Abstract
Introduction
To minimize the side effects of the central neuraxial blockade to obtain postoperative pain relief, there has been an increasing preference for targeting the peripheral structures in patients undergoing total hip arthroplasty (THA).
Patients and Methods
This prospective study was performed between September 2019 and September 2021 and involved 30 patients that were randomized to two groups. One group (n = 15) received combined nerve block (CNB) [obturator nerve, nerve to quadratus femoris, superior gluteal nerve, and femoral nerve], while another group (n = 15) received periarticular infiltrative analgesia (PIA). All the patients were given the same volume and composition of the drug cocktail (20 ml 0.5% ropivacaine, 1 ml (100 mcg) dexmedetomidine, and 29 ml normal saline).
Results
The patients in group CNB had a significantly lower visual analog score (VAS) at 6, 12, 18, 24, 30, 36, 42 and 48 h after surgery (p < 0.05). Patients in group CNB required fewer (p < 0.001) doses of the rescue analgesic (1.67 ± 0.90 doses) as compared to group PIA (3.53 ± 0.64 doses). Time to the first rescue analgesia was significantly longer (p = 0.01) in group CNB (6.71 ± 2.36 h) as compared to group PIA (4.80 ± 1.26 h). However, patients in group PIA had significantly faster sensory (p < 0.001) and motor recovery (p < 0.001) as compared to group CNB. It took significantly longer (p < 0.001) to administer the nerve block (16.87 ± 1.80 min) as compared to periarticular infiltration (6.53 ± 1.18 min). There were no complications in either group.
Conclusion
CNB registered significant superiority over PIA with respect to postoperative pain relief and time to rescue analgesia. However, the time taken to administer CNB was significantly higher and the patients in the PIA group had early recovery in sensory and motor modalities.
Level of Evidence
III (therapeutic).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considering the complexity of the perception and pathogenesis of pain, a multimodal approach appears logical [1]. Uncontrolled postoperative pain after total hip arthroplasty (THA) may lead to delayed rehabilitation, delayed discharge from the hospital, and poor patient satisfaction. The majority of the centers use epidural anesthesia with a top-up in the postoperative period, injectable opioids and/ or NSAIDS, or patient-controlled analgesia (PCA). Although both PCA and continuous epidural analgesia are technically less demanding and provide adequate joint analgesia, they are associated with multiple side effects such as hypotension, urinary retention, nausea, and vomiting related to the frequent use of opioids [2]. Recently, other modalities such as nerve blocks and periarticular infiltration are gaining popularity. Lumbar plexus block provides good analgesia, but it is a technically demanding procedure and may cause serious complications like retroperitoneal hemorrhage [2,3,4,5].
With recent advances, it has been the tendency to aim more peripherally located structures for pain relief. Though continuous femoral nerve block has fewer side effects, it is difficult to achieve adequate analgesia with an isolated nerve block. Thus, a combined block of all the nerves supplying the hip joint seems a comprehensive and logical modality of providing pain relief in patients undergoing THA [2,3,4,5]. Another alternative to the conventional techniques of providing analgesia in the postop period is periarticular infiltrative analgesia with a cocktail of drugs. Ropivacaine is gaining popularity for providing postoperative analgesia due to its differential sensory and motor blockade, wherein it provides good analgesia with minimal motor blockade [6]. There is a paucity of studies in the literature comparing two postoperative pain control modalities in patients undergoing THA [2, 7,8,9,10,11,12].
In the present study, we prospectively evaluated combined nerve block (CNB) and periarticular infiltrative analgesia (PIA) for obtaining pain relief in patients undergoing THA.
