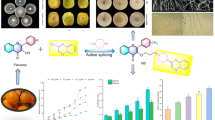
Abstract
Prenyl flavonoids are natural compounds with a relatively narrow distribution in plants with particular structural and pharmacological properties. A prenylated flavanone from the Dalea genus was reported as having potent antifungal action. As part of an extensive study on the chemistry and pharmacology of prenylated flavonoids from the Dalea genus, we report here on a new chromene flavanone isolated from the roots of Dalea boliviana Britton, Fabaceae. The results obtained from spectroscopic and spectrophotometric methodologies allowed us to elucidate the structure of compound 1 as (‒)-(2S)-5,2′-dihydroxy-6″,6″-dimethylchromene-(7,8:2″,3″)-flavanone, and provided new valuable data for the use of MS–MS as a tool for the elucidation and identification of prenylated flavonoids from the Dalea genus. In addition, the antifungal properties of 1 and other prenyl flavanones obtained from D. boliviana were investigated on multidrug-resistant Candida albicans.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prenyl flavonoids are compounds with a relatively limited distribution within the plant kingdom (Yang et al. 2015) and are notably present in the Dalea L. genus, Fabaceae. Prenyl flavonoids derived from the Dalea have been extensively studied for their reported antifungal and antibacterial properties (Peralta et al. 2019). This type of natural compound is characterized by its essential biological properties due to its particular structures (Yang et al. 2015). These relevant biological activities have encouraged new research about these compounds' chemical and pharmacological properties, with the aim of achieving their rapid identification and quantification in complex matrices such as plant extracts and to propose these extracts as potential raw materials for herbal medicines (Del Gaudio et al. 2020). In this sense, mass spectrometry (MS) provides high sensitivity and is well-suited for the high-throughput characterization of specialized metabolites in complex plant matrices. Ultra-high performance liquid chromatography in combination with tandem MS (UPLC-MS/MS) operating in a multiple reaction monitoring (MRM) mode is currently applied as a tool for quantitative metabolomics, due to its high sensitivity, specificity, and fast scanning speed. Fragmentation by electrospray ionization (ESI) in combination with collision cells in MS/MS constitutes an essential advance in plant metabolomics. In this way, ESI–MS/MS mass spectra provide relevant information about the molecular structures of metabolites, with many reports in recent years having focused on creating ESI–MS/MS-based database platforms to identify known and unknown metabolites from various sources (Limjiasahapong et al. 2021).
Due to their relevant pharmacological properties, the structures of prenylflavonoids have been investigated regarding their mass fragmentation by ESI–MS/MS to determine their chemical characteristics and generate a database to identify these compounds (Simons et al. 2009; Van Dinteren et al. 2021).
As part of the findings from extensive studies on the chemistry and pharmacology of metabolites from the Dalea genus, Peralta et al. (2011) described new prenyl and chromene flavanones from the roots of D. boliviana Britton (Fig. 1), a small shrub growing in northwestern Argentina, Bolivia, and Peru.
In other investigations about Dalea species, Peralta et al. (2012) reported a prenylated flavanone from D. elegans Gillies ex Hook.et Arn. as being a chemosensitizer against an azole-resistant Candida albicans strain with overexpression of membrane transporters as the mechanism of resistance.
Candida albicans is an opportunistic yeast species from the human microbiota, usually found on mucosal surfaces of the gastrointestinal, respiratory, and genitourinary systems. This yeast is a harmless commensal microorganism. However, under certain conditions it can cause infections known as candidiasis, acting as an opportunistic pathogen in immunocompromised or immuno-deficient patients (Meirelles et al. 2017; Triastuti et al. 2023). Resistant C. albicans (RCa) and its candidiasis have become a significant problem worldwide, to such an extent that in 2022 the World Health Organization declared this fungal pathogen as a priority for development and research of new therapeutics, among other actions that were proposed (WHO 2022).
Multidrug resistance (MDR) is a critical antifungal resistance mechanism mediated by drug transport through membrane proteins. Recently, prenyl flavonoids have been proposed as promising leaders in the search for new inhibitors of MDR in C. albicans (Santi et al. 2022).
