Abstract
Chronic wounds are important public health issues, affecting 1.51 to 2.21 per 1000 population. These hard-to-heal wounds affect the workers, due to pain, possibility of infection, amputation, and death, impacting around 2–4% of the health budget of countries. Since ancient times, humans have used plants to treat wounds. Intensive investigation of medicinal plants, their extracts, active molecules and respective formulations begin in the first half of the twentieth century. This review aims to present the definition of wounds and their healing mechanisms; the plants and their molecules with potential to heal wounds; the main developed and patented herbal formulations; additional therapies and future trends in this field. The scientific literature on the subject was investigated in the following databases: Pubmed, Science Direct, Scopus, Lilacs, and Scielo. The Derwent Innovations plataform was used for patent research. The study covered the description of wounds and the wound-healing process, medicinal plants employed in wound healing and active molecules, such as flavonoids, terpenes, polysaccharides, and proteases, along with many types of distinct formulations used to treat wounds, especially gels, creams, ointments and film membranes, and additional therapies that included skin substitutes and grafts, negative pressure wound therapy, and hyperbaric oxygen therapy. Great advancement has been observed over the main developed and patented herbal formulations based in the Green Chemistry principles. Classical formulations containing active plant molecules are commonly used to treat wounds. However, more sophisticated nanosystems and additional therapies enable important advances in the wound-healing.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wound is a disruption in continuity of the epithelial lining of the skin or mucosa, resulting in destruction of tissues, disruption of blood vessels, and extravasation of blood constituents and tissue hypoxia. Wounds cause pain, bleeding, disability, and can lead patients to death, especially because of sepsis (Fazil and Nikhat 2020). Chronic or hard-to-heal wounds do not reduce in size for more than 40% or heal in 30 days, affecting around 1.51 to 2.21 per 1000 population in the world. This incidence is increasing with ageing populations (Zhu et al. 2022). Furthermore, the impact of social and economic of this type of wound is 2–4% of the health budget of countries (Järbrink et al. 2017).
Prehistoric humans observed that bleeding decreased on covered wounds by plants, earth and clays (Bhattacharya 2012). Therefore, they developed a multitude of wound coverings, such as ointments, certain plant extracts, herbs, or clay, which can also relieve pain and accelerate healing. For a long time, these covers were selected empirically, by trial and error. The first written record containing medical information on wound treatment with clay tablets is dated at approximately 2500 B.C., in Mesopotamia. Many plant preparations were developed by primitive people in various parts of the world (Forrest 1982). The history of the topical application of herbal medicines goes back thousands of years, with records of the Egyptian and Babylonian use of salves and ointments in their traditional medical systems (Khogta et al. 2020).
The pharmaceutical industry has a long history of use of medicinal plants (Tariq et al. 2022), starting with the development of chemistry, which enabled the isolation, purification and characterization of plant-based pharmaceutical ingredients (Shakya 2016). Herbal medicine is a part of traditional medicine and can be used as simple herbal preparations, containing biologically active compounds, or as medicinal plant formulations which can be applied directly to the skin, promoting the healing process. There is evidence in literature that herbal medicines are also highly effective in combating microbial diseases and are promising in the treatment of persistent infections, especially skin wound infections. The most common topically applied formulations are gels, emulsions, films and ointments, or herbal extracts themselves (Pereira and Bártolo 2016). Herbs could be applied to skin wounds in a balsam, and leaves were frequently used as bandages; many were refreshing and calming (Forrest 1982; Fazil and Nikhat 2020).
A vast number of plants have been employed in wound treatment for thousands of years. Some were astringent, such as lady's mantle (Alchemilla vulgaris L., Rosaceae), others presented antimicrobial activities, such as comfrey (Symphytum officinale L., Boraginaceae) and St. John's wort (Hypericum perforatum L., Hypericaceae) (Sowa et al. 2018). The usefulness of various plants in folk medicine were also suggested by their aspect; for example, the perforated leaves of H. perforatum suggested its application on punctures. Currently, it is known that this plant contains a diversity of secondary metabolites, such as naphthodianthrones, phloroglucinols, flavonoids, and phenylpropanoids, that have antifungal, anti-inflammatory, and antimicrobial activities, which promote the wound-healing process by increasing fibroblast proliferation, collagen bundle synthesis, and tissue revascularization (Yadollah-Damavandi et al. 2015). On the other hand, the leaves of A. vulgaris, which has a high content of the astringent tannin, precipitates wound proteins, forming a temporary film over the wound surface that protects it from caustic agents and microorganisms, besides promoting vasoconstriction and arrest of haemorrhage (Maver et al. 2018). Besides, S. officinale has allantoin and phenolic acids, which are anti-bacterial and antioxidants, being excellent healing agents due to preserving fibroblasts and inducing granulation tissue (Sowa et al. 2018).
The removal of necrotic tissue from skin wounds and burns, called debridement, is an important step in healing. Autolytic debridement is the use of products to keep the wound moist and support the gradual softening of the eschar using the natural enzymes present in wound fluid (Thomas et al. 2021). Nowadays, many molecules, especially from plants, are employed with tremendous success in the healing of all types of wounds in different formulas. This work aims to study the concepts of wounds and their repair mechanisms, as well as to discuss the therapeutic potential of medicinal plants and their molecules in the healing of chronic or hard-to-heal wounds, since the first care for a wound is a topical formulation. However, they may need additional therapies. Therefore, we demonstrate that topical phytotherapeutic formulations, containing plants and their extracts, have been developed and patented as an accessible and effective solution in wound healing, since they have several active molecules that act synergistically. Finally, we present promising nanosystems and discuss additional treatments that are future trends in this field.
Search Strategy
The scientific literature for this review was investigated by searching Pubmed, Science Direct, Scopus, Lilacs, and Scielo databases. The research period was from 1993 to 2020. All the articles must be in English language. The keywords used were: “wound healing”, "wound repair", "wound regeneration", “plants in wound healing”, “plant proteases”, “secondary metabolites in wound healing”, “flavonoids in wound healing”, “polysaccharides in wound healing”, “terpenes in wound healing”, “medicinal plant formulations”, “wound dressing”, “tissue-engineered skin substitutes”, “negative-pressure wound therapy”, “skin grafts” and “hyperbaric oxygen therapy” which that were present in the title or in the abstract. Articles were chosen based on more recent works, followed by the selection and analysis of articles related to the theme of the present review. The exclusion criteria were no full text available, articles not in English language, topic unrelated to articles, and articles do not peer reviewed.
Patents were evaluated using the Derwent Innovations platform along with the appropriate keywords: “plant or plants and wound”, “extract or extracts and wound”, “protease or proteases and wound”, “flavonoid or flavonoids and wound”, “polysaccharide or polysaccharides and wound” and “terpene or terpenes and wound”. The results were refined for the years of 2012 to 2022, to decrease the number of patents found, and the keywords were researched in the title of each patent. All they must be in English language and with full text available.
Discussion
Wounds
Human skin is a specialized organ that acts as a primary defence barrier. It is highly adaptative and multifunctional, protecting us from the daily raid of chemicals, physical forces, and radiations that cause skin injury. Our skin is specialized to interface with the external environment and provides sophisticated and important homeostatic functions that allow it to heal quickly and efficiently. This organ consists of 3 layers: epidermis, dermis and hypodermis. Disruptions in skin integrity led to the formation of wounds, that are classified into two major categories: acute and chronic (Wilkinson and Hardman 2020).
Generally, acute wounds heal relatively faster. During the wound-healing process, injuries occurring in the border of blood vessels lead to the formation of blood clots. In this type of wound, a cascade of cellular and biochemical events follows, directing the repair process (Rezaie et al. 2019). Chronic wounds, on the other hand, may take 3 months or even years to heal after the initial injury. Thus, chronic wounds stand as a major concern in healthcare, and their treatment is one of the most challenging issues in the field of regenerative medicine. Many factors have been related with defective wound healing, such as maceration, ischemia, and infection (Walton 2014). These types of wounds are often associated with effects of an intrinsic disease state, or flawed healing due to a coexisting disease. Types of chronic wounds include diabetic foot ulcers, pressure ulcers, venous leg ulcers, and ischemic lower-extremity ulcers. The major characteristic of chronic wounds is the lack of progression via normal stages of healing, complicating the election of a suitable treatment (Morton and Phillips 2016).
Wound Healing
As mentioned before, the skin acts as a primary defence barrier, preventing damages to internal structures. For this, it has rapid and skilled mechanisms to close discontinuities, in a process known as wound-healing response. Complete wound repair is a dynamic, integrated and complex process involving many different cell types, special molecules and events that must be firmly coordinated to efficiently repair damaged tissue, that must all occur in the appropriate sequence and time frame, and that are commonly classified into four main phases: haemostasis, inflammation, proliferation and remodelling (Wilkinson and Hardman 2020).
Haemostasis
Immediately after injury, damaged blood vessels rapidly contract and expose degraded collagen and von Willebrand factor. These proteins then interact with platelets by glycoprotein Ia and VI receptors, promoting platelet activation and adherence to the blood vessel wall, forming blood clot and preventing the severe loss of blood from vascular damage (Rousselle et al. 2019). Activated platelets are crucial in the recruitment of immune cells to the tissue injury because its granules have more than 300 biologically active substances and, they are involved in immune cell recruitment and inflammation. Platelets secrete some growth factors that stimulate fibroblasts and keratinocytes that are crucial for wound healing, angiogenesis and remodelling. These growth factors are: platelet-derived growth factor (PDGF), which controls the growth of mesenchymal cells (fibroblasts and smooth muscle cells), stimulates collagen, glycosaminoglycan, and collagenase production by fibroblasts, promotes macrophages and neutrophils chemotaxis, and re-epithelialisation; transforming growth factor β (TGF-β), which inhibits macrophages and lymphocyte proliferation, mesenchymal stem cells proliferation, neutrophil and monocyte chemotaxis, and matrix formation; fibroblast growth factor (FGF), which has a mitogenic effect on fibroblasts, endothelial cells, mesenchymal stem cells, chondroblasts, osteoblasts, and promotes angiogenesis; epidermal growth factor (EGF), which promotes fibroblast migration and proliferation; vascular-endothelial growth factor (VEGF), which promotes angiogenesis and increases vessel permeability; and connective tissue growth factor (CTGF), which promotes platelet adhesion, white blood cell migration and angiogenesis, and regulates collagen synthesis (Golebiewska and Poole 2015).
When blood vessels are damaged, tissue factor is released to bind the blood factor VII, and the intrinsic blood cascade form the blood clot. The platelet surface is essential to activate many blood factors. Subsequently, thrombin-activated also triggers platelet activation, inducing a conformational change, reinforcing the blood clot. An insoluble clot known as eschar forms, made of fibrin, fibronectin, vitronectin and thrombospondin, and is the primary wound plug preventing bleeding. Eschars also fulfil a number of secondary functions: shielding against bacterial invasion, providing a scaffold for incoming immune cells, and reserving cytokines and growth factors to guide the behaviour of wound cells in early repair (Gantwerker and Hom 2011). As soon as enough clot is formed, the coagulation process is switched off, preventing excessive thrombosis; at the same time, the injured vessel wall is repaired by smooth muscle cells and endothelial cells (Rousselle et al. 2019).
Inflammation
The inflammation response is complex and modulated by a myriad of intrinsic and extrinsic factors that activate resident immune cells, such as mast, Langerhans and T cells, and macrophages, by binding pattern recognition receptors to activate inflammatory pathways (Martin and Nunan 2015). Afterwards, pro-inflammatory molecules, attracted by chemoattractants, including interleukin 1 (IL-1), tumour necrosis factor-alpha (TNF-α) and bacterial endotoxins, such as lipopolysaccharide (LPS), are released, attracting circulating leucocytes to the site of injury and stimulating vasodilatation, which facilitates neutrophil and monocyte adhesion, such as selectins, and diapedesis. Neutrophils, arriving early after injury, remove necrotic tissue and pathogens through phagocytosis and the release of antimicrobial peptides, a reactive oxygen species [ROS, that are superoxide anion (O2·−), hydrogen peroxide (H2O2) and hydroxyl radical (HO•)], prostaglandins, and proteolytic enzymes (Wang et al. 2018).
The blood monocytes migrate to the wound tissue and differentiate into macrophages, the most important cells in tissue repair. They are activated by LPS and interferon-gamma (IFN-γ), and promote inflammation by releasing ROS, inflammatory cytokines (e.g., IL-1, IL-6 and TNF-α), and growth factors such as VEGF and PDGF. They phagocytose necrotic debris, bacteria, and apoptotic neutrophils, while also stimulating reparative processes to enable an effective wound resolution (Wilkinson and Hardman 2020). T-cells are also critical for the early injury response, being recruited in the blood to deal with the inflammation caused by the damaged tissue. Additionally, mast cells release histamine in wounds in order to aid neutrophil recruitment during early inflammation (Zhang and Zhang 2020).
Proliferation
This stage begins with fibroblast migration to the wound site, induced by PDGF, TGF-β, TNF-α, IL-1, IL-6 and IL-2, and is characterized by the extensive activation of keratinocytes, fibroblasts, macrophages and endothelial cells to orchestrate wound closure, matrix deposition, and angiogenesis. The proliferative phase starts approximately 12 h post-injury. Keratinocytes are activated by changes in mechanical tension and electrical gradients, culminating in their migration across the wound to reform the epidermal layer, a process known as re-epithelialization. These cells remove debris and necrotic tissue from the wound bed due to the secretion of proteases, through their interactions with the structural protein of the preliminary matrix via integrin receptors. Matrix metalloproteases (MMPs), especially MMP-1 and MMP-9, are crucial for keratinocyte migration, as they help the dissociation of the integrin receptor. When keratinocytes from opposing edges meet, migration terminates, and a thin epithelial layer is established, and they suffer the terminal differentiation, to stratify and regenerate the epidermis. Besides, TGF-β stimulates fibroblasts to create the myofibroblasts, forming thick actin bundles below the plasma membrane, which are necessary for wound contraction (Stolzenburg-Veeser and Golubnitschaja 2018). In addition to MMPs, serine proteases, such as neutrophil elastase, cathepsin G, and urokinase-type plasminogen activator, are also involved in the wound-healing process. MMPs and neutrophil elastase are often present and may persist longer in the proteolytic environment of a non-healing chronic wound (Westby et al. 2020).