Patients and Methods
This prospective, interventional, comparative study was performed in a tertiary-level, referral, teaching institute attached to a reputed medical college between September 2019 and September 2021. Approval from the institutional ethics committee was granted and it was registered under the Clinical Trial Registry of India (CTRI). Written informed consent was obtained from all the patients for treatment, radiological investigations, and photographic documentation. All ASA I–II patients in the age group 18–60 years that underwent uncemented primary THA via the posterior approach in the stipulated time period were included in our study. Patients having ASA grade III–IV physical status, neurological disorders, coagulopathy, allergy to local anesthetics, or not willing to participate in the study were excluded from the study. All the patients that underwent bilateral THA in the same sitting were also excluded from the study.
At 95% confidence level and 80% power, taking mean analgesia consumption within the first 24 h as 16.4 ± 10.7 in the periarticular infiltration group and 30.0 ± 16.6 in the femoral nerve block group (as reported by Kuchálik et al8), the sample size for our study was calculated as 32 per group. However, due to time constraints in our study, we enrolled a total of 30 patients that were randomized in either of the following two groups using an odd and even number system: (1) group CNB (n = 15): the patients received blocks for obturator nerve, nerve to quadratus femoris, superior gluteal nerve, and femoral nerve; (2) group PIA (n = 15): the patients received periarticular infiltrative analgesia in this group. The patient, as well as the clinician who was evaluating postoperative pain scores, was not made aware of the respective group allocation.
Technique
All the patients were operated on through the posterior approach by the same team of surgeons (authors at serial numbers 2 and 6) using uncemented hip prostheses. All were administered spinal anesthesia alone and none of them received any pre-emptive analgesia. We used the same volume and composition of drug cocktail in both the groups that were made using 20 ml 0.5% ropivacaine, 1 ml (100 mcg) dexmedetomidine, and 29 ml normal saline (to make a total of 50 ml).
1. Group CNB: Obturator nerve, nerve to quadratus femoris, superior gluteal nerve, and femoral nerve were blocked in this group. We exercised due precautions while giving nerve blocks: documentation of pre-existing sensory/motor deficit in the distribution of the proposed block; sensitivity testing with the drugs; negative aspiration of blood before injecting the drug cocktail; and incremental administration of drug cocktail under vital monitoring.
The obturator nerve, nerve to quadratus femoris, and superior gluteal nerve were blocked intraoperatively by the surgeon. The femoral nerve, however, was blocked by the anesthetist after the patient was made supine on wound closure. These nerve blocks were guided by a nerve stimulator (Stimuplex Dig RC, B Braun Melsungen AG, Germany). Nerve stimulation was begun using a current intensity of 2–3 mA (2 Hz). The desired placement of the needle tip was marked by the presence of contraction of the muscles supplied by the respective nerve even at a current intensity of 0.3–0.5 mA. These nerves were blocked sequentially as mentioned below:
A. Obturator nerve: It was blocked after the acetabular component was placed. Taking the anterior cotyledon as the landmark (Fig. 1A–D), the needle attached to the nerve stimulator was inserted from the anteroinferior aspect of the transverse acetabular ligament and directed 40° anteriorly, 20° inferiorly, and medially (Fig. 2). The drug cocktail (15 ml) was then infiltrated after confirming the correct position of the needle by looking for the contraction of the adductors.
B. Nerve to quadratus femoris: It was blocked before the removal of Charnley’s retractor. It was identified as a thin branch running parallel to the sciatic nerve at the superior border of the quadratus femoris just below the obturator externus, approximately 4 cm medial to the intertrochanteric crest. The drug cocktail (8 ml) was administered after confirming the correct position of the needle by looking for the contraction of the quadratus femoris muscle.
C. Superior gluteal nerve: It was also blocked before the removal of Charnley’s retractor. The drug cocktail (7 ml) was administered at the superior border of the piriformis after checking for the contraction of the gluteus medius.
D. Femoral nerve: It was blocked by an anesthetist (author at serial number 3) on the operating table in the supine position after the completion of the surgery. The block was given with the ipsilateral extremity abducted 15–20° and slightly externally rotated. The site of needle insertion was located immediately lateral (1–1.5 cm) to the pulse of the femoral artery and 1–2 cm below the inguinal crease. The needle was introduced at a 30–45° angle to the skin in a cephalad direction after connecting it to a nerve stimulator. Loss of resistance was felt as the needle pierced the fasciae. The drug cocktail (20 ml) was administered after checking for the contraction of quadriceps muscle (patellar twitch) (Fig. 3).