As part of our bioprospecting Argentine native flora to search for new antifungals, we present the isolation and elucidation of a new chromene flavanone from D. boliviana. In addition, we provide new valuable data for utilizing MS/MS as a tool for identifying this chromene and other prenyl flavonoids from D. boliviana within complex plant matrices. Endorsing the biological importance and pharmacological potential of prenylated flavanones from D. boliviana, we present the antifungal properties of these compounds against multidrug-resistant C. albicans.
Materials and Methods
General Experimental Procedures
1H and 13C nuclear magnetic resonance (NMR) spectra were obtained in CDCl3 on a Brüker Advance II 400 (400 MHz for 1H and 100 MHz for 13C) spectrometer using tetramethylsilane (TMS, Sigma-Aldrich Co, St Louis, MO, USA.) as an internal standard. UV spectra were obtained in MeOH from Merck (Darmstadt, Germany), using quartz cells in a CARY WIN 50 (Varian) spectrophotometer. Column chromatography was performed on Merck (Darmstadt, Germany) silica gel. Thin layer chromatography (TLC) was carried out using 20 × 20 cm plates with a 0.5 mm layer of silica gel GF254 (Merck, Darmstadt, Germany). Spots were visualized by UV at 254 nm.
HPLC experiments were carried out to assess the purity of the compounds. A ProStar Varian HPLC machine (Palo Alto, CA) coupled with a UV detector (Varian) was used. Elution was carried out on a Varian C18 column (Ø 250 mm × 4.6 mm × 5 µm particle size).
Analyzes of UPLC-MS/MS were performed using an Acquity UPLC H-Class (Waters) with a quaternary pump chromatograph equipped with an autosampler, and coupled to a triple quadrupole (Waters Xevo TQ-S MS/MS) with an ESI source mass spectrometer used in the negative ion mode. For targeted analysis of compounds, the mass transitions were monitored in MRM mode using the Intellstart application. Argon 99.999% (Linde) at a pressure of 3.4 × 10−3 mbar served as the collision gas in the collision cell. Mass Lynx software version 4.1 controlled the LC–MS-MS system. A circular dichroism (CD) spectrum was obtained with a Jasco J-810 spectropolarimeter.
Solvents and Reagents
The acetonitrile, MeOH, and formic acid used for HPLC and UPLC experiments were purchased from Merck (Darmstadt, Germany). Fluconazole (purity ≥ 98%) was purchased from Sigma-Aldrich Co, St Louis, MO, USA.
Plant Material
Dalea boliviana Britton, Fabaceae, roots were collected during the flowering period in February 2019, near the town of Iturbe in the Humahuaca department, Jujuy province, Argentina (GPS coordinates 22°58′44″ S, 65°21′13″ W at 3223 m.a.s.l.). Prof. Dr. Gloria Barboza, from the Botanical Museum-UNC, identified the plant material. This specimen is on deposit as CORD 1066 at the Botanical Museum-UNC.
Extraction and Isolation
The plant material was dried at room temperature. Roots were separated from the aerial parts and 20 g were pulverized with a blade mill and extracted with a Soxhlet extractor using 500 ml of hexane (anhydrous, 95%, Sigma-Aldrich Co, St Louis, MO, USA). Subsequently, the solvent was eliminated with a rotary evaporator at reduced pressure, yielding the crude extract (1.02 g).
The secondary metabolites were isolated from the hexane extract of D. boliviana by column chromatography. Silica gel 60 (0.063–0.200 mm) for column chromatography (70–230 mesh, Merck, Darmstadt, Germany) was used as the stationary phase and successively eluted with hexane/EtOAc (anhydrous, 95%, Sigma-Aldrich Co, St Louis, MO, USA) (100:0 to 50:50) and EtOH (absolute for analysis, Sigma-Aldrich Co, St Louis, MO, USA). Eight fractions were collected and tested by TLC (hexane/EtOAc, 70:30) to compare their profiles, with the spots being visualized by UV light at 254 and 365 nm.
The compound (2S)-5,2′-dihydroxy-6″,6″-dimethylchromene-(7,8:2″,3″)-flavanone (1) was purified from fraction 5 (9.1 mg, Rf 0.77) by preparative TLC with CHCl3 (analytical grade, Sigma-Aldrich Co, St Louis, MO, USA)/EtOH, 98:2, yielding 5.1 mg of pure compound (1) (Rf 0.56).
