Abstract
Background
Lumbar disc herniation (LDH) can cause lumbar nerve root compression, which can lead to denervated atrophy of paraspinal muscles theoretically, however, the conclusions of morphological alteration in multifidus with LDH remain controversial. Transforming growth factor-beta 1 (TGF-β1) plays an essential role in the development of tissue fibrosis and is a molecular marker in the study of muscle fibrosis, but no relevant studies on TGF-β1 expression in multifidus have been reported so far. This study is to observe altered morphology of multifidus in patients with LDH, and to explore the correlation between multifidus fibrosis and TGF-β1 expression.
Materials and Methods
46 LDH patients with low back pain combined with unilateral leg radiation pain and/or numbness were selected. Patients were divided into four groups according to their medical histories. Group 1: medical history less than 6 months (15 cases); group 2: a medical history of 6–12 months (10 cases); group 3: a medical history of 12–24 months (13 cases); and group 4: medical history > 24 months (8 cases). Bilateral multifidus specimens were taken from compressed nerve root segments, and morphological changes in multifidus were determined. Multi-parameter changes in TGF-β1 expression in multifidus were observed by immunohistochemistry and immunofluorescence.
Results
HE staining showed that the cross-sectional area (CSA) of multifidus in the involved sides decreased and muscle fibers atrophied. Masson’s trichrome staining showed a decrease in the sectional area ratio of myofibers to collagen fibers in the involved side. In groups 1 and 2, there were no significant differences in the aforementioned parameters. In groups 3 and 4, statistically significant differences in the sectional area ratio of myofibers to collagen fibers in both sides were seen (P < 0.05). TGF-β1 expression was significantly enhanced in both muscle cells and the matrix of the involved side, while no expression or a little expression was found in the matrix in the uninvolved side. In group 1, there was no statistically significant difference in TGF-β1 expression in both sides. In the remaining three groups, TGF-β1 expression in the involved sides was higher than were found in the uninvolved sides.
Conclusions
Nerve root compression by LDH leads to multifidus atrophy, fibrosis, and increased TGF-β1 expression, which might promote multifidus fibrosis.
Trials registration All Clinical Trials done in India should preferably be registered with the Clinical Trials Registry of India, set up by the Indian Council of Medical Research (website: http://ctri.nic.in). Authors should provide the CTRI number along with the manuscript.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intervertebral disc degeneration gradually occurs with aging and is characterized by changes in the intervertebral disc structure, function, and mechanical loading capacity, which eventually leads to lumbar disc herniation (LDH). The pathogenesis of LDH is still unclear, which is widely considered to include developmental abnormalities, hereditary manifestations, trauma, degeneration, inflammation, and immune pathways. Studies of the lumbar paraspinal muscles were also rare until the end of the last century [1].
In recent years, an increasing number of scholars have begun to pay greater attention to the paraspinal muscles. They have studied paraspinal muscles in the context of their morphology, electrophysiology, physical testing, muscle biopsy and other aspects with the intent of exploring its relationship to LDH; however, the conclusions remain controversial [2,3,4,5,6,7,8].
Studies have shown that TGF-β1 plays an essential role in the development of tissue fibrosis and is a molecular marker in the study of muscle fibrosis [9]. Precisely how TGF-β1 is expressed in the paraspinal muscle of patients with LDH and their relative significance remain unanswered questions at this time. No relevant studies have been reported so far.
The multifidus is exclusively innervated by the medial branch of the posterior lumbar nerve, and each muscle bundle is innervated by a single branch, without communication branches [10]. Our study selected the multifidus from patients presenting with LDH as a research focus. In this study, multifidus morphological alterations and TGF-β1 expression were observed, and the correlation between multifidus fibrosis and TGF-β1 expression was also explored and reported.
Materials and Methods
Clinical Data
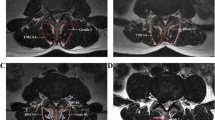
Forty-six patients with LDH were included in this study. The inclusion criteria were as follows: (1) low back pain accompanied by unilateral leg radiation pain and/or numbness. (2) Image examination showed that the lesion was a single level and unilateral lumbar radiculopathy, and the levels were located from L1–L2 to L4–L5 of the intervertebral discs (Fig. 1). (3) Patients that had presented with surgical indications of LDH and underwent surgical treatment. Exclusion criteria included the following: image examination indicated spinal deformity, infection, and tumor or severe osteoporosis. The gender, age, length of medical history, and lesion segments of each patient were recorded.
