Abstract
A new flavonoid glycoside, herbacetin-8-O-methyl glucuronide, together with nine known flavonoids herbacetin-8-O-β-d-xylopyranoside, kaempferol, herbacetin-8-methylether, naringenin, tricin, aromadendrin, quercetin, myricetin, and 5,7,3',4',5'-pentahydroxy-flavanone, was isolated from Rhodiola fastigiata (Hook.f. & Thomson) Fu, Crassulaceae. Their structures were identified by comprehensive analyses of physicochemical properties and spectrometric data (NMR and HR-ESI–MS). According to the bioactive study, herbacetin-8-O-β-d-xylopyranoside showed significant in vitro antimalarial activity against Plasmodium falciparum 3D7 at 50 µM. Further studies indicated that herbacetin-8-O-β-d-xylopyranoside was able to reduce the mitochondrial membrane potential of P. falciparum 3D7 considerably.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhodiola fastigiata (Hook.f. & Thomson) Fu, a perennial herb belong to the Crassulaceae family, is mainly distributed in high altitude areas (2500 to 5400 m above sea level) in southwestern China (Xizang, Sichuan, and Yunnan provinces), and found also in Kashmir, Nepal, Sikkim, and Bhutan. This plant is commonly used as folk medicine in China. According to the Standard of Tibetan Medicinal Materials of Sichuan Province (2020 Edition), R. fastigiata has the actions of clearing heat and draining the lung, and is used to treat pneumonia caused by cold, tracheitis, and halitosis. In our preliminary research on screening antimalarial plants from southwestern China, R. fastigiata was found to show in vitro antimalarial activity (Table 1). For the purpose to deeply understand the substance basis against malaria, the chemical constituents of ethyl acetate fraction of ethanol extract from R. fastigiata were investigated. As a result, a new flavonoid glycoside, herbacetin-8-O-methyl glucuronide (1), along with nine known flavonoids herbacetin-8-O-β-d-xylopyranoside (2), kaempferol (3), herbacetin-8-methylether (4), naringenin (5), tricin (6), aromadendrin (7), quercetin (8), myricetin (9), and 5,7,3',4',5'-pentahydroxy-flavanone (10), was isolated and identified from R. fastigiata. Herein, we reported the extraction, isolation, structural elucidation, and in vitro antimalarial activities of compounds 1–10, as well as the possible antimalarial mechanism of compound 2.

Materials and Methods
General Experimental Procedures
UV data were obtained on a TU-1901 UV/Vis spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, People’s Republic of China). NMR spectra (1D and 2D NMR) were recorded on a Bruker Avance III-400 instrument (Bruker, Faellanden, Switzerland) with TMS as an internal reference. HR-ESI–MS data were obtained on an Agilent G6230 TOF–MS spectrometer (Agilent Technologies Inc., Santa Clara, USA). Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden), Silica gel (Qingdao Marine Chemical Ltd., Qingdao, People’s Republic of China), polyamide (Sinopharm Chemical Reagent Co., Ltd., Shanghai, People’s Republic of China), and MCI gel CHP20/P120 (75–150 µm; Mitsubishi Chemical Corp., Tokyo, Japan) were used for open column chromatography. Silica gel GF254 plates (Qingdao Marine Chemical Ltd.) were used for TLC analyses.
Plant Material
Rhodiola fastigiata (Hook.f. & Thomson) Fu, Crassulaceae, was collected in September 2020 from Deqing county with an altitude of approximately 4000 m, Yunnan, People’s Republic of China. The plant material was identified by Yong-Zeng Zhang at Dali University, People’s Republic of China. A voucher specimen (No. 20200922–7) was deposited at the Yunnan Key Laboratory of Screening and Research on Anti-pathogenic Plant Resources from Western Yunnan, Dali University.