The structure of compound 1 was elucidated by 1H and 13C NMR. Samples were prepared using CDCl3 (Sigma-Aldrich Co, St Louis, USA), and TMS was used as the internal standard. The data was analyzed through the TopSpin 4.0.7 program.
The compounds (2S)-5,7,2′-trihydroxy-8,3′-diprenylflavanone (2) (6.5 mg), and (2S)-5,7,2′-trihydroxy-5′-(1′′′,1′′′-dimethylallyl)-8-prenylflavanone (3) (1.6 mg) were purified and identified as previously reported by Peralta et al. (2011).
(‒)-(2S)-5,2ʹ-Dihydroxy-6ʹʹ,6ʹʹ-dimethylchromene-(7,8:2ʹʹ,3ʹʹ)-flavanone (1)
Amorphous yellow solid. Optical rotation—39 (c 0.09, MeOH); UV(MeOH) λmax (log ε) 271 (3.95), 293 (3.41), 339 (sh) (2.89) nm; CD (c 0.020, MeOH) [θ]330 + 1.90, [θ]293 -4.75; 1H NMR (CDCl3,400 MHz) and 13C NMR (CDCl3,100 MHz) data are shown in Table 1.
Liquid Chromatography Experiments
The purity of the compounds was determined by HPLC, according to a methodology reported by Peralta et al. (2015). In brief, ultrapure water was used as the mobile phase A, and MeOH as the mobile phase B. The elution program started with a linear gradient from 50% B to 85% B in 60 min, followed by 100% B (maintaining this percentage for 10 min), and finished with a linear gradient from 100% B to 50% B in 10 min, flow rate at 1 ml/min, wavelength used for detection at 290 nm, injection volume: 20 µl, temperature: 30 °C.
The UPLC-MS/MS was performed on the compounds, with an UPLC equipment coupled to a mass detector. Analyzes were performed using an Acquity UPLC H-Class (Waters), with a quaternary pump equipped with an autosampler. An isocratic method was used: 20% ultrapure H2O 0.1 v/v formic acid (LC–MS quality, Merck, Darmstadt, Germany)/80% ACN 0.1 v/v formic acid, flow 0.20 ml/min, column temperature 30 ºC, injection volume 5 µl. Column used: Waters BEH C-18 (2.1 mm × 50 mm × 1.7 particle size).
Antifungal Evaluation
Fungal Strains
The Candida albicans strains were isolated from the oral cavity and kindly ceded by Dr. T. White. Two clinical strains of Candida albicans were used, one of these (SCa) was sensitive and the other (RCa) was resistant to imidazole antifungals. The SCa strain (2.76) is sensitive to imidazole antifungals, while RCa strain (12.99) overexpresses CDR1, CDR2, and MDR1 type genes, which are involved in multidrug resistance (MDR) (White et al. 2002). Both strains were grown in yeast peptone dextrose (YPD) broth and were preserved as frozen stocks containing 15% glycerol at -80 °C. Before each experiment, cells were sub-cultured from this stock onto YPD to guarantee purity and viability.
Antifungal Assay
The growth of SCa and RCa was quantified in the absence and presence of different concentrations of compounds 1–3. This was carried out following the standardized microdilution protocol in a 96-well plate, M27-A4 of the Clinical and Laboratory Standards Institute (CLSI 2017), with some modifications according to Peralta et al. (2012). A starting inoculum of 103 colony-forming units per ml (CFU/ml) was grown in a liquid medium using a 96-well microdilution plate. Compounds were added from stock solutions (40 mM in dimethyl sulfoxide; DMSO) and then diluted in Roswell Park Memorial Institute (RPMI) 1640 synthetic medium with glutamine but without sodium bicarbonate, buffered with 0.164 M morpholino propane sulfonic acid (MOPS), adjusted at pH 7 ± 0.1, and with 0.2% glucose to give different concentrations in the incubation medium. Samples were compared to the respective control containing the same solvent. Fluconazole, an azole antifungal, was used as a positive control. Absorbance was measured at 540 nm with a MicroQuant microplate spectrophotometer (Tecan Sunrise Model, TECAN, AUS).
The compounds’ minimum inhibitory concentration (MIC) was defined as the lowest concentration that produces an optical density of 50% or less relative to the growth control measured at 540 nm in a microplate reader.
