org/10.1186/s13287-021-02694-y
Bieback, K., & Netsch, P. (2016). Isolation, culture, and characterization of human umbilical cord blood-derived mesenchymal stromal cells (pp. 245–258). NY: Springer.
Koh, P.-O., Cho, J.-H., Nho, K.-H., Cha, Y.-I., Kim, Y.-K., Cho, E.-H., et al. (2009). Chondrogenesis of Mesenchymal Stem Cells Derived from Human Umbilical Cord Blood. Journal Veterinary Clinics., 26(6), 528–533.
Avercenc-Léger, L., Guerci, P., Virion, J.-M., Cauchois, G., Hupont, S., Rahouadj, R., et al. (2017). Umbilical cord-derived mesenchymal stromal cells: Predictive obstetric factors for cell proliferation and chondrogenic differentiation. Stem Cell Research & Therapy, 8(1), 161. https://doi.org/10.1186/s13287-017-0609-z
Gómez-Leduc, T., Hervieu, M., Legendre, F., Bouyoucef, M., Gruchy, N., Poulain, L., et al. (2016). Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Scientific Reports, 6(1), 32786. https://doi.org/10.1038/srep32786
Li, X., Duan, L., Liang, Y., Zhu, W., Xiong, J., & Wang, D. (2016). Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. BioMed Research International, 2016, 1–9. https://doi.org/10.1155/2016/3827057
Wang, H., Yan, X., Jiang, Y., Wang, Z., Li, Y., & Shao, Q. (2018). The human umbilical cord stem cells improve the viability of OA degenerated chondrocytes. Molecular Medicine Reports. https://doi.org/10.3892/mmr.2018.8413
Salehinejad, P., Moshrefi, M., & Eslaminejad, T. (2020). An overview on mesenchymal stem cells derived from extraembryonic tissues: supplement sources and isolation methods. Stem Cells and Cloning : Advances and Applications, 13, 57–65. https://doi.org/10.2147/SCCAA.S248519
Gu, C., Feng, J., Waqas, A., Deng, Y., Zhang, Y., Chen, W., et al. (2021). Technological advances of 3D scaffold-based stem cell/exosome therapy in tissues and organs. Frontiers in Cell and Developmental Biology, 9, 709204. https://doi.org/10.3389/fcell.2021.709204
Prakash, N., Kim, J., Jeon, J., Kim, S., Arai, Y., Bello, A. B., et al. (2023). Progress and emerging techniques for biomaterial-based derivation of mesenchymal stem cells (MSCs) from pluripotent stem cells (PSCs). Biomaterials Research, 27(1), 31. https://doi.org/10.1186/s40824-023-00371-0
Yang, G., Shao, J., Lin, J., Yang, H., Jin, J., Yu, C., et al. (2021). Transplantation of human umbilical cord blood-derived mesenchymal stem cells improves cartilage repair in a rabbit model. BioMed Research International, 2021, 1–8. https://doi.org/10.1155/2021/6380141
Rim, Y. A., Nam, Y., & Ju, J. H. (2019). Application of cord blood and cord blood-derived induced pluripotent stem cells for cartilage regeneration. Cell Transplantation, 28(5), 529–537. https://doi.org/10.1177/0963689718794864
Park, Y.-B., Ha, C.-W., Lee, C.-H., Yoon, Y. C., & Park, Y.-G. (2017). Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Translational Medicine, 6(2), 613–621. https://doi.org/10.5966/sctm.2016-0157
Liang, J., Liu, P., Yang, X., Liu, L., Zhang, Y., Wang, Q., et al. (2023). Biomaterial-based scaffolds in promotion of cartilage regeneration: Recent advances and emerging applications. Journal of Orthopaedic Translation, 41, 54–62. https://doi.org/10.1016/j.jot.2023.08.006
Munir, N., McDonald, A., & Callanan, A. (2020). Integrational Technologies for the Development of Three-Dimensional Scaffolds as Platforms in Cartilage Tissue Engineering. ACS Omega, 5(22), 12623–12636. https://doi.org/10.1021/acsomega.9b04022
Reddy, M. S. B., Ponnamma, D., Choudhary, R., & Sadasivuni, K. K. (2021). A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers, 13(7), 1105. https://doi.org/10.3390/polym13071105
Suamte, L., Tirkey, A., Barman, J., & Jayasekhar, B. P. (2023). Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Materials in Manufacturing, 1, 100011. https://doi.org/10.1016/j.smmf.2022.100011
