Abstract
Medicinal plants have long been used for phyto therapy, and various herbal medicines have been used for diabetes treatment. The bioactive components of several medicinal plants are known to critically impact on carbohydrate metabolism. High-fat diet and eventual type 2 diabetes are associated with several comorbidities, such as hepatic and cardiac disorders. This study was aimed at assessing the antihyperglycemic effects of biochanin A in a high-fat diet–streptozotocin–induced type 2 diabetes rat model. The levels of different hyperglycemic markers were assessed. A significant reduction in the levels of glucose, insulin resistance, hemoglobin, and glucose-6-phosphate, and activities of fructose-1,6-bisphosphatase and glycogen phosphorylase, was found after administration of 10 mg/kg bw orally. Furthermore, the biochanin A–treated hyperglycemic rats exhibited significantly higher activities of hexokinase and glycogen synthase and hepatic levels of glycogen. Histopathological analysis of the liver and pancreas revealed that biochanin A significantly protected these organs from glucotoxicity. Thus, reductions in blood glucose levels and modification of the activity of the carbohydrate-metabolizing enzymes revealed that biochanin A exhibits potential antihyperglycemic effects.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being a chronic metabolic disorder, worldwide prevalence of diabetes mellitus (DM) is expected to reach 4.4% by 2030 (Arjunan et al. 2020). The International Diabetes Federation estimates around 693 million people to suffer from DM globally by 2045, with the highest prevalence in China, India, and the USA (Cho et al. 2018). DM patients are characterized by impaired insulin signaling, which leads to abnormally high blood glucose levels. DM patients usually suffer from insulin deficiency. Type 1 DM (T1DM) patients suffer from β-cell destruction, while type 2 DM (T2DM) patients exhibit reduced β-cell function and insulin resistance. Globally, 90–95% of the DM patients suffer from T2DM (Idu et al. 2021).
Obesity, a highly prevalent ailment of the modern society, is closely associated with DM (Balaji et al. 2017). Previous studies have demonstrated that prolonged consumption of high-fat diet (HFD) leads to several metabolic syndromes, including DM (Idu et al. 2021; Balaji et al. 2017). HFD consumption is also associated with a rise in oxidative stress, hyperlipidemia, inflammation, hypertension, etc. (Parim et al. 2015; Uddandrao et al. 2020b).
Despite effective T2DM management through various non-pharmacological (diet and exercise) and pharmacological (antidiabetic agents) approaches, its morbidity and mortality rates are still of grave concern. This has warranted the discovery of novel antidiabetic agents derived from plant sources and with better efficacy and safety profiles. Recently, several nutraceuticals and natural therapeutics have emerged to counter T2DM (Uddandrao et al. 2022). Several plant-based compounds have attracted a lot of attention as potentially viable hypoglycemic agents (Uddandrao et al. 2020a) owing to their effectiveness, low costs, and minimal side effects (Gothandam et al. 2019). The beneficial effects of phytoconstituents in this context (Kotturu et al. 2021) suggest that the consumption of biochanin A may be of benefit in metabolic diseases.
Biochanin A (1) is an O-methylated isoflavonoid found in various plants, including soy, peanuts, chickpea, alfalfa sprouts, etc. (Harini et al. 2012). Biochanin A has been strongly associated with numerous biological and pharmacological activities, including antidiabetic (Oza and Kulkarni 2018), antiobesity (Rathinasamy et al. 2020), anticancer (Yu et al. 2019), antiallergic, and anti-inflammatory (Ko et al. 2011). A previous study has demonstrated the preliminary cardio-protective effect of biochanin A against myocardial infarction (MI) via attenuation of isoproterenol-induced oxidative stress (Sangeethadevi et al. 2021). Recently, biochanin A has been shown to be involved in the alleviation of impairment in insulin signaling in HFD-fed mice (Arjunan et al. 2020). Another study by Rani et al. (2021) showed that biochanin A helps alleviate insulin resistance and hyperglycemia and modify HO-1 and hepcidin. The current study was aimed at elucidating the effects of biochanin A on the activities of the enzymes implicated in glucose metabolism in order to determine its antihyperglycemic effects and the underlying mechanism of action.

Materials and Methods
Chemicals
Biochanin A was obtained from Sigma Chemical Company (Lot # S39234V). Insulin and fasting blood glucose kits were obtained from Bio-Merieux (RCS, Lyon, France) and Stanbio Laboratory (USA), respectively. All the reagents were of analytical grade.
Animals
Twenty-four male Sprague-Dawley rats (body weight: 180–200 g) were obtained from Arulmigu Kalasalingam College of Pharmacy, Virudhunagar, India, and kept under standard laboratory conditions (temperature: 22 ± 2 °C; moisture: 40–60%). All experiments were performed in accordance with the Institute Animal Ethical Committee of Arulmigu Kalasalingam College of Pharmacy, Virudhunagar, Tamil Nadu, India (Approval No: AKCP/IAEC/83/20-21).
High-Fat Diet Composition
The preparation of HFD was followed as per standard procedure (Rani et al. 2021).