Fibroblasts are the most important cells for replacing collagen, elastin, fibronectin, proteoglycans and other components of extracellular matrix (ECM), forming substantial granulation tissue. These cells degrade the provisional matrix and replace it with a granulation tissue rich in immature collagens, fibronectin and proteoglycans. This new granulation tissue enables the migration and differentiation of wound cells, supporting both the formation of new blood vessels and the deposition of mature ECM. New blood vessels are created during the process of angiogenesis to supply the metabolic demands of the highly proliferative healing tissue by the action of VEGF, TGF-βB and FGF-β. VEGF induces nitric oxide (NO) production and promotes the mobilisation of endothelial progenitor cells (EPCs) from the bone marrow into circulation. MMPs are involved in neovasculogenesis: increased NO levels promote MMP-9 secretion, helping to release EPCs from the bone marrow into circulation (Stolzenburg-Veeser and Golubnitschaja 2018).
Macrophages are also crucial in angiogenesis because they produce proteases such as MMPs, to degrade the dense fibrin network and chemotactic factors, e.g., TNF-α, VEGF and TGF-β, driving endothelial migration. Therefore, microvascular endothelial cells proliferate and migrate into the wound bed, sprouting new vessels that fuse with others to develop stable, tubular networks (Wang et al. 2018).
The skin contains a consistent network of sensory and autonomous nerve fibres, whose regeneration is essential in wound repair. Though wound innervation remains understudied, it is known that neuropeptides, such as substance P, are released from sprouting neurons and immune cells during healing, influencing various cellular processes, such as proliferation and angiogenesis (Kumar et al. 2021).
Remodelling
Fibroblasts are the major cell type responsible for ECM remodelling, replacing the initial fibrin clot with hyaluronan, fibronectin and proteoglycans, and forming mature collagen fibrils later in repair. Proteoglycans assist in the construction of mature, cross-linked collagen fibrils and help cell migration (Xue and Jackson 2015).
As healing advances, collagen type III is replaced by collagen type I, increasing the tensile strength of the forming scar. Sequential changes in the ECM require balance between collagen degradation and synthesis, obtained by temporal regulation of MMPs. These collagenases, expressed by anti-inflammatory macrophages, fibroblasts and keratinocytes, cleave local helical collagens during repair. The degradation of the normal dermal matrix causes the release of elastin fragments; elastin, being a crucial dermal component, must reform elastic fibres to retain skin elasticity. Then, myofibroblasts bind to one another, attach to matrix fibrils, and draw the matrix together, a process called contracture. Wound repair diminishes when macrophages, endothelial cells and fibroblasts suffer apoptosis or withdraw from the injury site, leaving a scar (Reinke and Sorg 2012).
Despite the myriads of physiological responses, healing can be reduced by multiple cellular aspects of an individual that affect wound closure. This reduction is most often a result of pathological systemic changes, such as those associated with advanced age or uncontrolled diabetes. Skin wounds have a negative effect in people’s lives, and their management and care generate high costs to the healthcare system every year. For that reason, many studies have been working on new approaches to skin wound healing, including investigations on potential phytopharmacological methods (Poljšak et al. 2019).
Wound Healing Plants
Since 1978, the World Health Organization recognizes the benefits of medicinal plants. The organization defines herbal agents as active compounds from plant parts or herb materials in crude or processed state, as well as excipients (WHO 2019). Phytotherapy is a field of medicine that uses plants to treat different conditions or as health-promoting agents. It is a popular method for intervention and prevention of various diseases. Around 4 billion people worldwide use plants as alternative medicine, due to their affordability, availability, and safety; nearly 80% of people living in developing countries depend on herbal medicine for healthcare, including the treatment of wounds, infectious and metabolic diseases (Ribeiro Neto et al. 2020).
As stated before, the use of medicinal plants is rooted in cultural heritage and traditionalism since ancient times. Considerable knowledge of medicinal plants has been compiled from historical documents, which were used as the basis for pharmaceutical research to discover new agents in plant extracts or fractions to accelerate wound healing (Falzon and Balabanova 2017). The use of phytotherapies grew exponentially in recent years, due to their relatively low cost, natural origin, and fewer adverse effects than chemical drugs. Numerous pharmaceuticals used in conventional medicine were originally derived from plants, such as aspirin® (acetylsalicylic acid, extracted from Salix alba L., Salicaceae); acheflan® (volatile oil, extracted from Cordia curassavica Jacq. Roem. & Schult, Cordiaceae); and imunomax® (obtained from Uncaria tomentosa Willd. ex Schult. DC., Rubiaceae) (Ghochikyan et al. 2014; Calixto 2019). Some examples of medicinal plants and their medicinal uses are listed in Table S1.
Wound Healing Plant Molecules
Phytotherapy is the preferred choice over conventional therapy using synthetic medicines, particularly in terms of perceived side effects, and it has been proven to be effective in skin wound healing (Ferreira et al. 2014). Traditional medicine has employed medicinal plants in many areas for millennia, and humans have learnt by experience and observation to use plants, building up an extensive traditional culture of their application (Hardy 2021). Over the last century, scientific and technical progress, and specifically molecular, metabolomic, and genomic pharmacognosy, have enabled the discovery of a large number of new molecules and their mechanisms of action, enabling the creation of a wide repertoire of medicinal plant-based medications. Whereas modern pharmacology began with the study of traditional medicinal plants, moving on to study their individual molecules and standardised extracts, modern pharmacognosy focuses on improving research and discovering new properties and assaying the potency of an increasingly large number of medicinal plants by high-throughput screening, target-based drug discovery, and in silico methods for virtual ligand screening (Colalto 2017).
In modern science, plant species that are traditionally used to cure diseases have been extensively studied for the identification of their bioactive constituents and development of new drugs. Studies concerning mechanisms of action and effectiveness have shown that many of these plant compounds are pharmacologically safe, suggesting additional tests should be carried out in preclinical studies and clinical trials (Atanasov et al. 2021). Besides, a significant portion of the world population cannot afford industrialized medicines, and the use of herbal remedies can benefit these patient groups (Lordani et al. 2018).
Secondary plant metabolites have been essential in the discovery of new potential wound-healing agents, since these molecules offer an alternative to aid this process. Experimental evidence and many surveys sustain that plant molecules have beneficial properties on wound healing, as well as on various types of skin diseases (Moeini et al. 2020). The effectiveness of their active principles for wound treatment has been recently observed by many biochemical, molecular and pharmacological investigations. Volatile oils from Lavandula, Croton, Blumea, Pinus, Cedrus, Abies, Eucalyptus, Cymbopogon, Rosarinus, Origanum, Salvia and Plectranthus species demonstrated faster closure wound size, improved collagen deposition and higher fibroblasts proliferation (Pérez-Recalde et al. 2018). Many volatile oils are associated with biopolymers, such as gelatine, collagen, alginate, chitosan, elastin and dextran, creating nanofibrous dressings with antioxidant, anti-inflammatory or antimicrobial activities (Huesca-Urióstegui et al. 2022).
Organic, aqueous and hydro-alcoholic extractions have been the primary approach to obtain active ingredient molecules from plants. Alerico et al. (2015) demonstrated that an ethanolic extract of Achyrocline satureioides (Lam.) DC., Asteraceae, containing flavonoids, coumarins, phenolic acids and polysaccharides, had antimicrobial and anti-inflammatory properties and improved fibroblasts and keratinocytes proliferation. Ponnusamy et al. (2015) observed beneficial effects in wounds using a rich polyphenol fraction from Dicranopteris linearis (Burm. f.) Underw., Gleicheniaceae, that increased fibroblast proliferation and migration. Finally, methanolic extract from Pongamia pinnata L. Merr., Fabaceae, leaves, rich in alkaloids, flavonoids, steroids and glycosides, demonstrated antimicrobial and antioxidant effects in skin ulcers, wounds, inflammations and infections (Dwivedi et al. 2017).
Flavones and Flavonoids
Flavonoids form one of the major groups of specialized metabolites or secondary metabolites. Today, more than 6,000 different compounds have already been described in nature, specially from plants (Luna et al. 2020). They can also be classified as polyphenols, because they often have one or more hydroxyl substituents in their structure, that also have a flavan nucleus of 15 carbon atoms (C6-C3-C6) and are diphenyl-propanoids (Salehi et al. 2019). In plants, these compounds perform a great diversity of functions, such as regulating cell growth, attracting pollinator insects, protecting against biotic and abiotic stresses, UV filtration, interfering with signalling pathways and gene expression, ROS scavenging (antioxidant), as anti-inflammatories, and having many roles in heat, drought, and frost tolerance (Dias et al. 2020, 2021). They stand out for being generally abundant in natural extracts, and different flavonoid classes have been studied as wound-healing treatment alternatives, and some examples of which are outlined below.
A chitosan hydrogel containing a flavonoid fraction from Passiflora edulis Sims, Passifloraceae, leaf extract demonstrated a beneficial effect on the treatment of skin lesions in diabetic rats, especially in the first few days after injury. This hydrogel was able to stimulate the antioxidant defence system, resulting in a potential formulation to be employed as dressings in the treatment of diabetic wounds (Soares et al. 2020).
The extract from French maritime pine tree (Pinus pinaster Aiton, Pinaceae) had shown, in experimental and in vitro studies, to exert photoprotective, antimicrobial, antioxidant, anti-inflammatory and anticarcinogenic effects, which were associated to its proanthocyanidin content. These molecules present high affinity to collagen and elastin and are able to inhibit the enzymatic hydrolysis by MMP in vitro. In vivo studies demonstrated that applying the extract over wounded tissues significantly reduced wound size, due to the presence of these flavonoids (Dogan et al. 2017).
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a well-known flavanol, with anticancer, antiproliferative, anti-inflammatory, antibacterial and antioxidant activities. Several studies have demonstrated its important effect in wound healing (Kim and Park 2020; Hofer et al. 2020). Özay et al. (2019) reported wound repair and regeneration action in diabetic excisional and nondiabetic incisional wounds treated with 1% (w/w) of kaempferol ointment for 14 days. The wound showed higher levels of hydroxyproline than those in the control groups. This hydroxylated amino acid is found in abundance in collagen and elastin structures and is an important re-epithelization indicator.
Quercetin is a polyhydroxy flavonoid frequently found in flowers, leaves, and fruits of various plants. It has various pharmacological activities, such as being anticancer, anti-oxidation, anti-fibrosis, and anti-inflammatory, and therefore has high medicinal value (Xu et al. 2019; Salehi et al. 2020). It proved to be a good tool in wound repair management in different wound-healing conditions due to the presence of catechol and hydroxyl groups, that can neutralise free radicals escaping from the skin’s endogenous antioxidant system, consequently inhibiting lipid peroxidation (Ozgen et al. 2016). Topical application of quercetin was also able to increase fibroblast proliferation, while decreasing fibrosis and scar formation and increasing the glutathione levels preventing the generation of novel ROS. Besides, it can chelate metal ions that could catalyse ROS formation (Fu et al. 2020). Vale et al. (2021) reported that pectin and casein microcapsules containing quercetin had an enhanced topical efficacy in blocking UVB-induced biochemical, cellular and pathological changes in the skin. Topical 3% quercetin ointment w/w demonstrated to be effective in accelerating wound repair by increasing collagen deposition and cell proliferation, due to modulating cytokines and growth factors. Besides, daily topical application of this quercetin ointment on diabetic wounds for 21 days resulted in increased expressions of interleukin-10 (IL-10), VEGF and tissue growth factor-b1 (TGF-b1) and decreased the expressions of tumour necrosis factor-α (TNF-α), IL-1b, MMP-9. The modulations of these cytokines, growth factors and proteases led to granular tissue formation, suppression of persistence of inflammatory cells, fibroblast proliferation, angiogenesis, thick collagen fibre synthesis, axonal regeneration, and faster regeneration of the epithelial layer, all of which significantly reflect the improvements in repair and regeneration of diabetic wounds in rats (Kant et al. 2020, 2021). Other findings indicate that quercetin reduces the production of inflammatory mediators such as IL-6, playing a role by inducing hemeoxygenase-1 (HO-1) and destroying the activation of inflammatory signalling pathway components, thereby modulating macrophage polarization from M1 to M2 phenotype and further inhibiting inflammation, accelerating diabetic wound repair (Fu et al. 2020).
Apigenin naturally occurs as 4´,5,7-trihydroxyflavone, and exists as apigenin-7-O-glucoside and various acylated derivatives. It is one of the most widespread flavonoids in plants, with countless nutritional and organoleptic characteristics, belonging to the flavone sub-class. Plants belonging to the Apium, Artemisia, Achillea, Hypericum, Matricaria, and Tanacetum genera are the main sources of this compound (Shankar et al. 2017). The large number of studies indicate that this flavone has many interesting pharmacological activities and nutraceutical potential. Apigenin was proved to be an antioxidant and can also be an important therapeutic agent for inflammation, autoimmune and neurodegenerative diseases, and even several types of tumours (Salehi et al. 2019). Topical treatment with Apigenin gel demonstrated to improve the degree of reepithelialisation and inflammation and favoured neo-vascularisation of the wounds (Lopez-Jornet et al. 2014). Besides, extracts from Matricaria chamomilla L., Asteraceae, and Punica granatum L., Lythraceae, which have high apigenin content, induced wound contraction and accelerated wound closure more than the conventional treatment with chemical compounds (Niknam et al. 2021).
Terpenes and Terpenoids
Isoprenoids are one of the largest and most diverse group of natural compounds, mostly found in plants, totalling more than 55,000 molecules described, and are a rich reservoir of candidate compounds for drug discovery. They are classified according to the organization and number of five-carbon (isoprene) units it contains, including hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), and polyterpenes (> C30). These compounds have been employed in cancer chemotherapy and have antimicrobial, antifungal, antiviral, antihyperglycemic, anti-inflammatory, antioxidants, antiparasitic, and immunomodulatory effects. However, many terpenes are found in low levels in nature (Cox-Georgian et al. 2019).