2. Group PIA: The structures around the hip, thought to be responsible for postoperative pain in patients undergoing THA, were infiltrated intraoperatively by the surgeon with the drug cocktail. We infiltrated the iliopsoas insertion (7 ml) (Fig. 4) and anterior capsule (5 ml) before the final reduction of hip prosthesis. The posterior capsule (5 ml), short external rotators (5 ml), gluteus maximus with its insertion (8 ml), abductors (5 ml), and fascia lata (15 ml) (Fig. 5) were infiltrated after the final reduction of the hip prosthesis.
Aftercare
The patients received standard postoperative care. They were given an injection of diclofenac 75 mg intramuscularly for rescue analgesia (VAS ≥ 4) unless contra-indicated. In those cases where injection of diclofenac was contraindicated, we used injection of paracetamol (1 gm intravenous). They were observed for any complications like respiratory depression, bradycardia, renal insufficiency, and urinary retention.
As a part of deep vein thrombosis (DVT) protocol, the following measures were taken: (1) injection of enoxaparin 0.4 ml s/c 24 hourly (prophylactic dose) was started on POD1, which was continued for a week; (2) compression stockings were used; and (3) all the patients underwent venous duplex ultrasonography on postoperative day 3 to rule out/ help early diagnose DVT.
Outcome Variables
(1) visual analog score (VAS): it was measured in the recovery room and then 6 hourly over the next 48 h by the author at serial number 1; (2) time to rescue analgesia: it was the time from the completion of CNB/ PIA till the time VAS was 4 or more; (3) number of doses of rescue analgesia needed in next 48 h; (4) time taken to administer CNB or PIA; (5) time taken for complete sensory and motor recovery; (6) complications observed in any of the patients such as intravascular injections, hypotension, hypersensitivity, prolonged motor blockade, bradycardia (heart rate < 60 bpm), respiratory depression (SpO2 < 88%), renal insufficiency (serum creatinine increases more than 0.5 above the baseline value in 24 h), and DVT.
Data Analysis
Data were analyzed and statistically evaluated using the SPSS-PC-25 version. Quantitative data were expressed as mean ± standard deviation or median with interquartile range which depends on normality distribution. The difference between the two comparable groups was tested by Student’s t test (unpaired) or Mann–Whitney ‘U’ test. Qualitative data were expressed in percentages and statistical differences between the proportions were tested by Chi-square test or Fisher’s exact test.
Results
Age and Gender
The study cohort had an identical gender distribution with 12 men and 3 women in each group. The mean age of patients in the two groups [34.80 ± 10.80 years in group CNB and 39.33 ± 12.24 years in group PIA] were comparable (p = 0.29).
VAS
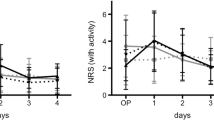
The values, in the recovery room and then at 6, 12, 18, 24, 30, 36, 42, and 48 h after surgery, were 1.53 ± 0.64, 2.80 ± 0.77, 3.87 ± 0.99, 3.0 ± 1.0, 2.93 ± 1.16, 2.13 ± 0.64, 1.20 ± 0.41, 1.20 ± 0.41, and 1.0 ± 0.0 in group CNB versus 1.60 ± 0.51, 4.93 ± 1.38, 4.73 ± 0.79, 4.40 ± 1.35, 3.67 ± 0.90, 3.0 ± 1.31, 2.33 ± 1.11, 1.73 ± 0.70, and 1.33 ± 0.48 in group PIA, respectively. It was significantly less at 6 h (p < 0.001), 12 h (p = 0.02), 18 h (p < 0.01), 24 h (p < 0.05), 30 h (p = 0.04), 36 h (p < 0.01), 42 h (p = 0.02), and 48 h (p = 0.01) in group CNB as compared to that measured in group PIA (Table 1). However, VAS in the recovery room was comparable in both the groups (p = 0.62).