Extraction and Isolation
The dried whole herb of R. fastigiata (9 kg) was milled and extracted four times with 95% ethanol at room temperature (50 l, each for 24 h), and the extract solutions were combined and concentrated under reduced pressure. The resulting residue (1.7 kg) was extracted by solid phase extraction with petroleum ether (PE), ethyl acetate, acetone, and methanol. The ethyl acetate fraction (326 g) was subjected to a silica gel column chromatography (CC) eluting with a gradient solvent system of CHCl3/(CH3)2CO (1:0 to 0:1) and finally with MeOH to give eight major fractions (Fr. A-Fr. H). Fr. B (48 g) was subjected to an MCI gel CC (10 to 100% MeOH) to give nine subfractions Fr. B1 to Fr. B9. Fr. B4 was subjected to repeated silica gel CC [PE/(CH3)2CO 35:1 to 4:1 and CHCl3/MeOH 25:1, respectively] to yield compound 5 (30 mg). Fr. B5 was subjected to a silica gel CC with CHCl3/EtOAc (10:1), and then was further purified by a polyamide CC (CHCl3/MeOH, 25:1 to 0:1) and a silica gel CC (CHCl3/MeOH, 1:0 to 0:1) to yield compounds 3 (100 mg), 4 (92 mg), and 6 (13 mg). Fr. C (35 g) was separated by a silica gel CC with CHCl3/(CH3)2CO (1:0 to 0:1) to give eight subfractions (Fr. C1-Fr. C8). Fr. C1 was subjected to a silica gel CC with CHCl3/(CH3)2CO (100:1–0:1) and a Sephadex LH-20 CC (CHCl3/MeOH, 1:1) to yield compounds 7 (90 mg) and 8 (8 mg). Fr. C7 was subjected to a Sephadex LH-20 CC ((CH3)2CO) and a silica gel CC (CHCl3/(CH3)2CO, 20:1) to yield compound 10 (12 mg). Fr. C8 was subjected to a Sephadex LH-20 CC (CHCl3/MeOH, 1:1) to give compound 9 (12 mg). Fr. E (24 g) was subjected to a silica gel CC eluting with CHCl3/EtOAc (1:3–0:1) and finally eluting with MeOH to give nine subfractions Fr. E1 to Fr. E9. Fr. E1 was purified by a Sephadex LH-20 CC (CHCl3/MeOH, 1:1) to yield compounds 1 (60 mg) and 2 (9 mg).
Herbacetin-8-O-methyl glucuronide (1): yellow powder; \([\alpha]^{D}_{20}\) + 203.8 (c = 0.1, MeOH); UV (MeOH) λmax (log ε): 203 (3.51), 222 (3.30), 271 (3.31), 321 (3.07), 375 (3.26) nm; 1H and 13C NMR spectroscopic data, see Table 2; HR-ESI–MS m/z: 491.0840 [M − H]⁻ (calcd. for C22H19O13, 491.0831).
Antimalarial Activity Assay
The strain of Plasmodium falciparum 3D7 (chloroquine-sensitive) was kindly provided by the Shanghai Institute of Immunity and Infection, Chinese Academy of Sciences. P. falciparum 3D7 was cultivated in human erythrocytes suspended in RPMI-1640 medium at 2% hematocrit supplemented with 0.2% w/v glucose, 0.5% w/v AlbuMax II, 0.22% w/v NaHCO3, 50 µg/ml gentamycin, and 50 µg/ml hypoxanthine (Trager and Jensen 1976). Antimalarial activity of the test samples was evaluated by a modified SYBR green I fluorescence method described previously (Huang et al. 2020). After synchronization of the parasite culture to ring stage using 5% sorbitol, P. falciparum 3D7 (1% parasitemia and 2% hematocrit) was exposed to test samples for 72 h in 96-well plates (37 °C, 5% CO2). Negative controls were treated with vehicle alone, and positive controls with chloroquine diphosphate treatment. Uninfected erythrocytes with vehicle were used for background determination. Then, erythrocyte lysis buffer (5 mM EDTA, 20 mM Tris pH 7.5, 0.12% v/v Triton X-100, and 0.012% w/v Saponin) containing the SYBR green I was distributed to each well and incubated for 2 h at room temperature in dark. Finally, the parasites growth was determined by DNA quantitation using fluorescent dye SYBR green I (excitation: 485 nm, emission: 535 nm), and the percent inhibition was calculated as follows: inhibition (%) = 100 × (fluorescence intensity of negative control – fluorescence intensity of test group)/(fluorescence intensity of negative control – fluorescence intensity of background). The IC50 value of compound 2 was calculated by dose–response curve from nonlinear regression analysis.