Carvacrol and thymol are important monoterpenes found in volatile oils of species from the Origanum genus and are investigated regarding their application as wound-healing agents. Carvacrol incorporated to chitosan films (0.5 or 1%) had a potential use on skin wound healing by promoting granulation tissue formation and enhancing cell epithelization and proliferation in rats, leading to reduction of oedema and wound areas (Costa et al. 2019). Volatile oils containing thymol and carvacrol modulate the production and release of reactive species, such as NO, pro-inflammatory cytokines and growth factors involved in the primary stages of the repair process. Besides, carvacrol inhibits the expression of different types of cytokines, such as IL-4, IL-17, IL-1β and TNF-α, and the isoenzyme cyclooxygenase 2 (COX-2), but also increases IL-10, a crucial anti-inflammatory cytokine (Cicalău et al. 2021; Dantas et al. 2021). Injury treatment with thymol showed a promotion of type III to type I collagen replacement in 14 days. Also, both isomers were able to downregulate MMP-9 and MMP-2, suggesting a complete collagenation and favourable regeneration, indicating it is an interesting alternative in the management of chronic wounds (Lv and Chen 2017; Costa et al. 2019).
The volatile oil of Bursera morelensis Ramírez, Burseraceae, demonstrated promotion of fibroblast migration, which leads to collagen production and remodelling, indicating an alternative use for scars with tensile strength. This activity was associated with the presence of important anti-inflammatory terpenes found in the volatile oil of B. morelensis, α-pinene and α-phellandrene (Salas-Oropeza et al. 2020). It was demonstrated that α-pinene has anti-inflammatory activity for decreasing COX-2 production and inhibiting TNF-α, IL-1β and IL-6. Further, α-phellandrene also inhibits the production of TNF-α and IL-6 and revealed antinociceptive effects. Moreover, both molecules induce cell cycle arrest and cell apoptosis, and are able to accelerate wound closure and promote collagen deposition (Salas-Oropeza et al. 2021).
Polysaccharides
This is a group of complex carbohydrates, ubiquitous high-molecular weight polymers that occur widely in nature. They are formed by repeated monosaccharide units joined by glycosidic bonds (Imre et al. 2019). These saccharides are extensively used in various healthcare products and medicines for their notable biological activities, such as antimicrobial, antioxidant, antitumor and neuroprotective effects. They are among the natural biopolymers found on Earth which are widely used as biomaterials for wound healing and tissue engineering (Gao et al. 2018). Acemannan is the major bioactive polysaccharide of Aloe vera (syn. of Aloe succotrina Weston, Asphodelaceae), which is extracted from the plant’s gel and skin. This molecule has been reported to have many pharmacological and biological applications in medical and industrial areas (Sierra-García et al. 2014). Recently, acemannan has been investigated for employment in the treatment of wounds and alveolar osteitis, using protocols approved by the US Food and Drug Administration (Liu et al. 2019). This polysaccharide was found to induce oral wound-healing functions in human gingival fibroblasts. This effect could be explained because acemannan stimulated the expression of collagen production by keratinocytes. A clinical trial showed that a skin patch of 5% acemannan reduced ulcer size and pain (Xing et al. 2015).
Another plant polysaccharide was isolated and purified from the rhizomes of Curcuma zedoaria (Christm.) Roscoe, Zingiberaceae, and promoted wound contraction, re-epithelialization, dermal angiogenesis, and collagen synthesis and deposition in diabetic rats, speeding up wound repair (Xu et al. 2018) Another polysaccharide, purified from roots of Sanguisorba officinalis L., Rosaceae, also demonstrated efficiency in experimental and mice burn wounds by accelerating wound contraction, decreasing the epithelialization period and hydroxyproline content, and promoting collagen synthesis and angiogenesis (Zhang et al. 2018a, b).
Proteases
All living organisms have several types of proteases or peptidases in all parts of their cells, which irreversibly cleave peptide bonds in proteins, polypeptides and peptides, in different environments and under different conditions. They have important therapeutic applications in certain diseases and conditions and are named as therapeutic enzymes, which are enzymes used in the treatment and diagnosis of diseases, and also in surgical procedures (Silva-López and Gonçalves 2019).
At the end of last century, proteases were initially used as therapeutic agents in haemostasis disorders such as coagulation factors VIII and IX to treat, respectively, haemophiliacs A and B, and urokinase, which converts plasminogen into plasmin, which in turn degrades fibrin polymers, being used in cases of thrombosis. These enzymes were obtained from natural sources, human and animal plasma, but large amounts of material were required to obtain a small amount of these enzymes. In addition to the low yield of these proteases, there was the imminent risk of contamination of the source material with pathogenic infectious agents, which could cause secondary infections and immunological reactions in patients who received them (Wolberg et al. 2012). Nowadays, they are produced by recombinant DNA technology, where the gene encoding of interesting proteases are inserted into suitable expression vectors and overexpressed in specialized vectors according to the complexity of the protein. Then, they are purified, standardized and finally formulated for human or veterinary use. Although it is a complex and expensive procedure, it guarantees quality and absence of pathogenic infectious agents (Swiech et al. 2017). However, therapeutic plant proteases are not heterologous proteins. They are obtained directly from the plant organs or latex, and it is possible to extract them without affecting the plant viability. These proteases are structurally simple and relatively easy to obtain, at low cost of production (Matagne et al. 2017). They are widely used as therapeutic enzymes in the treatment of wound healing, cancer, digestive disorders and infectious diseases (Rathnavelu et al. 2016; Ferreira and Silva-López 2021).
Wound repair is majority mediated by MMPs and serine proteases. In normal wound healing, proteases are active in all phases, via a cascade of precisely regulated steps and events that align with the appearance of various cell types in the wound bed during four distinct phases: haemostasis, inflammation, proliferation and epithelialization/remodelling. They are necessary for removal of denatured collagen, elastin, fibronectin, proteoglycans and other proteins from damaged matrix (debridement), contraction of the ECM, migration of epidermal cells, and remodelling of the scar (Westby et al. 2020). The most important therapeutic plant proteases employed in wound repair are bromelain and papain, which are discussed hereafter.
Bromelain is a crude aqueous extract rich in cysteine proteases obtained from the stem and fruit of species of the Bromeliaceae family, of which the pineapple (Ananas comosus (L.) Merr. and Ananas sativus Schult. & Schult.f. (syn. Ananas comosus var. comosus)) is the better-known species. The non-proteolytic constituents of this extract are escharase, glycosidases, peroxidases, ribonucleases, cellulases, carbohydrates, protease inhibitors, and other substances (Varilla et al. 2021). The escharase is a nonproteolytic enzyme with 45 kDa, that has no hydrolytic activity against normal protein substrates, but is responsible for debridement of necrotic tissue. It helps in the digestion, dissection and separation of non-viable, devitalized tissue, especially eschar (Silva-López 2017).
Pineapple tissues contain at least four proteases that belong to the papain superfamily: stem bromelain (EC 3.4.22.32), fruit bromelain (EC 3.4.22.33), ananain (EC 3.4.22.31), and comosain. They represent the whole protease activity of bromelain that showed expressive stability at high temperatures. Bromelain is prepared from pineapple juice by centrifugation, ultrafiltration, and lyophilization, yielding a yellowish powder, and the protease activity is determined (Rathnavelu et al. 2016). Pineapple (chemically known since 1876) has been used as a medicinal plant by many cultures, and its medicinal properties are attributed to bromelain (Petrovska 2012). Because of its complex composition, this extract interacts with many host molecules and provides a great diversity of pharmacological effects. Bromelain has been used for the treatment of many diseases and conditions, such as rheumatoid arthritis, thrombophlebitis, wounds, bronchitis, sinusitis, and for enhancing the absorption of certain drugs. In all these situations, this extract expressively alleviates pain and swelling, and decreases the healing time required with conventional treatments (de Lencastre Novaes et al. 2016; Tap et al. 2016). Bromelain decreased inflammatory mediators and was an anti-inflammatory agent in various conditions, since it modulated surface adhesion molecules on T cells, macrophages, NK cells and induces secretion of IL-1β, IL-6, TNF-α. Besides, it has analgesic properties, since it decreased the levels of pain mediators PGE2 and bradykinin (Varilla et al. 2021).
Debridement is the procedure of removing dead, infected, senescent, and/or devitalized tissues, because they interfere in the healing of a wound. This procedure transforms a chronic wound into an acute one and reduces the bacterial burden. The bromelain proteases degraded necrotic debris and regulated cell maturation and multiplication, collagen synthesis, and development and removal of perivascular fibrin. They also hydrolysed collagen, elastin, laminin, fibronectin and other damaged components of the ECM, releasing the growth and angiogenic factors sequestered in this matrix, activating bioactive chemokines and cytokines (Silva-López 2017). The extract promoted the effective repair of wounds. The topical formulations containing 35% bromelain also contains escarase, which efficiently removed eschars. Debridement accelerated recovery of blood perfusion, controlled the expression of TNF-α, which provides a chemotactic gradient for additional leukocytes to enhance the inflammatory process, stimulates cytokine secretion, and enhances fibroblast and smooth muscle cell chemotaxis, reprogramming the wound microenvironment (Wu et al. 2012; Anghel et al. 2016). Bromelain debridement is much better than the surgical procedure, because the surgical incision is painful, non-selective, and exposes the patients to the risk of repeated anaesthesia and bleeding. Bromelain debridement reduced the time to complete wound healing and reduces morbidity and mortality of severely burned and infection patients (Krieger et al. 2016).
Papain (EC 3.4.22.2) is a cysteine protease obtained from the latex of Carica papaya L., Caricaceae, a tropical fruit known as papaya. The papain activity is related to the fruit maturation: the greener the fruits, the greater the protease activity. It was the second protein to be crystallized (1968) and is also a model of the papain-like cysteine protease family and helps in understanding the action mechanism of cysteine proteases (Liu et al. 2018). This protease has high activity, important stability at high temperatures, and concentrations of organic solvents/denaturant agents at a wide pH range (3–9), beside it is easy to purify (dos Anjos et al. 2016). Papain is used in pharmaceutical preparations in the treatment of many disorders (Xue and Jackson 2015). It was employed with great success in tissue debridement and wound healing in ulcers, such as pressure, diabetic, leprosy and chemotherapeutic ulcers, in Fournier’s syndrome, and other injuries. Papain was able to produce proteolysis in necrotic tissues and cell fragments, helping to join wound edges and accelerating tissue regeneration (Craik et al. 2011). In addition, papain also reduced wound pH, stimulating the production of cytokines that promote cell repair, and decreasing the growth of microorganisms and inflammatory responses (Azevedo et al. 2017).
Medicinal Plant Formulations
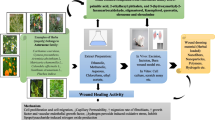
Simple herbal preparations can be used to heal many kinds of wounds, as the herbal extract itself, or can be administered to the skin as medicinal plant formulations, usually gels, emulsions, membrane films and ointments as stated in Fig. 1 (Anvisa 2011; Pereira and Bártolo 2016).
A gel is a semi-solid dosage form that contains a gelling agent to provide viscosity to a system in which colloidal-sized particles are uniformly distributed. Gels can be defined as a dosage form containing liquid phase constrained in a polymeric network held together by physical or chemical interactions, and capable of absorbing water or biological fluids to swell. Pharmaceutical gels contain medicinal, cosmetic or other agents, with sizes varying from nanometres to micrometres (Bolla et al. 2018). It can have a hydrophilic or hydrophobic character, depending on the base used in its composition. Hydrogels are three-dimensional networks formed by natural or synthetic polymers (e.g., collagen, chitosan, hyaluronic acid) which have high water content. The literature contains numerous studies of hydrogel dressing formulations aimed at wound healing, especially for chronic wounds, as they avoid microbial contamination but allow air and water vapor to reach the skin, not being occlusive (Thapa et al. 2019). However, the high-water content does not allow them to absorb a large number of exudates, and they are not suitable for treating very exuding skin wounds (Sarabahi 2012).
Polymeric films and membranes can be classified by their dense, porous or fibrous structure, often intended for topical application aimed at wound healing, which makes them useful as dressings (de Souza et al. 2020). This type of structure is widely used as a scaffold to support cell growth in tissue engineering as well; sometimes, in combination with engineered layers, it can improve mechanical properties and decrease the permeability of microbial contaminants. They have high load capacity, can be used for direct drug delivery to the wound site, and are usually permeable to gases and impermeable to liquids and bacteria. The films are flexible, which allows for easy accommodation to the patient's body, even in difficult areas such as joints, and endorse wound inspection without removing the dressing (Savencu et al. 2021). Nanofibers are highly valued in the field of wound healing because they can replicate the extracellular matrix structure in skin tissue, which cannot be fully achieved using traditional wound dressing materials (Juncos Bombin et al. 2020). Foam dressings are usually made of highly absorbent polyurethane, creating a moist environment and providing wound isolation. They are non-adherent, easy to apply and remove, and are intended for highly exuding wounds (Sarabahi 2012).
Ointments are semi-solid, spreadable and homogeneous bases that are used for external application on the skin or mucous membranes. From a pharmaceutical point of view, ointments are clearly distinguishable from creams and gels, as they consist of only one phase in which solid or liquid substances can be dispersed. Classic ointments are hydrophobic in nature, poorly absorbed, and traditionally used for local and topical application of medications, such as wound-healing agents (Osborne 1993). The mechanism for wound healing can be associated with the petroleum base used in the composition of the ointment, which can decrease epithelial proliferation (Uetsu et al. 2002). Due to their semi-occlusive character, ointments are not suitable for all biomedical applications, and some studies suggest that other topical therapies may have better benefits in tissue healing (Leise 2018; Rüther and Voss 2021). A cream is a semi-solid pharmaceutical form that consists of an emulsion, formed by a mixture of a lipophilic phase and a hydrophilic phase stabilized by the presence of a surfactant, and is normally used for external application on the skin or mucous membranes (Jaiswal et al. 2014). Like ointments, creams can present a degree of occlusion in wounds.
As reported before, some plants and their parts are popularly employed in the treatment of wounds. Here, examples of herbal formulations described in the scientific literature with potential to heal skin wounds are summarized.
Gels based on papain have antibacterial and anti-inflammatory activities and constitute a non-invasive technique for wound repair (Pinto et al. 2007). Topical formulations have been developed, such as hydrogels using carboxyvinyl polymer and oil-in-water (o/w) emulsions, containing papain for wound healing. The glycolic extract of Ananas comosus is usually administered as a gel, cream and lotion as its final dosage forms.
Aloe vera is traditionally one of the most used herbal medicines in topical formulations to heal skin lesions, being the most suitable for healing burn wounds (Maenthaisong et al. 2007). Aloe vera has been used in the preparation of pharmaceutical products, including hydroalcoholic gels prepared with its glycolic extract, 50% glycol extracts, simple A. vera ointments, tablets and capsules (Anvisa 2011).