Rescue Analgesia
Time to the first rescue analgesia was significantly longer (p = 0.01) in group CNB (6.71 ± 2.36 h) as compared to group PIA (4.80 ± 1.26 h). A mean of 1.67 ± 0.90 doses of intramuscular diclofenac was used in patients who were given CNB, which was significantly less (p < 0.001) than the number of doses required in patients who were given PIA (3.53 ± 0.64 doses).
Sensory and Motor Recovery
Time to complete sensory and motor recovery in patients of group PIA was 2.90 ± 0.43 h and 1.77 ± 0.45 h, respectively, which was significantly less (p < 0.001) as compared to patients in group CNB (7.63 ± 1.52 and 3.86 ± 0.67, respectively) (Table 2).
Time Taken to Administer Analgesia
It took significantly longer (p < 0.001) to administer CNB (16.87 ± 1.80 min) as compared to PIA (6.53 ± 1.18 min).
Complications
There were no complications in any of the patients.
Discussion
The International Association for the Study of Pain (IASP) suggested the definition of pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’. Various modalities such as parenteral injections of NSAIDS/ opioids, PCA, epidural top-up, lumbar plexus block, and newer alternatives like PIA and CNB can be used to provide pain relief in patients undergoing THA [2,3,4,5]. Multimodal analgesia is the cornerstone of enhanced recovery after surgery protocol (ERAS) [2,3,4,5]. It aims at using the synergistic and potentiating actions of various pharmacological agents to provide maximum analgesia with minimum side effects. The additive action of various drugs makes it possible to use a multitude of drugs in smaller doses for optimum action without an associated increase in adverse effects. It aims at replacing opioids as the chief analgesic agent in postoperative pain control protocols owing to its well-documented side effects. The multimodal protocol targets pain receptors at all levels, thereby decreasing the central and peripheral perception of pain that enables the patient to initiate the postoperative rehabilitation as early as possible for better functional outcomes. A review of the studies on the comparison of two postoperative pain control modalities in patients undergoing THA is summarized in Table 3 [2, 7,8,9,10,11,12].
Limitations of the Study
Despite it being a randomized blinded comparative study, we could identify the following limitations: (1) we did not record the preoperative VAS which could have a confounding effect, as patients with a higher preoperative VAS usually have higher scores postoperatively; (2) since this was a time-bound study, we could enroll 15 patients in each group. We recognize that a higher sample size would have further validated our results (Table 4).
Addressing the Anatomical Basis of Pain Relief
A comprehensive understanding of anatomical structures responsible for causing postoperative pain in patients undergoing THA is a prerequisite for providing optimum pain relief. In the present study, we compared CNB and PIA of structures thought to be responsible for the genesis of pain after surgery by administering the same drug cocktail in both groups. In the first group, nerve supply of the hip (obturator nerve, superior gluteal nerve, nerve to quadratus femoris, and the femoral nerve) was blocked after a detailed review of the article by Birnbaum et al. [13] on the sensory innervation of the hip joint and cadaveric dissection of the anatomical landmarks for the above nerves. The idea of targeting specific nerves and not administering a sciatic nerve block for blanket coverage of the sensory innervation of the hip was conceived with the thought that blocking the femoral nerve and the sciatic nerve would lead to complete paralysis of the limb in the postoperative period, thus increasing the risk of DVT and dislocation. Uppal [14] reported a case of permanent sciatic nerve injury caused by a preoperative intraneural injection of the local anesthetic agent. In the second group, various periarticular structures thought to be considered the source of postoperative pain, as outlined by Maheshwari et al. [15], were infiltrated with the drug cocktail.