Assessment of Mitochondrial Membrane Potential (MMP)
The membrane potential of healthy mitochondria is higher, and JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide) can accumulate in the matrix of mitochondria to form aggregates that can produce red fluorescence. However, when mitochondria are in an unhealthy condition, their membrane potential is low, and JC-1 cannot aggregate in its matrix and maintain the original monomer form that can produce green fluorescence. The experimental procedure was performed according to a mitochondrial membrane potential assay kit with JC-1 (Beyotime Biotechnology, Shanghai, People’s Republic of China). Parasite mitochondria (10 μl), purified using a cell mitochondria isolation kit (Beyotime Biotechnology), were added to 90 μl solution of JC-1, and then exposed to 100 μM compound 2 for 30 min. Finally, the red fluorescence (excitation: 525 nm, emission: 590 nm) of aggregates of JC-1 was measured by a Varioskan LUX microplate reader (Thermo Fisher Scientific, Vantaa, Finland). Carbonyl cyanide3-chlorophenylhydrazone (CCCP), a potent uncoupling agent for mitochondrial oxidative phosphorylation, was used as the positive control, and DMSO was used as the negative control. Three technical replicates were conducted in this experiment.
Results and Discussion
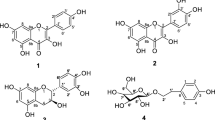
Compound 1 was obtained as yellow powder, and was established a molecular formula C22H20O13 (thirteen degrees of unsaturation) by its HR-ESI–MS m/z: 491.0840 [M − H]⁻ (calcd. for C22H19O13, 491.0831). According to 1H NMR spectrum (Table 2), 1 contained a methoxyl at δH 3.58 (3H, s), one anomeric proton of sugar at δH 4.82 (1H, d, J = 7.9 Hz), four proton resonances from oxygenated methines between δH 3.94 and 3.33, five proton signals resonances from the aromatic protons at δH 8.18 (2H, d, J = 8.9 Hz), 6.89 (2H, d, J = 8.9 Hz), and 6.26 (1H, s), three phenol groups at 12.35 (1H, s), 10.16 (1H, s), and 9.56 (1H, s), and two hydroxyl groups at 5.54 (1H, d, J = 5.9 Hz) and 5.50 (1H, d, J = 5.6 Hz). Analyses of the 13C NMR spectrum with the aid of DEPT experiments (Table 2) revealed the existence of 22 carbon resonances including a methoxyl (δC 52.1), one glucuronic acid moiety [δC 71.5, 73.8, 75.1, 75.6, 106.5, and 169.4], 14 olefinic carbons [δC 98.4, 102.9, 115.4 (× 2), 121.8, 125.1, 129.9 (× 2), 135.8, 147.0, 148.2, 156.6, 156.6, and 159.3], and one ketone carbonyl (δC 175.9). Therefore, 1 was considered as a flavonol glucuronide derivative. The HMBC correlation from the methoxy (δH 3.58) to C-6'' (δC 169.4) suggested that the methoxy was attached to C-6''. The HMBC correlation from an anomeric proton (δH 4.82, H-1'') to C-8 (δC 125.1) suggested that the glucuronic acid moiety was attached to C-8. Then the β-configuration of the glucuronic acid was determined by the coupling constant of H-1'' (δH 4.82, d, J = 7.9 Hz). Based on the further analyses of 1H-1H COSY, HMBC, and ROESY spectra (Fig. 1), compound 1 was finally named as herbacetin-8-O-methyl glucuronide.