Matricaria chamomilla is commercially available in the form of ointments containing the fluid extract of 10% M. chamomilla or tinctures (Martins et al. 2009; Yadav et al. 2021). A species of chamomile is marketed as a cream under the name Kamillosan® for healing skin lesions (Maver et al. 2015).
Avena sativa presents anti-inflammatory and healing activity, and its colloidal extracts are widely used in commercial formulations such as ointments, creams, gels, shampoos and soaps (Maver et al. 2015).
The main topical formulation with Ginkgo biloba L., Ginkgoaceae, consists of plant extracts applied directly to the injured tissue, but there is also scientific research with innovative formulations, such as carbomer gels, containing the herbal medicine carried in vesicles such as liposomes (Zhang et al. 2020).
Calendula officinalis L., Asteraceae, is usually administered as oils, infusions, tinctures, liquid extracts, creams or ointments (Maver et al. 2015; Gunasekaran et al. 2020). Camellia sinensis (L.) Kuntze, Theaceae, is commercially available as vegetable oil (Yadav et al. 2021).
In traditional Iranian medicine, people apply Citrus limon (L.) Osbeck, Rutaceae, leaves directly to the skin to treat skin wounds. Probably, the therapeutic properties of C. lemon are associated to its antioxidant compounds (Abbasi et al. 2021).
Curcuma longa L. is a phytotherapy species widely used in Asia. It is usually found in the form of talc and ointments and used in the treatment of chronic wounds, as it has antimicrobial, anti-inflammatory, antiviral and antifungal activities (Fazil and Nikhat 2020). There are formulations of pastes, ointments and powders containing the plant rhizome (Maver et al. 2015).
The scientific literature also reports the development of several new active release and targeting systems, aiming to improve some parameters related to plant-based pharmaceutical ingredients such as minimizing degradation and loss, preventing harmful side effects, improving the fraction of plant-based pharmaceutical ingredients accumulated in the necessary amount at the site of action, and increasing the bioavailability of the active. Among phytotherapy drug carriers, we can mention the development of soluble polymers, microparticles made of biodegradable and natural or synthetic polymers, microcapsules, lipoproteins, liposomes and micelles (Devi et al. 2010). Innovative formulations such as biocompatible membranes have been developed by numerous research groups reported in the literature. Skin wounds have been treated with great success with polymeric membranes of polylactic acid and babassu oil, developed through techniques such as electrospinning (Fernandes et al. 2021).
Due to its enormous therapeutic potential, recent research involving A. vera is focused on obtaining maximum benefits from this species (Maan et al. 2018). Various types of formulations, such as functional films, hydrogels and sponges, are being developed, mainly by combining the species with various biopolymers, such as cellulose, gel gum, alginate, chitosan or gelatine, in order to optimize its wound healing property (Maan et al. 2018). A gel formulation containing A. vera and insulin nanoemulsion developed for wound healing was tested in diabetic rats (Chakraborty et al. 2021).
Dutra et al. (2017) developed films for dressings containing papain, aiming at cellular healing. Ahlawat et al. (2019) reported the fabrication of new polyvinyl alcohol and gelatine nanofibers loaded with C. papaya by electrospinning technique for wound healing applications. Rad et al. (2019) investigated the incorporation of Calendula officinalis extract with poly (ε-caprolactone), zein and gum Arabic in nanofibers by electrospinning technique aiming at skin tissue regeneration. Banerjee et al. (2021) developed a topical formulation of neem oil (Azadirachta indica A.Juss., Meliaceae) with liquid crystals containing water deposits, aiming its application as an anti-inflammatory, healing and anti-methicillin agent resistant to Staphylococcus aureus. Ali et al. (2019) also studied the development of A. indica extract nanofibers using the electrospinning technique with polyvinyl alcohol and chitosan for antimicrobial activity. Debone et al. (2019) developed oily-resin films of chitosan and Copaifera langsdorffii Desf., Fabaceae, for application in skin dressings. Alsabeelah et al. (2021) prepared nanoemulsions using Achyrocline satureioides extract to increase skin protection. Maghimaa and Alharbi (2020) developed silver nanoparticles of C. longa for antimicrobial applications and wound healing activity. Studies involving M. chamomilla are also reported in the literature. Dogru et al. (2017) incorporated the plant extract into silver nanoparticles in order to increase its antimicrobial activity. Bayat et al. (2019) developed chitosan and bromelain fibres for application to skin burns aiming at their healing. Abbasi et al. (2021) developed an innovative formulation of a simple ointment and incorporated silver nanoparticles and the aqueous extract of C. limon for skin wound healing. El Hosary et al. (2019) developed a hydrogel based on polyvinyl alcohol and A. sativa powder, aiming at the healing of skin wounds. Table S2 presents some examples of medicinal plants and their developed formulations for wound healing.
Patents on Herbal Formulations
The principal tendency towards producing simpler and more economically viable pharmaceutical products can be confirmed by searching patents registered in different countries. Almost all of the inventions are owned by Asian countries, followed by Europe and America, which is not surprising, given the widespread use of plants in Asian medicine. Among the recent patents related to pharmaceutical formulations containing plant extracts and their active molecules for wound healing, a large number of documents describe the preparation of technologically simple and inexpensive formulations such as gels, creams and ointments. Most discoveries in this area associate more than one plant species in the same pharmaceutical preparation. Other patents describe the preparation of more economically viable nanosystems, which could be considered the future trend in wound repair, ensuring advantages to the medicine such as greater stability and efficacy. Furthermore, some nanofiber membranes have been patented. However, there is no guarantee that any of these patents have been licensed.
An example of patented nanofiber membrane is described by Feng et al. (2021), who developed a wound dressing from a plant extract, in the form of a fibre membrane that is formed directly on the surface of skin by using a portable or fixed electrostatic spinning device. The invention disclosed by Shin et al. (2019), describes a nanofiber for wound dressing, comprising a spirulina extract, alginate and polycaprolactone, which, according to the inventors, has a hydrophilic structure capable of retaining a large amount of water, has good skin adhesion, and can release the spirulina extract without cytotoxicity, thus being effective for the treatment of full-thickness skin wounds. Yarin et al. (2016) developed a biodegradable plant-based wound dressing composed of electrospun nanofibers formed of a blend of polymers. Table S3 describes other recent (2012 to 2022) intellectual products registered as patents in different countries.
Additional Therapies
Many molecules are employed to induce wound repair. However, some wounds, such as pressure ulcers, deep wounds, and cancerous and diabetic wounds, remain recalcitrant to all forms of treatments, or can only be slightly reduced, but remain chronic (Hassanshahi et al. 2019). In general, they are characterized by a prolonged inflammatory phase with elevated inflammatory markers, which results in high protease activity and subsequent degradation of growth factors and other positive wound healing factors; the overall consequence is impaired healing (Schultz and Cullen 2017). These wounds may be subjected to infections, which may stop or slow down wound healing, and may require the removal of the wounded limb or even cause the patient’s death (Hassanshahi et al. 2019). Therefore, approaches to wound management should target different aspects of each phase simultaneously. This strategy should involve protection from infection and free radicals, modulation of inflammation, support of cell proliferation, and acceleration of migration (Baron et al. 2020).
Conventional wound repair treatments, such as the application of different types of dressing (e.g., gauze, films, hydrogels, emulsions, ointments, foams, hydrofibres), tissue-engineered skin substitutes, negative-pressure wound therapy, autologous skin grafts, and hyperbaric oxygen therapy, have been effective for most wounds. They present distinctive characteristics, are more suitable to certain wounds, and have some limitations (Han and Ceilley 2017).
The purpose of a wound dressing is to protect the wound and to assist in the promotion of the healing process. Low-adherent dressings, semipermeable films, hydrocolloids and hydrogels are the most employed wound dressings, because they restrict liquid and microbial penetration while allowing air and water vapor through it (Weller 2009). All wound dressing types were discussed in section Medicinal Plant Formulations.
Hyaluronic acid is a non-sulphated glycosaminoglycan composed by repeated disaccharide β-1,4-d-glucuronic acid and β-1,3-N-acetylglucosamine units. It is one of the major components of animal ECM produced by fibroblasts, synoviocytes, chondrocytes, and keratinocytes. It is essential in the inflammation, granulation, and re-epithelialization wound repair phases, and its level is high in granulation tissue (Gupta et al. 2019). Many types of hyaluronic acid-based dressings have been employed over the last decade to treat various acute and chronic wounds, for example: Hyalosafe®, Hyalogran®, Hyalofill®, Hyalomatrix®, Hyiodine®, and Sorelex® (King and Sorooshian 2020). Although they present satisfactory results, recent research has investigated the association of hyaluronic acid with other biomaterials, such as collagen, polycaprolactone, poly(lactic-co-glycolic) acid, epigallocatechin-3-O-gallate, and growth factors such as FGF, EGF, VEGF and PDGF. These associations improve wound-healing outcomes, leading to significantly faster wound-healing rates due to fast vascularisation and wound closure; reduction of the risk of contractures, hypertrophy and keloids, optimise wound healing in deeper burn wounds (King and Sorooshian 2020). In addition to its wound healing and antimicrobial properties, the hyaluronic acid polymer yields good biocompatibility and minimal cytotoxicity within soft tissues, regardless of its concentration, when compared to other polymers, including carbomer and sodium alginate (King and Sorooshian 2020).
Tissue-engineered skin substitutes are types of wound dressing that are employed in cases of deep injuries and burns instead of surgical procedures with foreign tissues. They provide a barrier function for protection against micro-organisms, reduce pain, and promote wound healing by tissue regeneration. They can be classified in several ways, based on the duration of coverage, as permanent, semi-permanent or temporary; based on anatomical structure, they are epidermal, dermal, or dermo-epidermal (composite). They can also be cellular or acellular and, according to the type of biomaterial employed, they can be biological (autologous, allogeneic, xenogeneic) or synthetic (biodegradable, non-biodegradable). Despite their many advantages and applications, none of them can fully simulate native skin, due to the reduced vascularization, poor mechanical integrity, failure to integrate, scarring, and immune rejection of tissue-engineered skin substitutes (Vig et al. 2017).
Negative-pressure wound therapy refers to wound dressing systems that continuously or intermittently apply sub-atmospheric pressure, a differential suction (about 125 mm Hg), reducing the local pressure, creating macro- and microdeformations at the wound surface, that stretch cells and activate molecular pathways for angiogenesis and cell division. For wounds with oedema, these devices have the ability to remove a large amount of fluid. They have been used to treat pressure sores, open abdomen wounds, sternal wounds, traumatic wounds, diabetic foot infections, second-degree burns, and skin graft recipient sites. However, negative-pressure wound therapy is contraindicated in untreated fistulas, untreated osteomyelitis, or in a wound with malignant tumour (Huang et al. 2014).
Autologous skin grafts have been part of extensive wound and burn therapy for centuries. They provide wound coverage and closure by tissue replacement, pharmacological stimulus for healing, and promote the return of proper skin function. Their classification is based on the depth of the skin harvested. When the entire epidermis and dermis are obtained, with adnexal structures, the graft is called a full-thickness skin graft. It helps prevent skin contracture and thus can improve the cosmesis of the treated area. Alternatively, grafts that contain the entire epidermis but only parts of the dermis, also with adnexal structures, are called partial-thickness or split-thickness skin grafts. Although they are effective, grafts have a high cost, require an expert surgeon, use of anaesthesia and an operating room, and creating a wound at the donor site (Herskovitz et al. 2016).
Chronic wounds are characterized for being hypoxic, and tissue repair is disrupted. The low vascular supply, chronic inflammation, bacterial infection, and decreased local metabolic oxygen production contribute to chronic hypoxia. Acute, short-term hypoxia can stimulate angiogenesis; however, chronic hypoxia impedes angiogenesis, the release of VEGF and PDGF that stimulate endothelial cell division and migration to initiate angiogenesis, bacterial killing, and lymphocyte/leukocyte migration, in addition to fibroblast division and migration to synthesize new ECM, especially collagen. In chronic wounds, the production of ROS, O2· − , H2O2 and HO• are crucial for the upregulation of growth factors, cell signalling, and bacterial killing (Frykberg 2021). Therefore, hyperbaric oxygen therapy has been used as adjunctive to conventional wound care, especially in chronic wounds that do not respond to the usual clinical care, because it stimulates fibroblast proliferation and angiogenesis, enhance immune function, and accelerated wound closure, and reduces the incidence of infections (Han and Ceilley 2017). Moreover, the increase of pO2 oxygen induces to the production of ROS, performing on signalling pathways that modulate the synthesis of inflammation mediators, antioxidants and growth factors which can contribute to the healing process (Frykberg 2021).
Photo-biomodulation is an alternative technique that consists in placing light over wounds that are resistant to healing by other approaches, using light-emitting diodes (LEDs), lasers and other light sources, in the wavelength range from visual to infrared. Irradiation enhances healing and closure of these chronic wounds (e.g., pressure and diabetic ulcers) by enhancing cell migration, proliferation, angiogenesis and the rate of re-epithelialization, inducing skeletal muscle healing by reducing fibrosis, and enhancing the reorganization of myofibers, stimulation of collagen, cytokines and chemokines, growth factors production around the wound periphery, reducing oedema and the number of inflammatory cells, and exhibiting antibacterial actions. It can be employed on its own or conjugated with other devices (Kuffler 2016).
In order to circumvent the limitations of conventional approaches, the use of specific stem cells with skin-regenerative has emerged as a promising approach to accelerate the wound-healing process of acute and chronic wounds. Chronic wounds are caused by depletion of stem cells and a variety of other cellular and molecular mechanisms (Han and Ceilley 2017). Many stem cells have been investigated for their capacity to differentiate into fibroblasts, keratinocytes, epithelial and endothelial cells to repair chronic wounds. Among the most important are epidermal, dermal precursor of fibroblasts, melanocyte, mesenchymal-like, adipose-derived, bone marrow–derived mesenchymal, induced pluripotent, amniotic epithelial and mesenchymal cells (Hassanshahi et al. 2019). The activities of these stem cells are strictly regulated by many growth factors, such as EGF, FGF, PDGF, TGF, VEGF, and both stem cell and the specific growth factor have been used together (Nourian Dehkordi et al. 2019). However, these cells have some limitations, which may hamper their clinical applications for wound healing. These limitations may include ethical issues, risk of tumorigenicity of particular stem cells, and risk of cell rejection by the host immune system (Hassanshahi et al. 2019).