The Rationale for the Different Constituents of the Cocktail of Drugs
Over the years, there has been immense debate on the constituents of the drug cocktail to be used. It essentially has three components: NSAID/ local anesthetic, adjuvant/s, and diluent. It offers good pain control, reduced narcotic consumption, and early rehabilitation [2,3,4,5]. Keeping in mind the three basic components, we devised our own cocktail of drugs based on available evidence. The use of ropivacaine was backed by the better safety profile and the differential sensory motor blockade in comparison to bupivacaine [6]. The idea of using dexmedetomidine as an adjuvant was backed by its ability to prolong the duration of action of the local anesthetic with an additive effect and a better safety profile when compared to other adjuvants like epinephrine. We did not use a steroid in our drug cocktail considering its propensity of causing infection owing to its immune-suppressive effects. We did not use morphine in our drug cocktail owing to its propensity to cause adverse reactions such as nausea, vomiting, and urinary retention. Additionally, the use of morphine is associated with a high incidence of respiratory depression, necessitating intensive care postoperatively, which may be of concern in a resource-limited setting like ours. All the patients were given intravenous injection of ceftriaxone 1 g half an hour prior to surgery obviating the need for adding an antibiotic to our drug cocktail.
Surgeon-Administered Blocks
The conventional method of providing analgesia such as PCA, epidural top-up, or opioid injections often necessitates intensive monitoring. Anesthetist-administered nerve blocks are commonly employed for postoperative pain relief, but only those nerves that are accessible through a percutaneous approach can be blocked effectively. The concept of surgeon-administered blocks can be utilized especially for the nerves: (1) that are there in the vicinity of the surgical field; (2) that are relatively small or found in deeper planes, and not accessible via a percutaneous route, but still play an important role in the sensory supply, can be blocked effectively under vision providing optimum postoperative pain relief. A summary of all the surgeon-administered blocks, as described in the literature, is provided in Table [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. This encouraged us to block the obturator nerve, superior gluteal nerve, and the nerve to quadratus femoris intraoperatively by the surgeon and the femoral nerve by the anesthetist after the procedure.
A Novel Method of Giving Surgeon-Administered Nerve Blocks
To the best of our knowledge, there is no other published study that compares CNB (involving the femoral nerve, obturator nerve, superior gluteal nerve, and the nerve to quadratus femoris) and PIA. Additionally, we describe a novel intraoperative technique of blocking the obturator nerve, superior gluteal nerve, and the nerve to quadratus femoris in patients undergoing THA.
We used the anterior cotyledon of the acetabulum as the landmark to block the obturator nerve after placement of the acetabular cup (Fig. 6). The needle attached to the nerve stimulator was inserted from the anteroinferior aspect of the transverse acetabular ligament and directed 40° anteriorly, 20° inferiorly, and medially to block the obturator nerve. The surgeon should be aware of the branching pattern of the common obturator nerve at different levels. It has been suggested that its bifurcation (anterior and posterior) may be intrapelvic (23.22%), within the obturator canal (51.78%), or in the medial thigh (25%) [33]. In our study, we blocked the obturator nerve at its exit from the obturator canal, which in the majority of the patients is after its bifurcation. The correct positioning of the needle was determined by the contractions of thigh adductors.
After that, the nerve to quadratus femoris (branch of the anterior division of sacral plexus) was given by the surgeon. It courses almost parallel and posterior to the sciatic nerve above the piriformis. After emerging through the infra piriformis fossa, the nerve lies medial to the sciatic nerve just before it innervates the quadratus femoris and posterior aspect of the hip joint capsule. In our study, we blocked the nerve to the quadratus femoris in the interval between the obturator externus and quadratus femoris approximately 4 cm medial to the intertrochanteric crest, and correct positioning of the needle was determined by the contractions of the quadratus femoris [34].