Typical case: male, 53-years of age, with a history of complaining about low back pain accompanied by radiative pain and numbness in the left lower extremity for 9 months. MRI sagittal image showed L4–L5 disc prolapse and dissociation (a). Cross-sectional imaging showed that the L4–L5 left posterior disc had herniated and the left L5 nerve root was compressed significantly
The patients were divided into four groups according to their medical histories. Group 1: patients with a medical history of less than 6 months, which included 8 males and 7 females, aged 21–60 years of age, with an average age of 39.67 ± 13.35 years. Group 2: patients with a medical history of 6–12 months, including 3 males and 7 females, aged 28–68 years, with an average age of 49.70 ± 15.68 years. Group 3: patients with a history of 12–24 months, including 6 males and 7 females, aged 29–65 years, with an average age of 46.85 ± 11.72 years. Group 4: patients with a medical history greater than 24 months, including 3 males and 5 females, aged 28–63 years, with an average age of 51.00 ± 11.00 years. Lesion segments were found in the following: L1–L2 (1 case), L2–L3 (6 cases), L3–L4(12 cases), and L4–L5 (27 cases).
Muscle Specimens
All patients gave informed and written consent in compliance with the directives specified by the local ethics committee of the Affiliated Zhuzhou Hospital of Xiangya Medical College of Central South University. A bundle of multifidus with dimensions of approximately 0.5 cm × 0.5 cm × 2 cm was obtained from both sides of the involved segment respectively, and frozen in liquid nitrogen for later use.
Muscle Specimens Process and Research Methods
Muscle specimen assessment: routine hematoxylin and eosin (HE) and Masson’s trichrome staining was used to determine histological changes in muscle specimens. The muscle specimens were fixed in 10% formalin for 24 h and underwent routine dehydration and paraffin embedding. Muscle specimens were sectioned at a 3-μm thickness and stained by the HE and Masson’s trichrome methods using standard approaches. Under higher power magnification, 10 discontinuous visual fields of muscle specimens for each section were selected randomly by the LEICA image acquisition system (Leica Microsystems, Bannockburn, IL, USA). Ten muscle fibers were selected from each field of view for the HE stained section, and the CSA of each muscle fiber was calculated by Image J software version 1.8.0 (National Institutes of Health, Bethesda, MD, USA), following which, the mean CSA was automatically calculated. The ratio of myofibers to collagen fibers of each field of view from the Masson’s trichrome staining section was calculated by Image J software, and then the mean ratio was automatically calculated.
Immunohistochemistry and immunofluorescence staining was performed on paraffin-embedded sections as reported previously and the muscle specimens were sectioned at a thickness of 3-μm. The sections were blocked at room temperature using saline containing 0.1% BSA and 10% goat serum. Rabbit anti-TGF-β1 (Bioss, Beijing, China) and horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (H + L) (Zsbio, Beijing, China) were used for immunohistochemistry. Rabbit anti-TGF-β1 (Bioss, Beijing, China) and goat anti-rabbit IgG Cy3 conjugates (Cwbio, Beijing, China) were used for immunofluorescence analysis. 10 discontinuous visual fields were also randomly selected under the Olympus microscope and fluorescence microscope (Olympus Co., Tokyo, Japan) for each section respectively. The integrated optical density (IOD) of TGF-β1 expression in each visual field was determined using the Image J software, and then the mean IOD was automatically calculated.
Statistical Analysis
Statistical analyses were performed with the SPSS version 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± S.D. During the statistic analysis, the test of normality was conducted first. If the data followed a normal Poisson distribution, then a paired Student’s t test analysis was used. If not, the Wilcoxon signed-rank test was adopted. The difference between the involved side and the uninvolved side in the CSA of multifidus, and the sectional area ratio of myofibers to collagen fibers, as well as the IOD of TGF-β1 expression, were observed, wherein a significance level was set at an alpha value of P < 0.05.
Results
All data were normally distributed and paired Student’s t-tests were used.