Some approaches, such as the application of platelet-rich plasma and electrical stimulation, may also improve the rate of complete healing of chronic wounds in animal models. However, none of these approaches yield presented satisfactory results on their own, and many wounds remain recalcitrant to all techniques (Kuffler 2016; Baron et al. 2020).
Perspectives and Future Directions
The wound healing area is undergoing significant advances, because the integration of traditional knowledge with modern scientific techniques. The investigation of medicinal plants and their active molecules has already shown promising results, especially in the development of affordable and effective formulations such as gels, creams, ointments, and film membranes. However, the future of wound care is probably to prospect the most highlight with the employment of nanosystems, which offer enhanced stability and efficacy. These advanced formulations have the potential to revolutionize the treatment of chronic and hard-to-heal wounds by providing more targeted delivery of active compounds, reducing side effects, and improving patient outcomes and adherence.
Additionally, trends towards producing economically viable pharmaceutical products, particularly in regions with a rich history of herbal medicine such as Asia, suggests a growing global interest in accessible wound care solutions, as can be seen from the profile of patents registered in this area. In the future, there may be a greater focus on personalized medicine, where treatments are tailored to individual patient needs, potentially combining conventional therapies with phytotherapeutic approaches. Moreover, the association of additional technologies, such as tissue engineering and bioengineered skin substitutes, with phytotherapeutic formulations could offer synergistic effects, improving the wound healing process. It is mandatory that future researches must explore the potential of these approaches conducting rigorous clinical trials to validate their efficacy.
Conclusions
Wounds and particularly hard-to-heal wounds are important public health concern, causing pain, bleeding, disability, amputation, and may lead the host death. A vast number of plants have been empirically employed in wound treatment since ancient times. In the present day, these medicinal plants and their active molecules, especially flavonoids and proteases, have been investigated by their curative potential. Patented and scientifically developed herbal formulations, especially gels, creams, ointments, film membranes and nanosystems, are vehicles for medicinal plants, their extracts and active molecules, promoting patient adherence to the topical treatment, comfort and facilitating application to the skin and wound healing. Additional therapies were necessary to deal with hard-to-heal wounds in conjunction with topical formulations. The development of advanced and innovative herbal medicines generates better quality of life for the population, because they have good cost benefit ratio and fewer adverse effects than conventional chemical drug therapy. So far, these phytotherapeutic formulations are promising in the current scenario based in the Green Chemistry principles.
In conclusion, the future of wound healing stands at an exciting crossroads, where traditional knowledge and modern science intersect. The traditional herbal medicines will continue to play a crucial role in wound care. The future lies in the development of more sophisticated and economically feasible nanosystems, as well as in combined therapies that can provide superior outcomes for patients with chronic and hard-to-heal wounds.
Data Availability
Data will be made available on request.
References
Abbasi N, Ghaneialvar H, Moradi R, Zangeneh MM, Zangeneh A (2021) Formulation and characterization of a novel cutaneous wound healing ointment by silver nanoparticles containing Citrus limon leaf: a chemobiological study. Arab J Chem 14:103246. https://doi.org/10.1016/j.arabjc.2021.103246
Ahlawat J, Kumar V, Gopinath P (2019) Carica papaya loaded poly (vinyl alcohol) -gelatin nanofibrous scaffold for potential application in wound dressing. Mater Sci Eng C 2019:109834. https://doi.org/10.1016/j.msec.2019.109834
Ahmed AS, Taher M, Mandal UK, Jaffri JM, Susanti D, Mahmood S, Zakaria ZA (2019) Pharmacological properties of Centella asiatica hydrogel in accelerating wound healing in rabbits. BMC Complement Altern Med 19:213. https://doi.org/10.1186/s12906-019-2625-2
Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R (2014) Curcumin as a wound healing agent. Life Sci 116:1–7. https://doi.org/10.1016/j.lfs.2014.08.016
Alemzadeh E, Oryan A (2018) Effectiveness of a Crocus sativus extract on burn wounds in rats. Planta Med 84:1191–1200. https://doi.org/10.1055/a-0631-3620
Alerico GC, Beckenkamp A, Vignoli-Silva M, Buffon A, Von Poser GL (2015) Proliferative effect of plants used for wound healing in Rio Grande do Sul state, Brazil. J Ethnopharmacol 176:305–310. https://doi.org/10.1016/j.jep.2015.11.001
Ali A, Shahid MA, Hossain MD, Islam MN (2019) Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int J Biol Macromol 138:13–20. https://doi.org/10.1016/j.ijbiomac.2019.07.01
Alsabeelah N, Arshad MF, Hashmi S, Khan RA, Khan S (2021) Nanocosmeceuticals for the management of ageing: rigors and vigors. J Drug Deliv Sci Technol 63:102448. https://doi.org/10.1016/j.jddst.2021.102448
Amorim E, Matias JEF, Coelho JCU, Campos ACL, Stahlke HJ Jr, Timi JRR, Ferreira LM (2006) Efeito do uso tópico do extrato aquoso de Orbignya phalerata (babaçu) na cicatrização de feridas cutâneas: estudo controlado em ratos. Acta Cirur Bras 21:67–76. https://doi.org/10.1590/s0102-86502006000800011
Anghel EL, De Fazio MV, Barker JC, Janis JE, Attinger CE (2016) Current concepts in debridement: science and strategies. Plast Reconstr Surg 138:82–93. https://doi.org/10.1097/PRS.0000000000002651
Anvisa (2011) Formulário de Fitoterápicos Farmacopeia Brasileira. Available from: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/formulario-fitoterapico/arquivos/8080json-file-1. Accessed 21 June 2023
Arun M, Satish S, Anima P (2016) Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna J Phytomed 6:295–304
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Disc 20:200–216. https://doi.org/10.1038/s41573-020-00114-z
Atiyah AG, Al-Falahi NHR (2021) The role of Helianthus tuberosus powder in healing of full-thickness wounds in mice. Vet World 14:1290–1298. https://doi.org/10.14202/vetworld.2021.1290-1298
Azevedo FF, Santanna LP, Bóbbo VC, Libert EA, Araújo EP, Saad MA, Lima MHM (2017) Evaluating the effect of 3% papain gel application in cutaneous wound healing in mice. Index Wounds 29:96–101
Balestrin LA, Kreutz T, Fachel FNS, Bidone J, Gelsleichter NE, Koester LS, Bassani VL, Braganhol E, Dora CL, Teixeira HF (2021) Achyrocline satureioides (Lam.) DC (Asteraceae) extract-loaded nanoemulsions as a promising topical wound healing delivery system: in vitro assessments in human keratinocytes (HACAT) and HET-CAM irritant potential. Pharmaceutics 13:1241. https://doi.org/10.3390/pharmaceutics13081241
Banerjee K, Chatterjee M, Sandur VR, Nachimuthu R, Madhyastha H, Thiagarajan P (2021) Azadirachta indica A.Juss (neem) oil topical formulation with liquid crystals ensconcing depot water for anti-inflammatory, wound healing and anti-methicillin resistant Staphylococcus aureus activities. J Drug Delivery Sci Technol 64:102563. https://doi.org/10.1016/j.jddst.2021.102563
Bardaa S, Makni K, Boudaouara O, Bardaa T, Ktari N, Hachicha S, Salah RB, Kallel R, Sahnoun Z, Boufi S (2021) Development and evaluation of the wound healing effect of a novel topical cream formula based on Ginkgo biloba extract on wounds in diabetic rats. Biomed Res Int 2021:6474706. https://doi.org/10.1155/2021/6474706
Baron JM, Glatz M, Proksch E (2020) Optimal support of wound healing: new insights. Dermatology 236:593–600. https://doi.org/10.1159/000505291
Bayat S, Amiri N, Pishavar E, Kalalinia F, Movaffagh J, Hahsemi M (2019) Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci 229:57–66. https://doi.org/10.1016/j.lfs.2019.05.028
Bhattacharya S (2012) Wound healing through the ages. Indian J Plast Surg 45:177–179. https://doi.org/10.4103/0970-0358.101255
Bi B (2016) Application of chloroform portion extract of Henry Woodbetony roots in preparation of medicine for promoting wound healing. Patent CN106109678 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20161116&CC=CN&NR=106109678A&KC=A#. Accessed 31 August 2023
Bolla PK, Rodriguez VA, Kalhapure RS, Kolli CS, Andrews S, Renukuntla JA (2018) Review on pH and temperature responsive gels and other less explored drug delivery systems. J Drug Deliver Sci Technol 46:416–435. https://doi.org/10.1016/j.jddst.2018.05.037
Bonab FS, Farahpour MR (2017) Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Pathol 26:885–892. https://doi.org/10.1007/s00580-017-2473-8
Calixto JB (2019) The role of natural products in modern drug discovery. Biol Sci 91:e20190105. https://doi.org/10.1590/0001-3765201920190105
Chakraborty T, Gupta S, Nair A, Chauhan S, Saini V (2021) Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J Drug Delivery Sci Technol 64:102601. https://doi.org/10.1016/j.jddst.2021.102601
Chang WL, Chang TC, Shi LS, Yang Y (2018) Uses of Osmanthus fragrans extracts for manufacturing a composition for wound healing. Patent TW201838644 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20181101&CC=TW&NR=201838644A&KC=A#. Accessed 15 October 2023
Chinnasamy G, Chandrasekharan S, Koh TW, Bhatnagar S (2021) Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Front Microbiol 12:611560. https://doi.org/10.3389/fmicb.2021.611560
Cho HW, An BK, Jung HK, Jang JH, Lee KH, Woo KW, Nho JH, Lee HJ, Kim AH, Bak H, Hwang TY, Yang BD, Seo JW, Kim SY (2020) Compositions for skin wound healing and regeneration comprising an extract Cirsium japonicum. Patent KR20200124570 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20201103&CC=KR&NR=20200124570A&KC=A#. Accessed 11 November 202
Cho JY, Kim SG, Park SH, Park KJ, Jang SG, Kim DS, Choi YI, Kim JH, Choi EJ, Lim HY, Lee CY, Hong YH, Kim HG (2020) A composition comprising extract of Prasiola japonica for wound healing. Patent KR20200081727 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20200708&CC=KR&NR=20200081727A&KC=A#. Accessed 11 November 202
Choi KY, Han GH, Lee CH, Seo SH (2018) A pharmaceutical composition for wound healing comprising extract of Euodia sutchuenensis Dode. Patent KR20180119941 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20181105&CC=KR&NR=20180119941A&KC=A#. Accessed 15 October 2023
Chokpaisarn J, Chusri S, Amnuaikit T, Udomuksorn W, Voravuthikunchai SP (2017) Potential wound healing activity of Quercus infectoria formulation in diabetic rats. PeerJ 5:e3608. https://doi.org/10.7717/peerj.3608
Cicalău GIP, Babes PA, Calniceanu H, Popa A, Ciavoi G, Iova GM, Ganea M, Scrobotă I (2021) Anti-inflammatory and antioxidant properties of carvacrol and magnolol, in periodontal disease and diabetes mellitus. Molecules 26:6899. https://doi.org/10.3390/molecules26226899
Colalto C (2017) What phytotherapy needs: Evidence-based guidelines for better clinical practice. Phytother Res 32:413–425. https://doi.org/10.1002/ptr.5977
Costa MF, Durço AO, Rabelo TK, Barreto RSS, Guimarães AG (2019) Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: a systematic review. J Pharmacy Pharmacol 71:141–155. https://doi.org/10.1111/jphp.13054
Cox-Georgian D, Ramadoss N, Dona C, Basu C (2019) Therapeutic and medicinal uses of terpenes. In: Joshee N, Dhekney S, Parajuli P (eds) Medicinal Plants, Springer, Cham. https://doi.org/10.1007/978-3-030-31269-5_15
Craik CS, Page MJ, Madison EL (2011) Proteases as therapeutics. Biochem 435:1–16. https://doi.org/10.1042/BJ20100965
Dagotturen H, Hoeberechts G (2021) Commiphora molmol (myrrh) resin extracts and uses thereof for wound healing and treatment and prevention of mucositis and other diseases. Patent US2021353700 (A1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20211118&CC=US&NR=2021353700A1&KC=A1#. Accessed 11 November 2023
Dantas AGB, Souza RL, Almeida AR, Xavier Júnior FH, Pitta MGR, Rêgo MJBM, Oliveira EE (2021) Development, characterization, and immunomodulatory evaluation of carvacrol-loaded nanoemulsion. Molecules 26:3899. https://doi.org/10.3390/molecules26133899
Davoodi-Roodbordeii F, Afshar M, Tabrizi FHA, Choopani S, Torkaman G, Moayer F, Salimi M (2019) Topical hydrogel containing Fumaria vaillantii Loisel. extract enhances wound healing in rats. BMC Compl Altern Med 19:254. https://doi.org/10.1186/s12906-019-2645-y
De Lencastre Novaes LC, Jozala AF, Lopes AM, Santos-Ebinuma VC, Mazzola PG, Pessoa Junior A (2016) Stability, purification, and applications of bromelain: a review. Biotechnol Prog 32:5–13. https://doi.org/10.1002/btpr.2190
De Souza RFB, Bombaldi de Souza FC, Bierhalz ACK, Pires ALR, Moraes ÂM (2020) Biopolymer-based films and membranes as wound dressings. Biopolymer Membr Films 2020:165–194. https://doi.org/10.1016/B978-0-12-818134-8.00007-9
Debone H, Santos PL, Severino P, Yoshida C, Souto EB, da Silva CF (2019) Chitosan/Copaiba oleoresin films for wound dressing application. Int J Pharmaceutics 555:146–152. https://doi.org/10.1016/j.ijpharm.2018.11.054