Subsequently, the superior gluteal nerve (branch of the dorsal division of sacral plexus) was blocked by the surgeon before the removal of Charnley’s retractor. It crosses the supra-piriform foramen and runs in the plane between the the gluteus medius and minimus. It is accompanied by superior gluteal vessels and innervates the gluteus medius, minimus, and tensor fascia lata [35, 36]. The drug cocktail (7 ml) was administered at the superior border of the piriformis after checking for the contraction of the gluteus medius.
Conclusions
Combined nerve block and periarticular infiltrative analgesia are safe procedures. The combined nerve block technique provides superior postoperative analgesia; however, it is more time-consuming and takes more time until motor and sensory recovery when compared with periarticular infiltration. Future studies may be directed toward comparing the efficacy of different drug cocktails (including liposomal preparations).
Data availability
Not applicable.
References
Ranawat, A. S., & Ranawat, C. S. (2007). Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. Journal of Arthroplasty, 22(7 Suppl 3), 12–15.
Tetsunaga, T., Tetsunaga, T., Fujiwara, K., Endo, H., & Ozaki, T. (2016). Combination therapy with continuous three-in-one femoral nerve block and periarticular multimodal drug infiltration after total hip arthroplasty. Pain Research & Management, 2016, 1425201.
Pagnano, M. W., Hebl, J., & Horlocker, T. (2006). Assuring a painless total hip arthroplasty: a multimodal approach emphasizing peripheral nerve blocks. Journal of Arthroplasty, 21(4), 80–84.
Parvataneni, H. K., Shah, V. P., Howard, H., Cole, N., Ranawat, A. S., & Ranawat, C. S. (2007). Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. Journal of Arthroplasty, 22(6), 33–38.
Peters, C. L., Shirley, B., & Erickson, J. (2006). The effect of a new multimodal perioperative anaesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. Journal of Arthroplasty, 21(6), 132–138.
Kaur, A., Singh, R. B., Tripathi, R. K., & Choubey, S. (2015). Comparison between bupivacaine and ropivacaine in patients undergoing forearm surgeries under axillary brachial plexus block: a prospective randomized study. Journal of Clinical and Diagnostic Research, 9(1), UC01.
Jiménez-Almonte, J. H., Wyles, C. C., Wyles, S. P., Norambuena-Morales, G. A., Baez, P., Murad, M. H., & Sierra, R. J. (2016). Is local infiltration analgesia superior to peripheral nerve blockade for pain management after THA: a network meta-analysis. Clinical Orthopaedics and Related Research, 474(2), 495–516.
Kuchálik, J., Magnuson, A., Lundin, A., & Gupta, A. (2017). Local infiltration analgesia or femoral nerve block for postoperative pain management in patients undergoing total hip arthroplasty. A randomized, double-blind study. Scandinavian Journal of Pain, 16(1), 223–230.
Johnson, R. L., Amundson, A. W., Abdel, M. P., Sviggum, H. P., Mabry, T. M., Mantilla, C. B., et al. (2017). Continuous posterior lumbar plexus nerve block versus periarticular injection with ropivacaine or liposomal bupivacaine for total hip arthroplasty: a three-arm randomized clinical trial. Journal of Bone and Joint Surgery American, 99(21), 1836–1845.
Fahs, A. M., Koueiter, D. M., Kurdziel, M. D., Huynh, K. A., Perry, C. R., & Verner, J. J. (2018). Psoas compartment block vs periarticular local anesthetic infiltration for pain management after anterior total hip arthroplasty: a prospective, randomized study. Journal of Arthroplasty, 33(7), 2192–2196.
Gasanova, I., Alexander, J. C., Estrera, K., Wells, J., Sunna, M., Minhajuddin, A., & Joshi, G. P. (2019). Ultrasound-guided suprainguinal fascia iliaca compartment block versus periarticular infiltration for pain management after total hip arthroplasty: a randomized controlled trial. Regional Anesthesia and Pain Medicine, 44(2), 206–211.