Histological Observation of the Multifidus
HE staining showed that multifidus fibers of the uninvolved side presented as normal, and was characterized by a neat arrangement, clear horizontal lines, and an obvious nucleus without cell swelling. An abundant capillary network and a small number of fibroblasts could be seen between muscle cells. While the CSA of multifidus fibers in the involved side became smaller, the intermuscular space became larger. The number of inter-fascicular fibroblasts and connective tissues also increased. Masson’s trichrome staining showed that the muscle fibers were stained red and collagen fibers were stained dark blue. The muscle fibers in the uninvolved side were neatly arranged, without obvious atrophy of the muscle cells, and there was a small amount of collagen connective tissue seen between the muscle cells. Also, in the involved side, muscle fibers shrank in the CSA due to atrophy, and the distance between the muscle fibers widened due to the proliferation of collagen connective tissue. The amount of collagen connective tissue was found to have increased significantly (Fig. 2).
In groups 1 and 2, there were no significant differences in the CSA of bilateral multifidus and there was no significant difference found in the context of the sectional area ratio of myofibers to collagen fibers (P > 0.05). In groups 3 and 4, paired Student’s t-tests revealed significant differences in the CSA of bilateral multifidus and the sectional area ratio of myofibers to collagen fibers (P < 0.05). With an extended medical history, the CSA of the multifidus and the sectional area ratio of myofibers to collagen fibers were found to have decreased gradually (Tables 1 and 2).
TGF-β1 Detection
The positive results of a TGF-β1 immunohistochemical reaction were identified as stained brown granules or patches. No obvious brown or very low levels of brown staining particles were observed, in other words, no obvious positive TGF-β1 reaction was observed in the uninvolved side. With an extended medical history, brown staining material appeared in the multifidus of the involved side and took the form of a scattered granular distribution to a uniform patchy distribution, which suggested a positive TGF-β1 reaction (Fig. 3).
The result of immunofluorescence was similar to that of immunohistochemistry assays, and the positive result was that of a red fluorescence pattern. A small amount of TGF-β1 was expressed in the multifidus of the uninvolved side, which was mainly located on the muscle cell membrane. While increased expression of TGF-β1 could be seen on the cell membrane and in the matrix of the involved side, we found that TGF-β1 was also strongly expressed in muscle cells in the group of patients with a medical history of more than 12 months (Fig. 4).
There was no significant difference in the expression of TGF-β1 in group 1 (P > 0.05). In the other three groups with a history of more than 6 months, paired Student’s t-tests revealed that TGF-β1 was more significantly expressed in the involved side than it was on the non- involved side, with statistically significant differences found (P < 0.05) (Tables 3 and 4).
Discussion
Long-term denervation causes stage demyelination, and axonal degeneration of the peripheral nerves, which results in denervation of skeletal muscle and a gradual decrease in the number of muscle nuclei [11]. With the extension of the denervation time, the muscle fibers atrophy, and the muscle cells, which are unable to maintain basic functions, eventually differentiate to fibroblasts. In addition, the proliferation of fibroblasts leads to the proliferation of connective tissue in the denervated muscles. Capillaries and other small blood vessels found in the muscles are surrounded by layers of dense collagen that separate them from their neighboring muscle fibers. It potentially affects material exchanges between the vascular bed and the muscle fiber, and also affects the insertion and extension of axons. Fibrosis is inevitable in the skeletal muscles.
There are many studies on the changes seen in paraspinal muscle in patients with LDH. Histological studies suggested that denervation of the multifidus may result in atrophy and structural changes of muscle fibers after nerve root compression [3]. However, any conclusions drawn from image changes after LDH remain quite controversial. Yaltirik et al. [4] believed that long-term compression of nerve roots could lead to atrophy and degeneration of multifidus and erector spinae in LDH. Ploumis et al. [5] found that muscle atrophy of the multifidus, erector spinae, quadratus lumborum and psoas major occurred in the involved side of the corresponding segments. By contrast, according to Kader [6], muscle atrophy is not associated with compression of nerve roots. Kang et al. [7] found that there was no significant difference in multifidus CSA when comparing the uninvolved and involved sides in LDH. Therefore, it remains impossible to draw any conclusions that nerve compression causes atrophy of multifidus or other paraspinal muscles [8].