Devi VK, Jain N, Valli K (2010) Importance of novel drug delivery systems in herbal medicines. Pharmacognosy Rev 4:27. https://doi.org/10.4103/0973-7847.65322
Dias MC, Pinto DCGA, Freitas H, Santos C, Silva AMS (2020) The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochem 170:112199. https://doi.org/10.1016/j.phytochem.2019.112199
Dias MC, Pinto DCGA, Silva AMS (2021) Plant flavonoids: chemical characteristics and biological activity. Molecules 26:5377. https://doi.org/10.3390/molecules26175377
Dogan E, Yanmaz L, Gedikli S, Ersoz U, Okumus Z (2017) The effect of pycnogenol on wound healing in diabetic rats. Ost Wound Manag 63:41–47
Dogru E, Demirbas A, Altinsoy B, Duman F, Ocsoy I (2017) Formation of Matricaria chamomilla extract-incorporated Ag nanoparticles and size-dependent enhanced antimicrobial property. J Photochem Photobiol B: Biol 174:78–83. https://doi.org/10.1016/j.jphotobiol.2017.07.024
Dos Anjos MM, Da Silva AA, De Pascoli IC, Mikcha J, Machinski M, Peralta R, Abreu Filho BA (2016) Antibacterial activity of papain and bromelain on Alicyclobacillus spp. Int J Food Microbiol 216:121–126. https://doi.org/10.1016/j.ijfoodmicro.2015.10.007
Dutra JAP, Carvalho SG, Zampirolli ACD, Daltoé RD, Teixeira RM, Careta FP, Villanova JCO (2017) Papain wound dressings obtained from poly(vinyl alcohol)/calcium alginate blends as new pharmaceutical dosage form: preparation and preliminary evaluation. Eur J Pharm Biopharm 113:11–23. https://doi.org/10.1016/j.ejpb.2016.12.001
Dwivedi D, Dwivedi M, Malviya S, Singh V (2017) Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J Tradit Complement Med 7:79–85. https://doi.org/10.1016/j.jtcme.2015.12.002
El Hosary R, El-Mancy SMS, El Deeb KS, Eid HH, El Tantawy ME, Shams MM, Samir R, Assar NH, Sleem AA (2019) Efficient wound healing composite hydrogel using Egyptian Avena sativa L. polysaccharide containing β-glucan. Int J Biol Macromol 149:1331–1338. https://doi.org/10.1016/j.ijbiomac.2019.11.046
Elnahas RA, Elwakil BH, Elshewemi SS, Olama ZA (2021) Egyptian Olea europaea leaves bioactive extract: antibacterial and wound healing activity in normal and diabetic rats. J Tradit Complement Med 11:427–434. https://doi.org/10.1016/j.jtcme.2021.02.008
Falzon CC, Balabanova A (2017) Phytotherapy: an introduction to herbal medicine. Prim Care Clin Off Pract 44:217–227. https://doi.org/10.1016/j.pop.2017.02.001
Faraji A, Aghdaki M, Hessami K, Hosseinkhani A, Roozmeh S, Asadi N, Vafaei H, Kasraeian M, Bagheri R, Bazrafshan K, Foroughinia L (2021) Episiotomy wound healing by Commiphora myrrha (Nees) Engl. and Boswellia carteri Birdw. in primiparous women: a randomized controlled trial. J Ethnopharmacol 264:113396. https://doi.org/10.1016/j.jep.2020.113396
Fatima F, Aldawsari MF, Ahmed MM, Anwer K, Naz M, Ansari MJ, Hamad AM, Zafar A, Jafar M (2021) Green synthesized silver nanoparticles using Tridax procumbens for topical application: excision wound model and histopathological studies. Pharmaceutics 13:1754. https://doi.org/10.3390/pharmaceutics13111754
Fazil M, Nikhat S (2020) Topical medicines for wound healing: a systematic review of Unani literature with recent advances. J Ethnopharmacol 257:112878. https://doi.org/10.1016/j.jep.2020.112878
Feng B, Su D, Li H, Shi J (2021) Plant extract-containing wound dressing and application thereof. Patent CN112316196 (A). https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20210205&DB=EPODOC&locale=en_EP&CC=CN&NR=112316196A&KC=A&ND=4#. Accessed 11 November 2023
Fernandes DM, Barbosa WS, Rangel WSP, Valle IMMM, Matos APS, Melgaço FG, Monteiro MSSB (2021) Polymeric membrane based on polyactic acid and babassu oil for wound healing. Mater Today Comm 26:102173. https://doi.org/10.1016/j.mtcomm.2021.102173
Ferreira PF, Silva-López RE (2021) Plant therapeutic proteases: chemical aspects, applications and pharmaceutical formulations. Eur J Med Plant 32:46–63. https://doi.org/10.9734/EMJP/2021/v3211130429
Ferreira TS, Moreira CZ, Cária NZ, Victoriano G, Silva Junior WF, Magalhães JC (2014) Phytotherapy: an introduction to its history, use and application. Rev Bras Plant Med 16:290–298. https://doi.org/10.1590/S1516-05722014000200019
Forrest RD (1982) Early history of wound treatment. J R Soc Med 75:198–205
Frykberg RG (2021) Topical wound oxygen therapy in the treatment of chronic diabetic foot ulcers. Medicina 57:917. https://doi.org/10.3390/medicina57090917
Fu J, Huang J, Lin M, Xie T, You T (2020) Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J Surg Res 246:213–223. https://doi.org/10.1016/j.jss.2019.09.011
Gallagher AB, Hartmann RW, Engel M, Koch A, Van Koppen CJ (2018) A plant extract and compounds for use in wound healing. Patent US2018228858 (A1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180816&CC=US&NR=2018228858A1&KC=A1#. Accessed 15 October 2023
Gantwerker EA, Hom DB (2011) Skin: Histology and physiology of wound healing. Facial Plast Surg Clin N Am 19:441–453. https://doi.org/10.1016/j.fsc.2011.06.009
Gao QH, Fu X, Zhang R, Wang Z, Guo M (2018) Neuroprotective effects of plant polysaccharides: a review of the mechanisms. Int J Biol Macromol 106:749–754. https://doi.org/10.1016/j.ijbiomac.2017.08.075
Ghochikyan A, Pichugin A, Bagaev A, Davtyan A, Hovakimyan A, Tukhvatulin A, Davtyan H, Shcheblyakov D, Logunov D, Chulkina M, Savilova A, Trofimov D, Nelson EL, Agadjanyan MG, Ataullakhanov RI (2014) Targeting TLR-4 with a novel pharmaceutical grade plant derived agonist, Immunomax®, as a therapeutic strategy for metastatic breast cancer. J Trans Med 12:322
Givol O, Kornhaber R, Visentin D, Cleary M, Haik J, Harats M (2019) A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen 27:548–561. https://doi.org/10.1111/wrr.12737
Goh KC, Ashok G, Ramachandran, Hassan KB, John SSS, Murugaiah H, Karthikeyan M, Shivahirao RM (2021) Use of Adenia cordifolia extract for wound healing. Patent SG11202110005Y (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20211028&CC=SG&NR=11202110005YA&KC=A#. Accessed 11 November 2023
Golebiewska EM, Poole AW (2015) Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 29:153–162. https://doi.org/10.1016/j.blre.2014.10.003
Gong Y, Li H, Zhang M, Tian F, Tian Y (2021) Natural plant fresh leaf extract composition for promoting wound healing, preparation and preparation method of composition. Patent CN112336769 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210209&CC=CN&NR=112336769A&KC=A#. Accessed 11 November 2023
Gunasekaran S, Nayagam AAJ, Natarajan R (2020) Wound healing potentials of herbal ointment containing Calendula officinalis Linn. on the alteration of immunological markers and biochemical parameters in excision wounded animals. Clin Phytosci 6:45–56. https://doi.org/10.1186/s40816-020-00215-7
Gupta RC, Lall R, Srivastava A, Sinha A (2019) Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci 6:192. https://doi.org/10.3389/fvets.2019.00192
Hajhashemi M, Ghanbari Z, Movahedi M, Rafieian M, Keivani A, Haghollahi F (2018) The effect of Achillea millefolium and Hypericum perforatum ointments on episiotomy wound healing in primiparous women. J Matern Fetal Neonatal Med 31:63–69. https://doi.org/10.1080/14767058.2016.1275549
Hajiaghaalipour F, Kanthimathi MS, Abdulla MA, Sanusi J (2013) The effect of Camellia sinensis on wound healing potential in an animal model. Evid Based Compl Altern Med. https://doi.org/10.1155/2013/386734
Han D, Zhou Y (2021) Skin wound repairing composition containing plant extracts and preparation method thereof. Patent CN113144176 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210723&CC=CN&NR=113144176A&KC=A#. Accessed 11 November 2023
Han G, Ceilley R (2017) Chronic wound healing: a review of current management and treatments. Adv Ther 34:599–610. https://doi.org/10.1007/s12325-017-0478-y
Hardy K (2021) Paleomedicine and the evolutionary context of medicinal plant use. Rev Bras Farmacogn 31:1–15. https://doi.org/10.1007/s43450-020-00107-4
Hashemi SA, Madani SA, Abediankenari S (2015) The review on properties of Aloe vera in healing of cutaneous wounds. BioMed Res Int 2015:714216. https://doi.org/10.1155/2015/714216
Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini-Khah Z, Peymanfar Y, Ghalamkari S, Su Y, Xian CJ (2019) Adipose-derived stem cells for wound healing. J Cell Physiol 234:7903–7914. https://doi.org/10.1002/jcp.27922
Hemmati AA, Foroozan M, Houshmand G, Moosavi ZB, Bahadoram M, Maram NS (2015) The topical effect of grape seed extract 2% cream on surgery wound healing. Global J Health Sci 7:52–58. https://doi.org/10.5539/gjhs.v7n3p52
Herskovitz I, Hughes OB, Macquhae F, Rakosi A, Kirsner R (2016) Epidermal skin grafting. Int Wound J Suppl 3:52–56. https://doi.org/10.1111/iwj.12631
Hofer S, Geisler S, Lisandrelli R, Ngoc HN, Ganzera M, Schennach H, Fuchs D, Fuchs JE, Gostner JM, Kurz K (2020) Pharmacological targets of kaempferol within inflammatory pathways - a hint towards the central role of tryptophan metabolism. Antioxidants 9:180. https://doi.org/10.3390/antiox9020180
Hrytsyk LM, Neiko OV, Hrytsyk AR (2016) Ointment with yarrow extract which shows wound-healing properties. Patent UA104563 (U). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=1&ND=3&adjacent=true&locale=en_EP&FT=D&date=20160210&CC=UA&NR=104563U&KC=U#. Accessed 31 August 2023
Hsiao CY, Hung CY, Tsai TH, Chak KF (2012) A study of the wound healing mechanism of a traditional Chinese medicine, Angelica sinensis, using a proteomic approach. Evid Based Complement Alternat Med 2012:467531. https://doi.org/10.1155/2012/467531
Hu C, Gan Z (2018) Bromelain composition for skin wound decrustation and preparation method thereof. Patent CN108066754 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180525&CC=CN&NR=108066754A&KC=A#. Accessed 15 October 2023
Hu N, Xiong L, Li L, Li Y (2019) Sandalwood extract for promoting wound healing. Patent CN109925327 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190625&CC=CN&NR=109925327A&KC=A#. Accessed 15 October 2023
Huang C, Leavitt T, Bayer LR, Orgill D (2014) Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 51:301–331. https://doi.org/10.1067/j.cpsurg.2014.04.001
Huang Y, Chen L (2018) Self-heating film containing Radix salviae miltiorrhizae extract for promoting burn wound repair. Patent CN109010802 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20181218&CC=CN&NR=109010802A&KC=A#. Accessed 15 October 2023
Huesca-Urióstegui K, García-Valderrama EJ, Gutierrez-Uribe JA, Antunes-Ricardo M, Guajardo-Flores D (2022) Nanofiber systems as herbal bioactive compounds carriers: current applications in healthcare. Pharmaceutics 14:191. https://doi.org/10.3390/pharmaceutics14010191
Imre B, García L, Puglia D, Vilaplana F (2019) Reactive compatibilization of plant polysaccharides and biobased polymers: review on current strategies, expectations and reality. Carbohydr Polym 209:20–37. https://doi.org/10.1016/j.carbpol.2018.12.082
Jaiswal M, Dudhe R, Sharma PK (2014) Nanoemulsion: an advanced mode of drug delivery system. Biotech 5:123–127. https://doi.org/10.1007/s13205-014-0214-0
Jang HJ, Jung WY, Kim NK (2021) A composition for burn or wound treatment or prevention comprising the extract of Cynanchum atratum and phytosterol. Patent KR102285995 (B1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210805&CC=KR&NR=102285995B1&KC=B1#. Accessed 11 November 2023
Jang MJ, Baek SH, Pyun HO, Park GE (2021) Composition for anti-inflammation skin moisturizing skin wound healing or skin regeneration comprising extracts of black waxy rice with giant embryo. Patent KR20210066660 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210607&CC=KR&NR=20210066660A&KC=A#. Accessed 11 November 2023
Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J (2017) The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev 6:15. https://doi.org/10.1186/s13643-016-0400-8
Joe IH, Kim CT, Park EY, Shin MG (2021) Composition for skin regeneration and wound healing comprising the extract of Actinidia chinensis stem. Patent KR102248170 (B1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210507&CC=KR&NR=102248170B1&KC=B1#. Accessed 11 November 2023
Juncos Bombin AD, Dunne N, McCarthy HO (2020) Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C 114:110994. https://doi.org/10.1016/j.msec.2020.110994
Kang MH, Kim BH (2018) Oral wound healing effects of açai berry water extracts in rat oral mucosa. Toxicol Res 34:97–102. https://doi.org/10.5487/TR.2018.34.2.097
Kant V, Jangir BL, Kumar V, Nigam A, Sharma V (2020) Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 38:105–119. https://doi.org/10.1080/08977194.2020.1822830
Kant V, Jangir BL, Sharma M, Kumar V, Joshi VG (2021) Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol Immunotoxicol 43:536–553. https://doi.org/10.1080/08923973.2021.1950758
Khare CP (2007) Indian medicinal plants-an illustrated dictionary. Springer Science+Business Media, New York
Khogta S, Patel J, Barve K, Londhe V (2020) Herbal nano-formulations for topical delivery. J Herb Med 20:100300. https://doi.org/10.1016/j.hermed.2019.100300
Kim SK (2020) Cosmetic composition for skin wound healing and accelerating skin cell regeneration containing silk fibroin and mulberry leaf extracts. Patent KR102193102 (B1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20201218&CC=KR&NR=102193102B1&KC=B1#. Accessed 11 November 2023