Frassanito, L., Vergari, A., Nestorini, R., Cerulli, G., Placella, G., Pace, V., & Rossi, M. (2020). Enhanced recovery after surgery (ERAS) in hip and knee replacement surgery: description of a multidisciplinary program to improve management of the patients undergoing major orthopedic surgery. Musculoskeletal Surgery, 104(1), 87–92.
Birnbaum, K., Prescher, A., Hepler, S., & Heller, K. D. (1997). The sensory innervation of the hip joint-an anatomical study. Surgical and Radiologic Anatomy, 19(6), 371–375.
Uppal, H. S., Gwilym, S. E., Crawfurd, E. J., & Birch, R. (2007). Sciatic nerve injury caused by pre-operative intraneural injection of local anaesthetic during total hip replacement. Journal of Bone and Joint Surgery British, 89(2), 242–243.
Maheshwari, A. V., Blum, Y. C., Shekhar, L., Ranawat, A. S., & Ranawat, C. S. (2009). Multimodal pain management after total hip and knee arthroplasty at the Ranawat orthopaedic center. Clinical Orthopaedics and Related Research, 467(6), 1418–1423.
Lako, S. J., Steegers, M. A., van Egmond, J., Gardeniers, J., Staals, L. M., & van Geffen, G. J. (2009). Incisional continuous fascia iliaca block provides more effective pain relief and fewer side effects than opioids after pelvic osteotomy in children. Anesthesia and Analgesia, 109(6), 1799–1803.
Owen, D. J., Harrod, I., Ford, J., Luckas, M., & Gudimetla, V. (2011). The surgical transversus abdominis plane block—a novel approach for performing an established technique. Internat J Obst Gynaec, 118(1), 24–27.
Johns, N., O’neill, S., Ventham, N. T., Barron, F., Brady, R. R., & Daniel, T. (2012). Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Disease, 14(10), 635–642.
Wheble, G. A., Tan, E. K., Turner, M., Durrant, C. A., & Heppell, S. (2013). Surgeon-administered, intra-operative transversus abdominis plane block in autologous breast reconstruction: a UK hospital experience. Journal of Plastic, Reconstructive & Aesthetic Surgery, 66(12), 1665–1670.
Lapmahapaisan, S., Tantemsapya, N., Aroonpruksakul, N., Maisat, W., & Suraseranivongse, S. (2015). Efficacy of surgical transversus abdominis plane block for postoperative pain relief following abdominal surgery in pediatric patients. Pediatric Anesthesia, 25(6), 614–620.
Desroches, A., Klouche, S., Schlur, C., Bauer, T., Waitzenegger, T., & Hardy, P. (2016). Suprascapular nerve block versus interscalene block as analgesia after arthroscopic rotator cuff repair: a randomized controlled noninferiority trial. Arthroscopy, 32(11), 2203–2209.
Tamura, T., Mori, S., Mori, A., Ando, M., Yokota, S., Shibata, Y., & Nishiwaki, K. (2017). A randomized controlled trial comparing paravertebral block via the surgical field with thoracic epidural block using ropivacaine for post-thoracotomy pain relief. Journal of Anesthesia, 31(2), 263–270.
Lanier, S. T., Lewis, K. C., Kendall, M. C., Vieira, B. L., De Oliveira, G., Nader, A., et al. (2018). Intraoperative nerve blocks fail to improve quality of recovery after tissue expander breast reconstruction: a prospective, double-blinded, randomized, placebo-controlled clinical trial. Plastic and Reconstructive Surgery, 141(3), 590–597.
Obata, H., Naito, K., Sugiyama, Y., Nagura, N., Kinoshita, M., Goto, K., Iwase, Y., Obayashi, O., & Kaneko, K. (2019). Surgical treatment of distal radius fractures under the ultrasound-guided brachial plexus block performed by surgeons. The Journal of Hand Surgery Asian-Pacific Volume, 24(02), 147–152.