Graphs were made from the data obtained using version 5.00 of the GraphPad software (GraphPad Software San Diego, USA), and the standard error of three independent experiments was calculated. Duplicates for each experimental condition were included in all experiments. A statistical analysis was performed using the two-way ANOVA test, followed by a Bonferroni test. Values of p ≤ 0.05 represented a significant difference between the groups. Data were plotted as the mean ± standard error of the mean (SEM).
Results and Discussion
Compound 1 was isolated as an amorphous yellow solid that was optically active [α]D25 -39 (c 0.09, MeOH). Its molecular formula was deduced as C20H20O5, according to the spectral data obtained from MS/MS and elucidated by NMR spectroscopic data. The UV absorption maxima at 293 and 339 nm (Fig. S1) suggested a flavanone skeleton. The 1H and 13C NMR data (Table 1), together with the aid of the 2D Heteronuclear Single Quantum Coherence (HSQC) NMR spectrum (H-C resonances) allowed the complete structure of compound 1 to be determined. In the 1H spectrum, the signals of the ABX spin system characteristic of flavanone [δH 5.70 (1H, dd, J = 13.05, 2.76 Hz, H-2), 3.09 (1H, dd, J = 17.32, 13.05 Hz, H-3B) and 2.94 (1H, dd, J = 17.32, 2.76 Hz, H-3A)], and also the signal of a single aromatic proton in ring A at δH 6.03, can be observed (Fig. 2S). The signals at δH 6.88 (Fig. 2S.1) (1H, dd, J = 7.51, 1.02 Hz, H-3'), 7.53 (Fig. 2S.2) (1H, dt, J = 7.51, 1.02 Hz, H-4'), 7.00 (Fig. 2S.3) (1H, dt, J = 7.51, 1.02 Hz, H-5'), and 7.28 (1H, dd, J = 7.51, 1.02 Hz, H-6’) (Fig. 2S.4) were indicative of the substitution pattern at B-ring. Characteristic signals of the dimethylchromene ring were found: two doublets corresponding to olefinic protons with a cis coupling constant at δH 6.54 (1H, d, J = 10.00 Hz, H-4″) and 5.50 (1H, d, J = 10.00 Hz, H-5″), as well as two singlets assigned to gem-dimethyl at δH 1.43 (3H, s) and 1.45 (3H, s). In the 13C NMR spectrum of compound 1, signals corresponding to twenty carbon atoms were observed (Fig. 3S and Table 1).

The Heteronuclear Multiple Bond Connectivity (HMBC) spectrum (Fig. 4S) exhibited a correlation between the signals of the C5-OH hydroxyl proton (δH 12.11) and C-5. In addition, the signal of the proton of the dimethylchromene ring at δH 6.54 (H-4″) was correlated with the C-8 (δ 102.36), C-9 (δ 156.64), and C-7 (δ 162.19) atoms (Fig. 2 and 4S). These signals indicated that the chromene ring is attached to C-8. A correlation between C-8 and the methyl proton H-5″ (δH 5.50) of the dimethylchromene ring was also observed. The B-ring substitution pattern was determined by HMBC correlations. The H-6’ signal (δH 7.28) correlated with the signal at 153.25 ppm, which corresponds to a quaternary carbon bonded to a hydroxyl group. This signal was assigned to C-2’, confirming that the hydroxyl group is located in the B-ring at position C-2’. Other HMBC correlations of the H-6’ signal (δH 7.28) were observed with the signals at 135.02 ppm (CH, C-3’) and 129.84 ppm (CH, C-4’). The same B-ring pattern was observed for the other flavanones described for D. boliviana (Peralta et al. 2011). The key HMBC correlations are shown in Fig. 2. The 2D HMBC spectrum (H-C) of compound 1 can be observed in Fig. 4S of supplementary data. The correlations observed for the COSY spectrum were related to the dimethylchromene ring (H-5’’/H-4’’), with the COSY (H–H) spectrum being shown in Fig. 5S. The CD experiment indicated a positive cotton effect at [θ]330 and a negative cotton effect at [θ]293, indicating the S configuration of C-2. All these spectroscopic and spectrophotometric data allow us to propose the structure of (‒)-(2S)-5,2′-dihydroxy-6″,6″-dimethylchromene-(7,8:2″,3″)-flavanone for compound 1. As far as we are aware, this is the first report of this compound in the Dalea genus.