Kim BH, Kang MH (2017) Composition for wound-healing comprising acai berry extract. Patent KR20170014052 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20170208&CC=KR&NR=20170014052A&KC=A#. Accessed 31 August 2023
Kim BH, Hwang SJ, Jin MH, Roh HS (2018) Cosmetic composition for wound healing or skin regeneration comprising extract of Sophora japonica Linne. Patent KR20180084480 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180725&CC=KR&NR=20180084480A&KC=A#. Accessed 15 October 2023
Kim BH, Jin MH, Hwang SJ (2018) Cosmetic composition for wound healing or skin regeneration comprising extract of Selaginella tamariscina. Patent KR20180084482 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180725&CC=KR&NR=20180084482A&KC=A#. Accessed 15 October 2023
Kim CS, Kim JS, Jo GH, Lee IS, Hyun SW (2019) Composition for wound healing or skin regeneration comprising Aster koraiensis extract or fraction thereof. Patent KR20190132076 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20191127&CC=KR&NR=20190132076A&KC=A#. Accessed 15 October 2023
Kim JK, Park SU (2020) Recent studies on kaempferol and its biological and pharmacological activities. EXCLI J 19:627–634. https://doi.org/10.17179/excli2020-2162
King ICC, Sorooshian P (2020) Hyaluronan in skin wound healing: therapeutic applications. J Wound Care 29:782–787. https://doi.org/10.12968/jowc.2020.29.12.782
Kishan KG, Shekshavali T, Kuppast IJ, Kumar MAK, Patil J (2016) A review on Hydnocarpus wightiana. Res J Pharmacol Pharmacodyn 8:168–170. https://doi.org/10.5958/2321-5836.2016.00030.6
Krieger Y, Shoham Y, Bogdanov-Berezovsky A, Silberstein E, Sagi A, Levy A, Rosenberg N, Rubin G, Egozi D, Ullman Y, Haik J, Rosenberg L (2016) Review of 30 years of research and development of an enzymatic debridement agent for burns. Harefuah 155:281–285
Kuffler DP (2016) Photobiomodulation in promoting wound healing: a review. Regen Med 11:107–122. https://doi.org/10.2217/rme.15.82
Kumar S, Tan Y, Berthiaume F (2021) Neuropeptide substance P enhances skin wound healing in vitro and in vivo under hypoxia. Biomedicines 9:222. https://doi.org/10.3390/biomedicines9020222
Labib RM, Ayoub IM, Michel HE, Mehanny M, Kamil V, Hany M, Magdy M, Moataz A, Maged B, Mohamed A (2019) Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS One 14:e0219561. https://doi.org/10.1371/journal.pone.0219561
Lee J (2019) Composition containing sericin, Torilis japonica extract, and Viscum album extract for skin generation, skin soothing, or wound healing. Patent CN110248641 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190917&CC=CN&NR=110248641A&KC=A#. Accessed 15 October 2023
Leise BS (2018) Topical wound medications. Vet Clin North Am: Equine Pract 34:485–498. https://doi.org/10.1016/j.cveq.2018.07.006
Li X, Qiu Z (2016) Natural plant essential oil wound healing ointment and preparation method thereof. Patent CN105232993 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20160113&CC=CN&NR=105232993A&KC=A#. Accessed 31 August 2023
Lim JY, Lim JC (2019) Glucan Composition for accelerating wound healing comprising an Aquilaria crassna extract and β-Glucan. Patent KR101966374 (B1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190405&CC=KR&NR=101966374B1&KC=B1#. Accessed 15 October 2023
Lim YS, Kim YM, Park JE (2018) Composition comprising extract of Quamoclit angulata for wound healing. Patent KR20180025789 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180309&CC=KR&NR=20180025789A&KC=A#. Accessed 15 October 2023
Liu J, Sharma A, Niewiara MJ, Singh R, Ming R, Yu Q (2018) Papain-like cysteine proteases in Carica papaya: lineage-specific gene duplication and expansion. BMC Genom 19:26. https://doi.org/10.1186/s12864-017-4394-y
Liu C, Cui Y, Pi F, Cheng Y, Guo Y, Qian H (2019) Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from Aloe vera: a review. Molecules 24:1554. https://doi.org/10.3390/molecules24081554
Lopez-Jornet P, Camacho-Alonso F, Gómez-Garcia F, Miñano FM, Cañas X, Serafín A, Castillo J, Vicente-Ortega V (2014) Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int Wound J 11:489–495. https://doi.org/10.1111/j.1742-481X.2012.01114.x
Lordani TVA, Brenzan MA, Cortez LER, Lordani CRF, Honda PA, Lonardoni MVC, Cortez DAG (2014) Effect of a topical formulation containing Calophyllum brasiliense Camb. extract on cutaneous wound healing in rats. Nat Prod Res 29:953–957. https://doi.org/10.1080/14786419.2014.956742
Lordani TVA, Lara CE, Ferreira FBP, Monich MST, Silva CM, Lordani CRF, Bueno FG, Teixeira JJVV, Lonardoni MVC (2018) Therapeutic effects of medicinal plants on cutaneous wound healing in humans: a systematic review. Mediators Inflam. https://doi.org/10.1155/2018/7354250
Luna SLR, Ramírez-Garza RE, Saldívar SOS (2020) Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci World J 2020:1–38. https://doi.org/10.1155/2020/6792069
Luo Q (2021) Ilex pubescens extract gel for promoting skin ulcer wound repair and preparation method. Patent CN112315988 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210205&CC=CN&NR=112315988A&KC=A#. Accessed 11 November 2023
Lv R, Chen Z (2017) Thymol inhibits cell migration and invasion by downregulating the activation of PI3K/AKT and ERK pathways in human colon cancer cells. Trop J Pharmaceutic Res 16:2895–2901. https://doi.org/10.4314/tjpr.v16i12.13
Maan AA, Nazir A, Khan MKI, Ahmad T, Zia R, Murid M, Abrar M (2018) The therapeutic properties and applications of Aloe vera: a review. J Herbal Med 12:1–10. https://doi.org/10.1016/j.hermed.2018.01.002
Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C (2007) The efficacy of Aloe vera used for burn wound healing: a systematic review. Burns 33:713–718. https://doi.org/10.1016/j.burns.2006.10.384
Maghimaa M, Alharbi SA (2020) Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabric for antimicrobial applications and wound healing activity. J Photochem Photobiol B Biol 204:111806. https://doi.org/10.1016/j.jphotobiol.2020.111806
Maliughina EA, Mazulin OV, Smoylovska GP, Byelenichev IF (2015) A method of producing a lipophilic extract from plant material that has the wound-healing and anti-inflammatory activity. Patent UA103390 (U). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20151210&CC=UA&NR=103390U&KC=U#. Accessed 20 July 2023
Martin P, Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 173:370–378. https://doi.org/10.1111/bjd.13954
Martins MD, Marques MM, Bussadori SK, Martins MA, Pavesi VC, Mesquita-Ferrari RA, Fernandes KP (2009) Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother Res 23:274–278. https://doi.org/10.1002/ptr.2612
Matagne A, Bolle L, El Mahyaoui R, Baeyens-Volant D, Azarkan M (2017) The proteolytic system of pineapple stems revisited: purification and characterization of multiple catalytically active forms. Phytochemistry 138:29–51. https://doi.org/10.1016/j.phytochem.2017.02.019
Maver T, Maver U, Kleinschek S, Dragica MS, Samo K (2015) A review of herbal medicines in wound healing. Int J Dermatol 54:740–751. https://doi.org/10.1111/ijd.12766
Maver T, Kurečič M, Smrke DM, Kleinschek KS, Maver U (2018) Plant-derived medicines with potential use in wound treatment. Herb Med IntechOpen. https://doi.org/10.5772/intechopen.72813
Moeini A, Pedram P, Makvandi P, Malinconico M, d’Ayala GG (2020) Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym 233:115839. https://doi.org/10.1016/j.carbpol.2020.115839
Morton LM, Phillips TJ (2016) Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol 74:589–605. https://doi.org/10.1016/j.jaad.2015.08.068
Nho CW, Kim MS, Lee HJ, Moon KC, Oh SR, Dulamjav B (2018) Composition for wound treatment containing extract of Stellera chamaejasme or fraction thereof and method for treating wound of subject. Patent JP2018115202 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180726&CC=JP&NR=2018115202A&KC=A#. Accessed 15 October 2023
Niknam S, Tofighi Z, Faramarzi MA, Abdollahifar MA, Sajadi E, Dinarvand R, Toliyat T (2021) Polyherbal combination for wound healing: Matricaria chamomilla L. and Punica granatum L. DARU J Pharm Sci 29:133–145. https://doi.org/10.1007/s40199-021-00392-x
Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S (2019) Skin tissue engineering: wound healing based on stem-cell based therapeutic strategies. Stem Cell Res Ther 10:111. https://doi.org/10.1186/s13287-019-1212-2
Osborne DW (1993) Phase behavior characterization of ointments containing lanolin or a lanolin substitute. Drug Dev Ind Pharm 19:1283–1302
Özay Y, Güzel S, Yumrutaş O, Pehlivanoğlu B, Erdoğdu IH, Yildirim Z, Türk BA, Darcan S (2019) Wound healing effect of kaempferol in diabetic and nondiabetic rats. J Surg Res 233:284–296. https://doi.org/10.1016/j.jss.2018.08.009
Ozgen S, Kilinc OK, Selamoğlu Z (2016) Antioxidant activity of quercetin: A mechanistic review. Turk J Agricult Food Sci Technol 4:1134–1138. https://doi.org/10.24925/turjaf.v4i12.1134-1138.1069
Park ON (2021) A topical composition comprising an extract of combined herbs comprising Longanae arillus for the skin regeneration and the treatment or alleviation of skin wound and the use thereof. Patent WO2021182873 (A1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210916&CC=WO&NR=2021182873A1&KC=A1#. Accessed 11 November 2023
Park MH, Kim JH, Jeong JH, Lee JH (2019) Composition for treating skin wound comprising extract of Phragmitis rhizoma and grape seed oil. Patent KR20190021942 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190306&CC=KR&NR=20190021942A&KC=A#. Accessed 15 October 20
Park JS, Park YI, Cha YE, Jang MS (2021) Composition for wound-healing comprising Hibiscus abelmoschus L. Extract. Patent KR20210033422 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210326&CC=KR&NR=20210033422A&KC=A#. Accessed 11 November 2023
Peng C-L, Lee J-S (2012) Wound dressing containing citrus extract and fabrication method thereof. Patent TW201208717 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20120301&CC=TW&NR=201208717A&KC=A#. Accessed 21 June 202
Pereira RF, Bártolo PJ (2016) Traditional therapies for skin wound healing. Adv Wound Care 5:208–229. https://doi.org/10.1089/wound.2013.0506
Pérez-Recalde M, Arias IER, Hermidaab EB (2018) Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 38:57–65. https://doi.org/10.1016/j.phymed.2017.09.024
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6:1–5. https://doi.org/10.4103/0973-7847.95849
Pinto C, Green D, Baby R, Ruas G, Kaneko T, Marana S, Velasco M (2007) Determination of papain activity in topical dosage forms: single laboratory validation assay. Lat Am J Pharm 26:771–775
Poljšak N, Kreft S, Glavač NK (2019) Vegetable butters and oils in skin wound healing: scientific evidence for new opportunities in dermatology. Phytother Res 34:254–269. https://doi.org/10.1002/ptr.6524
Ponnusamy Y, Chear NJY, Ramanathan S, Lai CS (2015) Polyphenols rich fraction of Dicranopteris linearis promotes fibroblast cell migration and proliferation in vitro. J Ethnopharmacol 168:305–314. https://doi.org/10.1016/j.jep.2015.03.062
Prakoso YA, Rini CS, Wirjaatmadja R (2018) Efficacy of Aloe vera, Ananas comosus, and Sansevieria masoniana cream on the skin wound infected with MRSA. Adv Phar Sc 2018:4670569. https://doi.org/10.1155/2018/4670569
Rad ZP, Mokhtari J, Abbasi M (2019) Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int J Biol Macromol 135:530–543. https://doi.org/10.1016/j.ijbiomac.2019.05.20
Rathnavelu V, Alitheen NB, Sohila S, Kanagesan S, Ramesh R (2016) Potential role of bromelain in clinical and therapeutic applications. Biom Rep 5:283–288. https://doi.org/10.3892/br.2016.720
Reinke JM, Sorg H (2012) Wound Repair and Regeneration. Eur Surg Res 49:35–43. https://doi.org/10.1159/000339613
Rezaie F, Momeni-Moghaddam M, Naderi-Meshkin H (2019) Regeneration and repair of skin wounds: various strategies for treatment. Int J Lower Extrem Wounds 18:247–261. https://doi.org/10.1177/1534734619859214
Ribeiro Neto JA, Tarôco BRP, Santos HB, Thomé RG, Wolfram E, Ribeiro RIMA (2020) Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J Ethnopharmacol 260:112547. https://doi.org/10.1016/j.jep.2020.112547
Rousselle P, Braye F, Dayan G (2019) Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev 146:344–365. https://doi.org/10.1016/j.addr.2018.06.019
Rüther L, Voss W (2021) Hydrogel or ointment? Comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon 7:e06071. https://doi.org/10.1016/j.heliyon.2021.e06071
Salas-Oropeza J, Jimenez-Estrada M, Perez-Torres A, Castell-Rodriguez AE, Becerril-Millan R, Rodriguez-Monroy MA, Canales-Martinez MM (2020) Wound healing activity of the essential oil of Bursera morelensis, in mice. Molecules 25:1795. https://doi.org/10.3390/molecules25081795
Salas-Oropeza J, Jimenez-Estrada M, Perez-Torres A, Castell-Rodriguez AE, Becerril-Millan R, Rodriguez-Monroy MA, Jarquin-Yañez K, Canales-Martinez MM (2021) Wound healing activity of α-pinene and α-phellandrene. Molecules 26:2488. https://doi.org/10.3390/molecules26092488
Salehi B, Venditti A, Sharifi-Rad M, Kregiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, Antolak H, Azzini E, Setzer WN, Martins N (2019) The therapeutic potential of apigenin. Int J Mol Sci 20:1305. https://doi.org/10.3390/ijms20061305