Narasimhulu, D. M., Scharfman, L., Minkoff, H., George, B., Homel, P., & Tyagaraj, K. (2018). A randomized trial comparing surgeon-administered intraoperative transversus abdominis plane block with anesthesiologist-administered transcutaneous block. International Journal of Obstetric Anesthesia, 35, 26–32.
Caldwell, G. L., Jr., & Selepec, M. A. (2019). Reduced opioid use after surgeon-administered genicular nerve block for anterior cruciate ligament reconstruction in adults and adolescents. HSS Journal, 15(1), 42–50.
Laumonerie, P., Blasco, L., Tibbo, M. E., Panagiotis, K., Fernandes, O., Lauwers, F., et al. (2019). Ultrasound-guided versus landmark-based approach to the distal suprascapular nerve block: a comparative cadaveric study. Arthroscopy, 35(8), 2274–2281.
Wong, D. J., Curran, T., Poylin, V. Y., & Cataldo, T. E. (2020). Surgeon-delivered laparoscopic transversus abdominis plane blocks are non-inferior to anesthesia-delivered ultrasound-guided transversus abdominis plane blocks: a blinded, randomized non-inferiority trial. Surgical Endoscopy, 34(7), 3011–3019.
Peterson, J. R., Steele, J. R., Wellman, S. S., & Lachiewicz, P. F. (2020). Surgeon-performed high-dose bupivacaine periarticular injection with intra-articular saphenous nerve block is not inferior to adductor canal block in total knee arthroplasty. Journal of Arthroplasty, 35(5), 1233–1238.
Greenky, M. R., McGrath, M. E., Levicoff, E. A., Good, R. P., Nguyen, J., Makhdom, A. M., & Lonner, J. H. (2020). Intraoperative surgeon administered adductor canal blockade is not inferior to anesthesiologist administered adductor canal blockade: a prospective randomized trial. Journal of Arthroplasty, 35(5), 1228–1232.
Caldwell, G. L., & Selepec, M. A. (2020). Surgeon-administered nerve block during rotator cuff repair can promote recovery with little or no post-operative opioid use. HSS Journal, 16(2_suppl), 349–357.
James, D., Evans, F. M., Rai, E., & Roy, N. (2021). Delivering essential surgical care for lower-limb musculoskeletal disorders in the low-resource setting. World Journal of Surgery, 45(10), 2975–2981.
Yoshida, T., Nakamoto, T., & Kamibayashi, T. (2017). Ultrasound-guided obturator nerve block: a focused review on anatomy and updated techniques. BioMed Research International, 9, 2017.
Alejandra, M., Sofía, M., Joaquín, C., Joaquín, G., & Eduardo, O. (2020). Anatomical basis for the selective block of the nerve to quadratus femoris by way of percutaneous techniques. International Journal of Morphology, 38(6), 1549–1554.
Ohgoshi, Y., Usui, Y., Terada, S., Takeda, Y., & Ohtsuka, A. (2020). Superior gluteal nerve block: a cadaveric study to evaluate the optimal injection site. Braz J Anesthesiol., 69, 639–640.
Sá, M., Graça, R., Reis, H., Cardoso, J. M., Sampaio, J., Pinheiro, C., & Machado, D. (2018). Nervo glúteo superior: um novo bloqueio a caminho? Braz J Anesthesiol, 68, 400–403. article in Portugese.
Author information
Authors and Affiliations
Contributions
The manuscript has been read and approved by all the authors and the requirement for authorship of this document has been met. Each author certifies that the work and all investigations were conducted in conformity with the ethical principles of research. Each author believes that the manuscript represents honest work. They did not receive grants from any commercial entity in support of this work. Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
Granted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wadhawan, A., Arora, S., Krishna, A. et al. A Comparative Evaluation of Combined Nerve Block Versus Periarticular Infiltration on Postoperative Pain Relief in Total Hip Arthroplasty. JOIO 57, 1251–1266 (2023). https://doi.org/10.1007/s43465-023-00924-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-023-00924-4