Compounds 2 and 3 are prenyl flavanones isolated from the root extract of D. boliviana. Their spectroscopic and spectrophotometric data matched those previously reported by Peralta et al. (2011), thereby confirming their identification as (2S)-5,7,2′-trihydroxy-8,3′-diprenylflavanone (2) and (2S)-5,7,2′-trihydroxy-5′-(1′′′,1′′′-dimethylallyl)-8-prenylflavanone (3) (Peralta et al. 2011). These prenylflavanones have demonstrated significant biological activities, including the inhibition of tyrosinase and xanthine oxidase (Peralta et al. 2011; Santi et al. 2023).
The purity of compounds 1–3 was determined by HPLC with the UV detector technique, as previously described by Peralta et al. (2015). The retention times and purities of the three compounds respectively were: 44.90 min, 92.26% for compound 1 (Fig. 7S.1), 60.24 min, 94.45% for compound 2 (Fig. 7S.2), and 54.20 min, 95.31% for compound 3 (Fig. 7S.3).
Given that UPLC-MS/MS is a widely employed tool for structural elucidation and natural product identification, we performed a UPLC-MS/MS analysis on compound 1 (Fig. 8S.1 and 8S.2) to provide additional spectral evidence that supported the proposed structure of this compound (Simons et al. 2009; Limjiasahapong et al. 2021). The retention time for 1 under UPLC conditions was 1.24 min (Fig. 8S). Scheme 1 illustrates the proposed MS–MS fragmentation pathway of compound 1 using electrospray ionization (ESI–MS) (Fabre et al. 2001). Initially, minimal fragmentation was observed, and the molecular ion [M-H]− at m/z 337.10 (100) was observed (Fig. 8S.1). Subsequently, the MS spectra were obtained for the molecular ion at m/z 337.10 under collision energies of 20, 10, and 30 eV (Fig. 8S.2 (A), (B), and (C) respectively). At all collision energies, the predominant daughter mass was observed at m/z 191.03. This corresponded, as depicted in Scheme 1, to the A-ring fragment obtained from the proposed 1,4 cleavage (1,4A), following the terminology adopted for retrocyclization cleavage reactions involving a flavanone (Fabre et al. 2001; Simons et al. 2009).
From the UPLC-MS/MS experiments, we have also included the spectral data for compounds 2 (Fig. 9S.1 and 9S.2) and 3 (Fig. 10S.1 and 10S.2), which provide new spectroscopic data for the identification of these flavanones in plant extracts.
The retention times under UPLC conditions were 1.76 min and 1.29 min for 2 and 3, respectively. Once again, the predominant reaction observed at all collision energies was the 1,4 cleavage of the [M-H]− ion at m/z 407.15 for both compounds. Consequently, the major daughter ion detected was the 1,4A− fragment at m/z 193.03 (Figs. 9S and 10S). This fragmentation pattern highlights that the nucleophilic characteristics of the prenyl group render degradation within the A ring highly unfavorable. These distinctive structural features of prenyl flavonoids demonstrate the usefulness of MS/MS in supporting the elucidation and identification of these compounds within complex matrices, such as plant extracts, with the MS/MS spectral data supporting the structure proposed for compound 1 and providing contributions for subsequent identification and quantification of the three prenyl flavanones (compounds 1–3) from the D. boliviana in different matrices.