The nine known compounds were identified by analyses of their spectroscopic data, combined with comparison of the physicochemical properties with those reported in literature. They were finally determined to be herbacetin-8-O-β-d-xylopyranoside (2) (Thuong et al. 2007), kaempferol (3) (Pitchuanchom et al. 2022), herbacetin-8-methylether (4) (Olszewska et al. 2009), naringenin (5) (Pitchuanchom et al. 2022), tricin (6) (Jung et al. 2015), aromadendrin (7) (Minh et al. 2022), quercetin (8) (Liao et al. 2023), myricetin (9) (Abdel Bar et al. 2023), and 5,7,3',4',5'-pentahydroxy-flavanone (10) (Wei et al. 2014).
In vitro antimalarial activities of compounds 1–10 against P. falciparum 3D7 are shown in Table 3. Compounds 2, 3, 8, and 9 showed better activity with the inhibition rates of 60.3 ± 1.8%, 24.6 ± 2.3%, 32.1 ± 8.3%, and 26.6 ± 5.4%, and compounds 1, 4, and 10 exhibited weaker activity with the inhibition rates of 17.1 ± 6.3%, 16.5 ± 3.3%, and 20.5 ± 5.8% at a concentration of 50 μM, respectively. According to the results shown above, the flavonols with 5,7,4'-trihydroxyl groups all showed certain antimalarial activity. However, when the flavone skeleton changed to a flavanone skeleton, the activity was decreased (compounds 3 vs 7). In addition, the activity of flavonol containing C-8 phenol group was significantly increased after it formed glycoside with xylose (compounds 1 and 4 vs 2). Among the tested compounds, 2 showed the best antimalarial activity with an IC50 value of 39.6 µM (Fig. 2).
Mitochondria is a validated malaria drug target (Goodman et al. 2017). Mitochondrial dysfunction induces MMP depolarization and causes the parasite death. Unlike eukaryotes, blood-stage P. falciparum produces ATP mainly via glycolysis, rather than oxidative phosphorylation (Goodman et al. 2017). The main role of the blood-stage parasite mitochondrion was the provision of precursors for de novo pyrimidine synthesis (Painter et al. 2010), which was a process requiring a mitochondrional dihydroorotate dehydrogenase and a functioning electron transport chain to maintain turnover of ubiquinol (Goodman et al. 2017). The effect of compound 2 on MMP of P. falciparum 3D7 was measured using fluorescent probe JC-1 dye. The fluorescence intensity of parasite mitochondria with 100 µM compound 2 treated and with vehicle DMSO treated were 1.11 ± 0.06 and 7.51 ± 0.53, respectively, which indicated that compound 2 may exert antimalarial effects by reducing Plasmodium MMP (Fig. 3).
Flavonoids are important secondary metabolites produced by plants, and have a variety of physiological functions related to growth, development, and stress protection (Dias et al. 2021). Although there have been numerous reports on flavonoids so far, few studies on flavonoids from R. fastigiata have been conducted, and this study is the first report about antimalarial activity of the flavonoids from R. fastigiata. It should be useful to better understand R. fastigiata and therefore provide some scientific basis for the further development and utilization of R. fastigiata as well.
Data Availability
The data will be made available from the corresponding author upon reasonable request.