Establishment of HFD-STZ-Induced T2DM Rat Models
To establish the T2DM rat model, the rats were fed with HFD for 2 weeks. The control rats were administered normal diet. HFD-fed and control rats were administered with STZ (35 mg/kg i.p.) and vehicle (citrate buffer (pH 4.5); 1 ml/kg), respectively. Three days post-STZ injection, glucose levels of experimental rats were determined. The rats having a serum glucose level of ≥ 200 mg/dl were considered to have diabetes and were selected for further analysis. Once diabetes diagnosis was confirmed, biochanin A treatment was initiated.
Experimental Design
The rats were divided into the following four groups:
-
Group 1: normal control rats
-
Group 2: diabetic control rats
-
Group 3: diabetic +10 mg/kg bw of biochanin A administered in a vehicle solution (normal saline) orally for 30 days using an intragastric tube (Harini et al. 2012; Rathinasamy et al. 2020).
-
Group 4: Diabetic +5 mg/kg bw of gliclazide administered in a vehicle solution (normal saline) orally for 30 days using an intragastric tube.
Quantification of Hyperglycemic Markers
Plasma Glucose and Insulin Levels
The body weight and the total amount of food consumed by each rat were measured (data not shown). The animals were starved overnight and anesthetized prior to collecting blood samples by using the retro-orbital puncture strategy. The samples were then incubated at room temperature for 15 min. Thereafter, the collected samples were centrifuged for 15 min at 700 × g for plasma isolation. Commercially available kits were used to determine the insulin and glucose levels.
Fasting Blood Glucose, Hemoglobin, and Glycosylated Hemoglobin Levels
The oral glucose tolerance test (OGTT) was used to assess the fasting blood glucose (FBG) levels. Briefly, 2 g/kg bw glucose was administered using an orogastric tube to the overnight starved rats. The glucose level was estimated in the blood samples collected at 0, 30, 60, and 120 min after glucose administration. Hemoglobin (Hb) and glycosylated hemoglobin (GHb) levels were determined using commercially available kits.
Activity of Carbohydrate-Metabolizing Enzymes and Liver Glycogen Level
Liver, kidneys, and muscle of experimental rats were extracted, washed using ice-cold saline, and homogenized in 0.1 M Tris-HCl buffer (pH 7.4). For the determination of enzyme activity, the supernatant was used. Glucose-6-phosphatase, fructose-1,6-bisphosphatase, and hexokinase were quantified using methods previously described by Koida and Oda (1959), Gancedo and Gancedo (1971), and Kinney LaPier and Rodnick (2001), respectively. The activities of glycogen phosphorylase and synthase were examined using the methods previously described by Cornblath et al. (1963) and Leloir and Goldenber (1962), respectively. Finally, hepatic glycogen levels were quantified using the method previously described by Ong and Khoo (2000).
Histopathological Analysis
From the sacrificed rats, liver and pancreas tissue was collected and straightaway cleaned with saline to remove blood stains, and 10% formalin was used to fix the tissues. Then, the tissues were subjected to embedding in paraffin and 5-μm-thick sections were prepared from the paraffinized samples and stained using hematoxylin and eosin stain. The histoarchitectural changes of liver and pancreas in the prepared sections were examined using light microscope.
Statistical Analysis
SPSS (version 10.0) software was used to perform statistical analysis. The results were expressed as the mean ± SD. One-way analysis of variance (ANOVA) and least significant difference (LSD) test were used to assess the significance of intergroup differences. Statistical difference was defined as p value < 0.05.
Results and Discussion
Figure 1 shows the changes in bw of rats of all groups. HFD intake markedly elevated the BW of the rats (p < 0.05). Furthermore, compared to the HFD-STZ group, groups administered with BCA or glyclazide exhibited a significant reduction in the rise of the bw (p < 0.05).
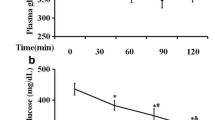
Table 1 depicts the plasma levels of glucose and insulin as well as degree of insulin resistance in all groups. The HFD-STZ group exhibited significantly higher insulin resistance and plasma glucose levels and lower plasma insulin levels. Treatment with BCA or glyclazide led reversed these alterations observed in diabetic rats.
Compared to the control group, the HFD-STZ group exhibited significantly higher glucose tolerance. In the control group, post-glucose load and blood glucose levels reached a peak at 60 min, followed by a decline to near basal levels at 120 min. On the contrary, in the HFD-STZ group, once the glucose levels reached a peak, they did not decline even at 120 min post-glucose load. Treatment with biochanin A or glyclazide led to significantly decreased blood glucose levels at 60 min post-glucose load compared to untreated diabetic rats (Fig. 2).
Table 2 summarizes the levels of hemoglobin and glycated hemoglobin in all groups. The HFD-STZ group exhibited significantly reduced levels of hemoglobin and higher levels of glycated hemoglobin compared to the normal control group. These altered levels were restored after biochanin A or glyclazide treatment.