Located in the dorsal lamina spinous groove in the lumbosacral segment, the multifidus mainly participates in the stretch movement of the spine and maintains the presence of lordosis of the lumbar spine, which is an important factor in terms of dynamic stability of the spine. The superficial layer of the multifidus originates from the medial side of the posterior sacro-iliac ligaments and the posterior superior iliac spine and ends at the L1–5 spinous process. It is uniquely innervated by the medial branch from the posterior branch of the lumbar nerve, and each muscle bundle is innervated by a single branch, without communicating branches [12, 13]. Thus, multifidus specimens above the level of L5 from patients with LDH were selected as the subjects of interest to our study.
The CSA of muscle fibers and the sectional area ratio of myofibers to collagen fibers are quite ideal indicators that reflect the morphology of muscle atrophy. In this study, it was found that multifidus fibers in the involved side of patients with LDH had atrophied, and the CSA of the fibers became smaller. There was a large amount of collagen proliferation between muscle fibers, which did not display any contractility. With an extended medical history, we found that the CSA of muscle cells and the sectional area ratio of myofibers to collagen fibers was significantly different from that of the uninvolved side, which supported the view of ipsilateral multifidus atrophy and fibrosis caused by nerve root compression by the herniated disc.
TGF-β is a polypeptide signaling molecule with a superfamily of more than 50 members that regulate a variety of cellular functions, including developmental and homeostatic processes [14]. TGF-β1 represents one of the most well-known members of the TGF-β super-family and was discovered more than 30 years ago. Since then it has been shown to play a role in a variety of cellular functions [15] with a gene location aligned to chromosome 19q13, which contains 7 exons and 6 introns, with 9 common gene polymorphism sites. This gene polymorphism affects the transcription and expression of TGF-β1. Mature TGF-β1 is comprised of 112 amino acids, and two polypeptide chains with a molecular weight of 12.5 kD that is connected by disulfide bonds [16].
TGF-β1 is synthesized and secreted by various cells that are derived from the bone marrow matrix, which includes T cells, macrophages, eosinophils, and neutrophils. Primary TGF-β1 is inactive, and various stimuli lead to changes in the microenvironment of tissues that activate primary TGF-β1 to generate various biological activities [14]. TGF-β1 is a core molecule regulating skeletal muscle fibrosis, which can promote the transverse differentiation of satellite cells into myofibroblasts and fibroblasts, and promotes the excessive synthesis of collagen in the extracellular matrix, which inhibits muscle healing [17].
In vivo experimental studies have shown that increased TGF-β1 activation leads to apoptosis of precursor cells of skeletal muscle cells, endomysium fibrosis, muscle atrophy, and myoblast differentiation into fibroblasts [18]. During the process of fibrosis, TGF-β1 expression in skeletal muscles was significantly up-regulated and was highly expressed for an extended period of time, and the deposition of type I collagen fibers were associated with TGF-β1 expression [19]. After stimulating denervated myogenic stem cells with TGF-β1 in vitro, cellular mRNA expression of type I and type III collagen was significantly enhanced, and the synthesis levels of type I and type III collagen were also significantly increased. It indicated that TGF-β1 can activate myogenic stem cells, change their biological activity, promote their massive synthesis and secretion of collagen fibers, strengthen extracellular matrix deposition and remodeling, and promote denervated skeletal muscle fibrosis [20].
Precisely how TGF-β1 is expressed in the paraspinal muscle of patients with LDH, and understanding its precise significance is currently unknown. No relevant studies have been reported so far. It is necessary to study altered TGF-β1 expression patterns in the multifidus of patients with LDH.
In this study, the expression levels of TGF-β1 in the involved side of the multifidus was significantly higher than that found in the uninvolved side, and the expression site extended from the muscle membrane and matrix to the muscle cells, which suggested that over-expression of TGF-β1 was closely related to multifidus fibrosis. The expression of TGF-β1 was found to be altered earlier as compared to any histological changes, which suggested that increased expression of TGF-β1 might promote the process of multifidus fibrosis.