References
Abdel Bar FM, Salkini AA, Amen Y, Sherif AE (2023) Acetylcholinesterase inhibitors from Conocarpus lancifolius Engl. (Combretaceae). Nat Prod Res 37:1668–1673. https://doi.org/10.1080/14786419.2022.2103124
Dias MC, Pinto D, Silva AMS (2021) Plant flavonoids: chemical characteristics and biological activity. Molecules 26:5377. https://doi.org/10.3390/molecules26175377
Goodman CD, Buchanan HD, Mcfadden GI (2017) Is the mitochondrion a good malaria drug target? Trends Parasitol 33:185–193. https://doi.org/10.1016/j.pt.2016.10.002
Huang Z, Li R, Tang T, Ling D, Wang M, Xu D, Sun M, Zheng L, Zhu F, Min H, Boonhok R, Ding Y, Wen Y, Chen Y, Li X, Chen Y, Liu T, Han J, Miao J, Fang Q, Cao Y, Tang Y, Cui J, Xu W, Cui L, Zhu J, Wong G, Li J, Jiang L (2020) A novel multistage antiplasmodial inhibitor targeting Plasmodium falciparum histone deacetylase 1. Cell Discov 6:93. https://doi.org/10.1038/s41421-020-00215-4
Jung YJ, Park JH, Cho JG, Seo KH, Lee DS, Kim YC, Kang HC, Song MC, Baek NI (2015) Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and neuroprotective activities. Arch Pharm Res 38:178–185. https://doi.org/10.1007/s12272-014-0387-4
Liao GF, Mo LY, Teng MX, Xu XH, Huang QX, Lu RM (2023) A new macrocyclic flavonoid from Onychium japonicum. Acta Pharm Sin 58:423–428. https://doi.org/10.16438/j.0513-4870.2022-1067
Minh TT, Toan HK, Quang LD, Hoang VD (2022) Myobontioids A-D and antifungal metabolites from the leaves of Myoporum bontioides A. Gray Nat Prod Res 36:5708–5714. https://doi.org/10.1080/14786419.2021.2016747
Olszewska MA (2009) Flavonoid profile of Sorbus intermedia. Chem Nat Compd 45:722–724. https://doi.org/10.1007/s10600-009-9409-8
Painter HJ, Morrisey JM, Vaidya AB (2010) Mitochondrial electron transport inhibition and viability of intraerythrocytic Plasmodium falciparum. Antimicrob Agents Chemother 54:5281–5287. https://doi.org/10.1128/aac.00937-10
Pitchuanchom S, Mahiwan C, Chotichayapong C, Kanokmedhakul S, Poopasit K, Nontakitticharoen M (2022) Phytochemicals from twigs of Afzelia xylocarpa and their antioxidation kinetics of oxymyoglobin. Nat Prod Res 36:2615–2619. https://doi.org/10.1080/14786419.2021.1912746
Thuong PT, Kang HJ, Na M, Jin W, Youn UJ, Seong YH, Song KS, Min BS, Bae K (2007) Anti-oxidant constituents from Sedum takesimense. Phytochemistry 68:2432–2438. https://doi.org/10.1016/j.phytochem.2007.05.031
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675. https://doi.org/10.1126/science.781840
Wei JG, Yang DS, Chen WY, Wang XM, Li XL (2014) Chemical constituents from Ampelopsis cantoniensis and their anti-angiogenic activities. Chin Tradit Herbal Drugs 45:900–905. https://doi.org/10.7501/j.issn.0253-2670.2014.07.002
Funding
This work was supported by the National Natural Science Foundation of China (81960637), the Basic Science Foundation of Yunnan Province Science and Technology Department (No. 202101AT070030), the Yunnan Key Laboratory of Screening and Research on Anti-pathogenic Plant Resources from Western Yunnan (APR2022Y02), and the Scientific Research Foundation of Yunnan Education Department (2023Y1031).
Author information
Authors and Affiliations
Contributions
BJ, CJX, and LS contributed to the conception and design. BCL, LG, HS, GJZ, and GL performed material preparation, data collection, and analysis. BCL and LG wrote the first draft of the manuscript. All authors commented on previous versions of the final manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, BC., Gou, L., Sun, H. et al. Flavonoids from Rhodiola fastigiata and their Antimalarial Activities. Rev. Bras. Farmacogn. (2024). https://doi.org/10.1007/s43450-024-00581-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43450-024-00581-0