To contribute to the current knowledge related to the properties and pharmacological potential of the prenylated flavanones from D. boliviana, compounds 1–3 were evaluated for their antifungal activity. For this purpose, we employed two C. albicans strains: one sensitive (SCa) and the other resistant (RCa) to imidazole antifungals. For each compound, the MIC was defined as the lowest concentration that resulted in an optical density of 50% or less than that of the growth control (without antifungals) (Peralta et al. 2012). Fluconazole, a well-known azole antifungal, was used as a positive control. The MIC values obtained for compound 1 against the SCa and RCa strains were both 0.062 mM, resulting in a growth inhibition of 52.52% for SCa, and 52.09% for RCa. For compound 2, the MIC value was 0.125 mM against SCa, leading to a growth inhibition of 61.66%, while the MIC for RCa exceeded 0.500 mM. Compound 3 exhibited a MIC of 0.062 mM for SCa, and 0.125 mM for RCa, resulting in growth inhibition percentages of 57.15% and 50.30%, respectively. For the reference antifungal fluconazole the MIC values were 0.026 mM and 0.21 mM for SCa and RCa, respectively. The detailed MIC values are available in Table 2. It is noteworthy that the activity of compound 1 was similar for both azole-susceptible and resistant strains, with the same MIC value being observed for RCa and SCa. These results suggest that the azole resistance mechanism by overexpression of membrane transporters is not influential in the antifungal activity of compound 1. This mechanism was evident in the differences observed in the MIC values of both strains for the antifungal fluconazole. At 0.125 mM, compound 1 exhibited significantly greater activity than compound 2 against the azole-resistant strain, with a p-value of < 0.001. Similarly, at 0.125 mM, compound 3 also displayed significantly higher activity than compound 2 against RCa, with a p-value of < 0.01. Figure 3 shows the percentages of growth inhibition of the SCa and RCa strains in the presence of compounds 1–3 at 0.125 Mm.
Percentage of growth inhibition of Candida albicans exerted by the three compounds at 0.125 mM. ***p < 0.001 denotes a significant difference between compounds 1 and 2 at 0.125 mM for the RCa strain; ##p < 0.01 denotes a significant difference between compounds 2 and 3 at 0.125 mM for RCa strain. SCa: Sensitive Candida albicans; RCa: Resistant Candida albicans
Conclusions
The isolation and chemical elucidation of the chromene flavanone (2S)-5,2′-dihydroxy-6″,6″-dimethylchromene-(7,8:2″,3″)-flavanone (1) has been described for the first time for D. boliviana. In addition, new data about MS/MS has been provided about prenylated flavonoids from this species, enabling their future identification and quantification in complex matrices such as extracts. Moreover, the antifungal activity of 1 and two other prenylated flavanones from D. boliviana was found for azole-resistant C. albicans. Our results provide evidence showing the potential of prenylated flavonoids 1 and 3 as a source of new drugs to combat MDR in C. albicans.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
CLSI (2017) Reference method for broth dilution antifungal susceptibility testing of yeasts, 4.ª edición. M27-A4. Clinical and Laboratory Standards Institute, Wayne, PA. https://clsi.org/standards/products/microbiology/documents/m27/. Accessed Nov 2022
Del Gaudio MP, Santi MD, Cabrera JL, Peralta MA, Ortega MG (2020) Dalea extracts as potential for phyto-ingredients: antioxidant, antityrosinase, antifungal and cytotoxicity in vitro evaluations. Atena Editora: Ponta Grossa. 130–143. https://doi.org/10.22533/at.ed.38220021012
Fabre N, Rustan I, De Hoffmann E, Quetin-Leclercq J (2001) Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J Am Soc Mass Spectr 12:707–715. https://doi.org/10.1016/S1044-0305(01)00226-4
Limjiasahapong S, Kaewnarin K, Jariyasopit N, Hongthong S, Nuntasaen N, Robinson JL, Nookaew I, Sirivatanauksorn Y, Kuhakarn C, Reutrakul V, Khoomrung S (2021) UPLC-ESI-MRM/MS for absolute quantification and MS/MS structural elucidation of six specialized pyranonaphthoquinone metabolites from Ventilago harmandiana. Front Plant Sci 11:602993. https://doi.org/10.3389/fpls.2020.602993
Meirelles GC, Pippi B, Hatwig C, Barros FMC, Oliveira LFS, Von Poser GL, Fuentefria AM (2017) Synergistic antifungal activity of the lipophilic fraction of Hypericum carinatum and fluconazole. Rev Bras Farmacogn 27:118–123. https://doi.org/10.1016/j.bjp.2016.08.001