Wasyłeczko, M., Sikorska, W., & Chwojnowski, A. (2020). Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes, 10(11), E348. https://doi.org/10.3390/membranes10110348
Fan, J., Abedi-Dorcheh, K., Sadat, V. A., Kazemi-Aghdam, F., Rafieyan, S., Sohrabinejad, M., et al. (2022). A review of recent advances in natural polymer-based scaffolds for musculoskeletal tissue engineering. Polymers, 14(10), 2097. https://doi.org/10.3390/polym14102097
Huang, Y., Seitz, D., Chevalier, Y., Müller, P. E., Jansson, V., & Klar, R. M. (2020). Synergistic interaction of hTGF-β3 with hBMP-6 promotes articular cartilage formation in chitosan scaffolds with hADSCs: Implications for regenerative medicine. BMC Biotechnology, 20, 48. https://doi.org/10.1186/s12896-020-00641-y
Ponta, S., Bonato, A., Neidenbach, P., Bruhin, V. F., Laurent, A., Applegate, L. A., et al. (2024). Streamlined, single-step non-viral CRISPR-Cas9 knockout strategy enhances gene editing efficiency in primary human chondrocyte populations. Arthritis Research & Therapy, 26(1), 66. https://doi.org/10.1186/s13075-024-03294-w
Chehelgerdi, M., Chehelgerdi, M., Khorramian-Ghahfarokhi, M., Shafieizadeh, M., Mahmoudi, E., Eskandari, F., et al. (2024). Comprehensive review of CRISPR-based gene editing: Mechanisms, challenges, and applications in cancer therapy. Molecular Cancer, 23(1), 9. https://doi.org/10.1186/s12943-023-01925-5
Ledo, A. M., Vining, K. H., Alonso, M. J., Garcia-Fuentes, M., & Mooney, D. J. (2020). Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta biomaterialia, 110, 153–163. https://doi.org/10.1016/j.actbio.2020.04.027
Ikeda, T., Kamekura, S., Mabuchi, A., Kou, I., Seki, S., Takato, T., et al. (2004). The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis & Rheumatism, 50(11), 3561–3573. https://doi.org/10.1002/art.20611
Bieback, K., Kern, S., Klüter, H., & Eichler, H. (2004). Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells (Dayton, Ohio), 22(4), 625–634. https://doi.org/10.1634/stemcells.22-4-625
Amati, E., Sella, S., Perbellini, O., Alghisi, A., Bernardi, M., Chieregato, K., et al. (2017). Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Research & Therapy, 8(1), 14. https://doi.org/10.1186/s13287-016-0465-2
Hassan, G., Bahjat, M., Kasem, I., Soukkarieh, C., & Aljamali, M. (2018). Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells. Cellular & Molecular Biology Letters, 23(1), 11. https://doi.org/10.1186/s11658-018-0080-6
Ha, C.-W., Park, Y.-B., Chung, J.-Y., & Park, Y.-G. (2015). Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Translational Medicine, 4(9), 1044–1051. https://doi.org/10.5966/sctm.2014-0264
Song, J.-S., Hong, K.-T., Kong, C.-G., Kim, N.-M., Jung, J.-Y., Park, H.-S., et al. (2020). High tibial osteotomy with human umbilical cord blood-derived mesenchymal stem cells implantation for knee cartilage regeneration. World Journal of Stem Cells, 12(6), 514–526. https://doi.org/10.4252/wjsc.v12.i6.514
Muthu, S., Korpershoek, J. V., Novais, E. J., Tawy, G. F., Hollander, A. P., & Martin, I. (2023). Failure of cartilage regeneration: Emerging hypotheses and related therapeutic strategies. Nature Reviews. Rheumatology, 19(7), 403–416. https://doi.org/10.1038/s41584-023-00979-5
Arrigoni, C., D’Arrigo, D., Rossella, V., Candrian, C., Albertini, V., & Moretti, M. (2020). Umbilical cord MSCs and their secretome in the therapy of arthritic diseases: a research and industrial perspective. Cells, 9(6), 1343. https://doi.org/10.3390/cells9061343
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is submitted for the special issue on Orthobiologics and Regenerative Orthopaedics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeyaraman, N., Jeyaraman, M., Muthu, S. et al. Chondrogenic Potential of Umbilical Cord-Derived Mesenchymal Stromal Cells: Insights and Innovations. JOIO (2024). https://doi.org/10.1007/s43465-024-01239-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43465-024-01239-8