TGF-β1 plays a significant role in denervated skeletal muscle fibrosis and is an important breakthrough point to explore the prevention and treatment of denervated muscular atrophy. When fibrosis occurs in the liver, kidney, lung and indeed other tissues, intervention in the production or biological effects of TGF-β1 with therapeutic drugs, cytokines, receptor antagonists, neutralizing antibodies, small interfering RNA interference technology, and other means can all serve to reduce the extent of fibrosis in the tissues [21].
The increased knowledge with regard to the participation of TGF-β1 in several muscular pathologies has attracted great interest in the evaluation of therapeutic alternatives to neutralize or diminish the deleterious effects of biologically active TGF-β1. Potential strategies to inhibit the effects of TGF-β1 include blocking TGF-β1 antibodies [22], and the different components of RAS [23] as well as several inhibitors of TGF-β1 receptors or the associated signaling pathways [24, 25]. However, the main challenge that is recognized with these treatments is their lack of specificity, which can lead to concerning side-effects. The major challenge in clinical therapy is to understand how to improve muscle regeneration, reduce muscle fibrosis and atrophy, and to do so without altering the normal functioning of TGF-β1 in other tissues, such as regulating cellular proliferation, hematopoietic pathways, migration, and inflammation.
There are also some shortcomings identified in this study that require a brief discussion. (1) Changes in other growth factors and their correlations with the process of multifidus fibrosis caused by LDH remain unknown. (2) The conclusion might be more convincing if a TGF-β1 blocking group had been introduced, and we recognize this as a potential weakness of the study. (3) Finally, we understand that this work described a single-center study, with limited specimens collected and the risk of possible bias in the selection of cases, most of which were obtained from the local area. In addition, height, weight, occupation, nutritional status, and other factors related to the recruited and studied subjects were not considered, which might have a certain impact on the reliability of the conclusions. Consequently, we need to expand the sample size. Further studies are required that address the mechanism of multifidus fibrosis and to promote tissue damage healing, and improved prognosis in LDH.
References
Hides, J. A., Stokes, M. J., Saide, M., Jull, G. A., & Cooper, D. H. (1994). Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine, 19, 165–172.
Zhao, W. P., Kawaguchi, Y., Matsui, H., Kanamori, M., & Kimura, T. (2000). Histochemistry and morphology of the multifidus muscle in LDH: Comparative study between diseased and normal sides. Spine (Phila Pa 1976), 25, 2191–2199.
Yoshihara, K., Shirai, Y., Nakayama, Y., & Uesaka, S. (2001). Histochemical changes in the multifidus muscle in patients with lumbar intervertebral disc herniation. Spine (Phila Pa 1976), 26, 622–626.
YaltiriK, K., Güdü, B. O., Isik, Y., Altunok, Ç., Tipi, U., & Atalay, B. (2018). Volumetric muscle measurements indicate significant muscle degeneration in single-level disc herniation patients. World Neurosurg, 116, e500–e504.
Ploumis, A., Michailidis, N., Christodoulou, P., Kalaitzoglou, I., Gouvas, G., & Beris, A. (2011). Ipsilateral atrophy of paraspinal and psoas muscle in unilateral back pain patients with monosegmental degenerative disc disease. British Journal of Radiology, 84, 709–713.
Kader, D. F., Wardlaw, D., & Smith, F. W. (2000). Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clinical Radiology, 55, 145–149.
Kang, J. I., Kim, S. Y., Kim, J. H., Bang, H., & Lee, I. S. (2013). The location of multifidus atrophy in patients with a single level, unilateral lumbar radiculopathy. Annals of Rehabilitation Medicine, 37, 498–504.
Farshad, M., Gerber, C., Farshad-Amacker, N. A., Dietrich, T. J., Laufer-Molnar, V., & Min, K. (2014). A symmetry of the multifidus muscle in lumbar radicular nerve compression. Skeletal Radiology, 43, 49–53.
Guadagnin, E., Narola, J., Bannemann, C. G., & Chen, Y. W. (2015). Tyrosine 705 phosphorylation of stat3 is associated with phenotype severity in TGFβ1 transgenic mice. BioMed Research International, 2015, 843743.
Bogduk, N. (1983). The innervation of the lumbar spine. Spine, 8, 286–293.