Peralta MA, Ortega MG, Agnese AM, Cabrera JL (2011) Prenylated flavanones with anti-tyrosinase activity from Dalea boliviana. J Nat Prod 74:158–162. https://doi.org/10.1021/np1004664
Peralta MA, Calise M, Fornari MC, Ortega MG, Diez RA, Cabrera JL, Pérez NC (2012) A prenylated flavanone from Dalea elegans inhibits rhodamine 6 G efflux and reverses fluconazole resistance in Candida albicans. Planta Med 78:981–987. https://doi.org/10.1055/s-0031-1298627
Peralta MA, Da Silva MA, Ortega MG, Cabrera JL, Paraje MG (2015) Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 22:975–980. https://doi.org/10.1016/j.phymed.2015.07.003
Peralta MA, Santi MD, Cabrera JL, Ortega MG (2019) Dalea genus, chemistry, and bioactivity studies. Stud Nat Prod Chem 62:307–341. https://doi.org/10.1016/B978-0-444-64185-4.00008-3
Santi MD, Ortega MG, Peralta MA (2022) A state-of-the-art review and prospective therapeutic applications of prenyl flavonoids as chemosensitizers against antifungal multidrug resistance in Candida albicans. Curr Med Chem 29:4251–4281. https://doi.org/10.2174/0929867329666220209103538
Santi MD, Aguirre EB, Negro MF, Zunini MP, Peralta MA, Ortega MG (2023) Prenylated flavonoids from Dalea genus as xanthine oxidase inhibitors: in vitro bioactivity evaluation and molecular docking studies. Res Chem 6:101115. https://doi.org/10.1016/j.rechem.2023.101115
Simons R, Vincken JP, Bakx EJ, Verbruggen MA, Gruppen H (2009) A rapid screening method for prenylated flavonoids with ultra-high-performance liquid chromatography/electrospray ionization mass spectrometry in licorice root extracts. Rapid Commun Mass Spectr 23:3083–3093. https://doi.org/10.1002/rcm.4215
Triastuti A, Vansteelandt M, Barakat F, Amasifuen C, Jargeat P, Haddad M (2023) Untargeted metabolomics to evaluate antifungal mechanism: a study of Cophinforma mamane and Candida albicans interaction. Nat Prod Bioprospec 13:1. https://doi.org/10.1007/s13659-022-00365-w
Van Dinteren S, Araya-Cloutier C, de Bruijn WJ, Vincken JP (2021) A targeted prenylation analysis by a combination of IT-MS and HR-MS: identification of prenyl number, configuration, and position in different subclasses of (iso) flavonoids. Anal Chim Acta 1180:338874. https://doi.org/10.1016/j.aca.2021.338874
White TC, Holleman S, Dy F, Mirels LF, Stevens DA (2002) Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Ch 46:1704–1713. https://doi.org/10.1128/AAC.46.6.1704-1713.2002
WHO (2022) WHO fungal priority pathogens list to guide research, development, and public health action. World Health Organization https://www.who.int/publications/i/item/9789240060241. Accessed in March 2023
Yang DS, Wang SM, Peng WB, Yang YP, Liu KC, Li XL, Xiao WL (2015) Minor prenylated flavonoids from the twigs of Macaranga adenantha and their cytotoxic activity. Nat Prod Bioprospec 5:105–109. https://doi.org/10.1007/s13659-015-0059-1
Acknowledgements
We gratefully acknowledge the generous support of the Universidad Nacional de Córdoba and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for providing the facilities used in this research. We extend our sincere gratitude to Drs. Guillermo Blanco and Ezequiel Falchi for their invaluable assistance with the UPLC-MS/MS experiments, Dr. Gloria Bonetto for conducting the NMR analysis, Dr. T. White (Washington University) for providing the Candida albicans strains, Prof. Dr. G. Barboza (IMBIV, CONICET) for the identification of the species under study.
Dr. Paul Hobson, a native speaker, for revising the manuscript.
Funding
This work was supported by ANPCyT BID–PICT 2017 N° 2565, BID–PICT 2019 N° 1576, and SECYT-Universidad Nacional de Córdoba (05/C375). M.F.N. is a doctoral fellow of CONICET. M.A.P. and M.G.O. are members of the Research Career of CONICET.
Author information
Authors and Affiliations
Contributions
MFN carried out isolation and elucidation of the compounds, antifungal activity assessments, writing—original draft. MGO and MAP polished the article. MAP designed and checked the whole manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no competing interests associated with this investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Negro, M.F., Ortega, M.G. & Peralta, M.A. Bioprospecting Prenyl Flavanones from Dalea boliviana: Structural Insights and Antifungal Properties Against Azole-Resistant Candida albicans. Rev. Bras. Farmacogn. 34, 785–792 (2024). https://doi.org/10.1007/s43450-024-00526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-024-00526-7