Salehi B, Machin L, Monzote L, Sharifi-Rad J, Ezzat SM, Salem MA, Merghany RM, El Mahdy NM, Kılıç CS, Sytar O, Sharifi-Rad M, Sharopov F, Martins N, Martorell M, Cho WC (2020) Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega 5:11849–11872. https://doi.org/10.1021/acsomega.0c01818
Samuelson R, Lobl M, Higgins S, Clarey D, Wysong A (2020) The effects of lavender essential oil on wound healing: A review of the current evidence. J Altern Compl Med 26:680–690. https://doi.org/10.1089/acm.2019.0286
Sangiovanni E, Fumagalli M, Pacchetti B, Piazza S, Magnavacca A, Khalilpour S, Melzi G, Martinelli G, Dell’Agli M (2019) Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res 33:2083–2093. https://doi.org/10.1002/ptr.6400
Sarabahi S (2012) Recent advances in topical wound care. Indian J Plast Surg 45:379. https://doi.org/10.4103/0970-0358.101321
Savencu I, Iurian S, Porfire A, Bogdan C, Tomuță I (2021) Review of advances in polymeric wound dressing films. React Funct Polym 168:105059. https://doi.org/10.1016/j.reactfunctpolym.2021.105059
Scheibe CL, Ribas-Filho JM, Czeczko NG, Malafaia O, Barboza LED, Ribas FM, Wendler E, Torres O, Lovato FC, Scapini JGS (2016) Schinus terebinthifolius raddi (aroeira) and Orbignya phalerata Mart. (babassu) effect in cecorrahphy healing in rats. Acta Cir Bras 31:402–410. https://doi.org/10.1590/S0102-865020160060000007
Schultz G, Cullen B (2017) Proteases Made Easy. Wounds International. Available at: https://woundsinternational.com/wp-content/uploads/sites/8/2023/02/01dc1613f536cd66dc09557085a48267.pdf. Accessed 06 January 2023
Shakya AK (2016) Medicinal plants: future sources of new drugs. Int J Herb Med 4:59–64
Shankar E, Goel A, Gupta K, Gupta S (2017) Plant flavone apigenin: an emerging anticancer agent. Curr Pharmacol Rep 3:423–446. https://doi.org/10.1007/s40495-017-0113-2
Sharifi-Rad M, Mnayer D, Morais-Braga MFB, Carneiro JNP, Bezerra CF, Coutinho HDM, Salehi B, Contreras MM, Soltani-Nejad A, Uribe YAH, Yousaf Z, Iriti M, Sharifi-Rad J (2018) Echinacea plants as antioxidant and antibacterial agents: from traditional medicine to biotechnological applications. Phytother Res 32:1653–1663. https://doi.org/10.1002/ptr.6101
Shin HS, Choi JI, Ki MS (2019) PCL Spirulina extract-impregnated alginate-PCL nanofiber wound dressing for skin regeneration. Patent KR20190051330 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=37&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190515&CC=KR&NR=20190051330A&KC=A#. Accessed 15 October 2023
Sierra-García GD, Castro-Ríos R, González-Horta A, Lara-Arias J, Chávez-Montes A (2014) Acemannan, an extracted polysaccharide from Aloe vera: a literature review. Nat Prod Commun 9:1217–1221
Silva-López RE (2017) Debridement applications of bromelain: a complex of cysteine proteases from pineapple. Adv Biotechnol Microbiol 3:124–126. https://doi.org/10.19080/AIBM.2017.03.555624
Silva-López RE, Gonçalves RN (2019) Therapeutic proteases from plants: biopharmaceuticals with multiple applications. J Appl Biotechnol Bioeng 6:101–109. https://doi.org/10.15406/jabb.2019.06.00180
Sirinthipaporn A, Jiraungkoorskul W (2017) Wound healing property review of siam weed, Chromolaena odorata. Pharmacogn Rev 11:35–38. https://doi.org/10.4103/phrev.phrev_53_16
Soares RDF, Campos MGN, Ribeiro GP, Salles BCC, Cardoso NS, Ribeiro JR, Souza RM, Leme KC, Soares CB, Oliveira CM, Elston LB, Fonseca CCP, Ferreira EB, Rodrigues MR, Duarte SMS, Paula FBA (2020) Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J Biomed Mater Res A 108:654–662. https://doi.org/10.1002/jbm.a.36845
Sowa I, Paduch R, Strzemski M, Zielińska S, Rydzik-Strzemska E, Sawicki J, Kocjan R, Polkowski J, Matkowski A, Latalski M, Wójciak-Kosior M (2018) Proliferative and antioxidant activity of Symphytum officinale root extract. Nat Prod Res 32:605–609. https://doi.org/10.1080/14786419.2017.1326492
Stolzenburg-Veeser L, Golubnitschaja O (2018) Mini-encyclopaedia of the wound healing - opportunities for integrating multi-omic approaches into medical practice. J Proteomics 188:71–84. https://doi.org/10.1016/j.jprot.2017.07.017
Swiech K, Picanço-Castro V, Covas DT (2017) Production of recombinant coagulation factors: are humans the best host cells. Bioengineered 8:462–470. https://doi.org/10.1080/21655979.2017.1279767
Tap FM, Majid FAA, Khairudin NBA (2016) Structure prediction of stem bromelain from pineapples (Ananas comosus) using procaricain enzyme as a modelling template. Int J Ap Engineer Res 11:6109–6111
Tariq RA, Vashisht R, Sinha A, Scherbak Y (2022) Medication dispensing errors and prevention. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK519065/ Accessed 20 July 2023
Thapa RK, Diep DB, Tønnesen HH (2019) Topical antimicrobial peptide formulations for wound healing: current developments and future prospects. Acta Biomater 103:52–67. https://doi.org/10.1016/j.actbio.2019.12.025
Thomas DC, Tsu CL, Nain RA, Arsat N, Fun SS, Lah NASN (2021) The role of debridement in wound bed preparation in chronic wound: a narrative review. Ann Med Surg 71:102876. https://doi.org/10.1016/j.amsu.2021.102876
Tomulewicz M (2019) Herbal preparation comprising an extract of Melittis melissophyllum for use in wound healing. Patent HRP20182012 (T1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190125&CC=HR&NR=P20182012T1&KC=T1. Accessed 15 October 2023
Tuhin RH, Begum MM, Rahman MS, Karim R, Begum T, Ahmed SU, Mostofa R, Hossain A, Abdel-Daim M, Begum R (2017) Wound healing effect of Euphorbia hirta Linn. (Euphorbiaceae) in alloxan induced diabetic rats. BMC Compl Altern Med 17:423. https://doi.org/10.1186/s12906-017-1930-x
Uetsu N, Okamoto H, Fujii K, Doi R, Horio T (2002) Treatment of chronic actinic dermatitis with tacrolimus ointment. J Am Acad Dermatol 47:881–884. https://doi.org/10.1067/mjd.2002.124703
Upadhyay NK, Kumar R, Siddiqui MS, Gupta A (2011) Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Ev Bas Compl Altern Med 2011(1):659705. https://doi.org/10.1093/ecam/nep189
Üstündağ Okur N, Okur ME, Karadağ AE, Ayla S, Demirci F, Sipahi H (2021) Gel formulations obtained from phlomis plant extract and their use in wound treatment. Patent WO2021242204 (A1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20211202&CC=WO&NR=2021242204A1&KC=A1. Accessed 11 November 2023
Vale DL, Martinez RM, Medeiros DC, Rocha C, Sfeir N, Lopez RFV, Vicentini FTMC, Verri WA, Georgetti SR, Baracat MM, Casagrande R (2021) A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: enhancement of activity by microencapsulation. J Drug Target 29:983–997. https://doi.org/10.1080/1061186X.2021.1898621
Varilla C, Marcone M, Paiva L, Baptista J (2021) Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 10:2249. https://doi.org/10.3390/foods10102249
Veerasubramanian PK, Thangavel P, Kannan R, Chakraborty S, Ramachandran B, Suguna L, Muthuvijayan V (2018) An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf B Biointerfaces 165:92–102. https://doi.org/10.1016/j.colsurfb.2018.02.022
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR (2017) Advances in skin regeneration using tissue engineering. Int J Mol Sci 18:789. https://doi.org/10.3390/ijms18040789
Walton EW (2014) Topical phytochemicals: applications for wound healing. Adv Skin Wound Care 27:382–332. https://doi.org/10.1097/01.ASW.0000450101.97743.0f
Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ (2018) Wound Healing. J Chin Med Assoc 81:94–101. https://doi.org/10.1016/j.jcma.2017.11.002
Weller C (2009) Interactive dressings and their role in moist wound management. Adv Tex Wound Care 2009:97–113. https://doi.org/10.1533/9781845696306.1.97
Wen T-C (2020) Use of leaf extract of Cinnamomum osmophloeum Kanehira in manufacture of wound healing composition. Patent TW202000224 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20200101&CC=TW&NR=202000224A&KC=A. Accessed 11 November 2023
Westby MJ, Norman G, Watson REB, Cullum NA, Dumville JC (2020) Protease activity as a prognostic factor for wound healing in complex wounds. Wound Repair Regen 28:631–644. https://doi.org/10.1111/wrr.12835
WHO (2019) WHO Global Report on Traditional and Complementary Medicine. Available at: https://iris.who.int/bitstream/handle/10665/312342/9789241515436-eng.pdf?sequence=1. Accessed 02 August 2024
Wilkinson HN, Hardman MJ (2020) Wound healing: cellular mechanisms and pathological outcomes. Open Biol 10:200223. https://doi.org/10.1098/rsob.200223
Wolberg AS, Aleman MM, Leiderman K, Machlus KR (2012) Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg 114:275–285. https://doi.org/10.1213/ANE.0b013e31823a088c
Wu SY, Hu W, Zhang B, Liu S, Wang JM, Wang AM (2012) Bromelain ameliorates the wound microenvironment and improves the healing of firearm wounds. J Surg Res 176:503–509. https://doi.org/10.1016/j.jss.2011.11.1027
Xie Y, Wu Y (2021) Application of walnut extract in preparation of burn wound treatment medicine. Patent CN112915117 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210608&CC=CN&NR=112915117A&KC=A#. Accessed 11 November 2023
Xing W, Guo W, Zou CH, Fu TT, Li XY, Zhu M, Qi JH, Song J, Dong CH, Li Z, Xiao Y, Yuan PS, Huang H, Xu X (2015) Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J Dermatol Sci 79:101–109. https://doi.org/10.1016/j.jdermsci.2015.03.016
Xoлявкa MГ, Apтюxoв BГ, Caзыкинa CM (2019) Method for obtaining heterogeneous preparation based on bromelain with the wound-healing properties. Patent RU2017123459 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20190109&CC=RU&NR=2017123459A&KC=A#. Accessed 15 October 2023
Xu N, Wang L, Guan J, Tang C, He N, Zhang W, Fu S (2018) Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol 117:102–107. https://doi.org/10.1016/j.ijbiomac.2018.05.066
Xu D, Hu MJ, Wang YQ, Cui YL (2019) Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24:1123. https://doi.org/10.3390/molecules24061123
Xue M, Jackson CJ (2015) Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care 4:119–136. https://doi.org/10.1089/wound.2013.0485
Yadav S, Abhay M, Prakash K, Negi S, Maurya AA, Kumar V (2021) Herbal wound healing agents. In: Egbuna C, Mishra AP, Goy MR (eds) Preparation of Phytopharmaceuticals for the Management of Disorders, pp. 169–184. https://doi.org/10.1016/B978-0-12-820284-5.00007-1
Yadollah-Damavandi S, Chavoshi-Nejad M, Jangholi E, Nekouyian N, Hosseini S, Seifaee A, Rafiee S, Karimi H, Ashkani-Esfahani S, Parsa Y, Mohsenikia M (2015) Topical Hypericum perforatum improves tissue regeneration in full-thickness excisional wounds in diabetic rat model. Ev Bas Compl Altern Med 2015:245328. https://doi.org/10.1155/2015/245328
Yarin AL, Sett S, Lee M, Sinha-Ray S (2016) Biodegradable plant wound dressing composed of electrospun nanofibers. Patent US2016219874 (A1). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20160804&CC=US&NR=2016219874A1&KC=A1#. Accessed 31 August 2023
Yen GC, Fan SL, Lin JH (2020) Use of Hsian-Tsao (Mesona procumbens Hemsl.) Extracts for preparing a pharmaceutical composition for promoting wound healing. Patent TW202000223 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20200101&CC=TW&NR=202000223A&KC=A#. Accessed 11 November 2023
Zanoschi IC (2013) Wound-healing ointment with Calendula officinalis extract. Patent RO128266 (A2). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20130430&CC=RO&NR=128266A2&KC=A2#. Accessed 21 June 2023
Zhang M, Zhang S (2020) T Cells in fibrosis and fibrotic diseases. Front Immunol 26(11):1142. https://doi.org/10.3389/fimmu.2020.01142
Zhang H, Chen J, Cen Y (2018a) Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int J Biol Macromol 112:862–867. https://doi.org/10.1016/j.ijbiomac.2018.01.214
Zhang CW, Li MF, Tao R, Peng MJ, Wang ZH, Qi ZW, Xue XY, Wang CZ (2020b) Physiochemical property and antibacterial activity of formulation containing polyprenol extracted from Ginkgo biloba leaves. Ind Crops Prod 147:112213. https://doi.org/10.1016/j.indcrop.2020.112213
Zhang D, Liang Y, Zhao W, Xiong Q, Ma H (2018) Application of extract of Dalbergia pinnata in preparing drug for healing skin wound. Patent CN108514569 (A). https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20180911&CC=CN&NR=108514569A&KC=A#. Accessed 15 October 2023
Zhu X, Olsson MM, Bajpai R, Järbrink K, Tang WE, Car J (2022) Health-related quality of life and chronic wound characteristics among patients with chronic wounds treated in primary care: a cross-sectional study in Singapore. Int Wound J 19:1121–1132. https://doi.org/10.1111/iwj.13708
Acknowledgements
We are grateful to Aline Brandão at BT Idiomas for her review of the English text of this article.
Funding
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
TAAA: conceptualization, data curation, investigation, methodology, writing—original draft; FRL: conceptualization, data curation, investigation, methodology, writing—original draft; RES-L: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing; FAC: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft; writing—review and editing. All authors read and approved the final version.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 392 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Araujo, T.A.A., Locatelli, F.R., da Silva-López, R.E. et al. Formulations with Active Plant Molecules and Additional Therapies in Wound Healing. Rev. Bras. Farmacogn. (2024). https://doi.org/10.1007/s43450-024-00593-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43450-024-00593-w