Rebolledo, D. L., González, D., Faundez-Contreras, J., Contreras, O., Vio, C. P., Murphy-Ullrich, J. E., et al. (2019). Denervation-induced skeletal muscle fibrosis is mediated by CTGF/CCN2 independently of TGF-β. Matrix Biology, 82, 20–37.
Macintosh, J. E., Valencia, F., Bogduk, N., & Munro, R. R. (1986). The morphology of the human lumbar multifidus. Clinical Biomechanics, 1, 196–204.
Kalimo, H., Rantanen, J., Viljanen, T., & Einola, S. (1989). Lumbar muscles: Structure and function. Annals of Medicine, 21, 353–359.
Ismaeel, A., Kim, J. S., Kirk, J. S., Smith, R. S., Bohannon, W. T., & Koutakis, P. (2019). Role of transforming growth factor-β in skeletal muscle fibrosis: A review. International Journal of Molecular Sciences, 20, 2446.
Attisano, L., & Wrana, J. L. (2002). Signal transduction by the TGF-beta superfamily. Science, 296, 1646–1647.
Vieira de Castro, J., Gonçalves, C. S., Costa, S., Linhares, P., Vaz, R., Nabiço, R., et al. (2015). Impact of TGF-beta1-509C/T and 869T/C polymorphisms on glioma risk and patient prognosis. Tumour Biology, 36, 6525–6532.
Li, H., Hicks, J. J., Wang, L., Oyster, N., Philippon, M. J., Hurwitz, S., et al. (2016). Customized platelet-rich plasma with transforming growth factor neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials, 87, 147–156.
Narola, J., Pandey, S. N., Glick, A., & Chen, Y. W. (2013). Conditional expression of TGF-β1 in skeletal muscles causes endomysial fibrosis and myofibers atrophy. PLoS ONE, 8, e79356.
Liu, F., Tang, W., Chen, D., Li, M., Gao, Y., Zheng, H., et al. (2016). Expression of TGF-β1 and CTGF is associated with fibrosis of denervated sternocleidomastoid muscles in mice. Tohoku Journal of Experimental Medicine, 238, 49–56.
Fanbin, M., Jianghai, C., Juan, L., Yang, W., Yuxiong, W., Yanhua, C., et al. (2011). Role of transforming growth factor-β1 in the process of fibrosis of denervated skeletal muscle. Journal of Huazhong University of Science and Technology [Medical Sciences], 31, 77–82.
Jain, M., Lam, A., & Gottardi, C. J. (2017). Tissue-specific knockout/knockdown of type 2 TGF-beta receptor and protection against bleomycin injury/fibrosis. American Journal of Respiratory and Critical Care Medicine, 184, 983.
Lamar, K. M., Bogdanovich, S., Gardner, B. B., Gao, Q. Q., Miller, T., Earley, J. U., et al. (2016). Overexpression of latent TGFbeta binding protein 4 in muscle ameliorates muscular dystrophy through myostatin and TGFbeta. PLoS Genetics, 12, e1006019.
Abrigo, J., Simon, F., Cabrera, D., & Cabello-Verrugio, C. (2016). Angiotensin-(1-7) Prevents skeletal muscle atrophy induced by transforming growth factor Type Beta (TGF-beta) via mas receptor activation. Cellular Physiology and Biochemistry, 40(1–2), 27–38.
Lemos, D. R., Babaeijandaghi, F., Low, M., Chang, C. K., Lee, S. T., Fiore, D., et al. (2015). Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature Medicine, 21, 786–794.
Davies, M. R., Liu, X., Lee, L., Laron, D., Ning, A. Y., Kim, H. T., et al. (2016). TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS ONE, 11, e0155486.
Author information
Authors and Affiliations
Contributions
DP, ZZ: concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing. DC: clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing. QH: definition of intellectual content, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis. TS: concepts, design, definition of intellectual content, manuscript preparation, manuscript editing, manuscript review, guarantor.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, D., Zhang, Z., Chen, D. et al. Morphological Alteration and TGF-β1 Expression in Multifidus with Lumbar Disc Herniation. JOIO 54 (Suppl 1), 141–149 (2020). https://doi.org/10.1007/s43465-020-00213-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-020-00213-4