Tables 3, 4, 5 and 6 depict the levels of hexokinase (Table 3), fructose-1,6-bisphosphatase (Table 4), and glucose-6-phosphatase (Table 5) in all groups. The HFD-STZ group exhibited significantly reduced levels of hexokinase and higher levels of fructose-1,6-bisphosphatase and glucose-6-phosphatase compared to the control group; however, these levels normalized post biochanin A or glyclazide treatment.
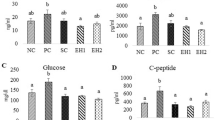
Table 6 and Fig. 3 A and B show the tissue glycogen levels and activities of glycogen phosphorylase and synthase in all groups. Compared to the normal group, the HFD-STZ group exhibited significantly reduced levels of glycogen in liver and muscle and higher glycogen levels in the kidneys. Furthermore, the HFD-STZ group exhibited significantly reduced activity of glycogen synthase and higher activity of glycogen phosphorylase compared to control rats (Fig. 3A, B). Treatment with biochanin A or glyclazide markedly restored the altered glycogen levels and activities of glycogen phosphorylase and synthase in diabetic rats.
Figures 4 and 5 indicate the histopathological changes in the liver and pancreas of control and experimental diabetic rats. Normal control rats showed normal nucleus with sinusoidal cards around the central vein (Fig. 4A). Diabetic rats showed severe morphological alterations in the liver such as hepatocyte hypertrophy, bile duct hyperplasia, and an increased number of intracytoplasmic acidophilus pellets (Fig. 4B). Diabetic rats treated with biochanin A or glyclazide showed no major alterations in liver structure and were near normalcy (Fig. 4C, D). On the other hand, there were no remarkable alterations in the pancreatic architecture observed in normal control rats (Fig. 5A), whereas severe necrosis and mild atrophy of islets were found in the diabetic rats. The islets were shrunken in the diabetic rats when compared with control rats (Fig. 5B). Diabetic rats treated with BCA or glyclazide showed notable expansion of islets and significantly reduced injuries in pancreas (Fig. 5C, D).
The current study was aimed at assessing the antihyperglycemic effects of biochanin A on HFD-STD-induced diabetic rat models and compared them with those of the second-generation sulfonylurea drug gliclazide, which has been demonstrated to be safe and effective in clinical trials. The results indicated that biochanin A exhibits the efficacy in lowering blood glucose levels and improving the antioxidant and lipid profiles of rats with diabetes.
The rats with HFD-STZ treatment–induced diabetes in this study showed significantly higher plasma glucose levels and insulin sensitivity and lower insulin than the normal controls, indicating that the diabetic rat model was established successfully. In humans, insulin plays a role in maintaining glucose homeostasis, which is mainly due to its direct effects on the liver, white adipocytes, and skeletal muscle, wherein it either increases glucose utilization and transport or suppresses gluconeogenesis gene expression or lipolysis and promotes glycogen synthesis (Hatting et al. 2018). The loss of sensitivity to insulin or insulin resistance is the most common manifestation of T2DM (Sathibabu Uddandrao et al. 2019). In the current study, biochanin A–treated diabetic rats exhibited lower plasma glucose levels and insulin resistance and higher glucose tolerance and insulin levels compared to the diabetic controls. These results indicated that the effect of biochanin A is comparable to that of gliclazide in lowering glucose levels and insulin resistance. Moreover, the findings corroborate those of Oza and Kulkarni (2018) who reported that a high dose of biochanin A (40 mg/kg) markedly reduced insulin resistance and increased glucose tolerance, in addition to increasing SIRT1 expression in pancreatic tissues, in diabetic rats. Future studies need to elucidate the underlying mechanism by which biochanin A increases insulin secretion to reduce plasma glucose levels and increase glucose tolerance and to determine the optimum biochanin A dose.
Uncontrolled DM is characterized by persistent hyperglycemia, which leads to protein glycation (non-enzymatic glycosylation) (Saravanan et al. 2014). During DM, the glycation of HB is possible due to overindulgence of glucose presence in the blood that reacts with hemoglobin. Previous studies have shown that glycation rate is associated with blood glucose levels (Harini et al. 2012). HbA1c accounts for 3.4 to 5.8% of total hemoglobin in normal individuals. However, in individuals with hyperglycemia, HbA1c levels increase significantly. A previous study showed that HbA1c increased up to 16% in diabetic patients and that HbA1c level can be used as a reliable glycemic index (Sherwani et al. 2016). Increased protein glycosylation is one of the consequences of insulin insufficiency, which leads to downregulation of Hb and upregulation of glycosylated Hb. According to the International Expert Committee and American Diabetes Association, the GHb levels of ≥ 48 nmol/mol and 39–46 nmol/mol are indicative of DM and prediabetes, respectively (Cho et al. 2018). In this study, diabetic rats exhibited higher GHb levels, which suggested poor glycemic regulation in these rats. HFD-STZ-fed rats treated with biochanin A or glyclazide showed a marked reduction in the GHb levels, which may be attributed to the antihyperglycemic effect of biochanin A. These results corroborated with the results of previous studies (Harini et al. 2012; Arjunan et al. 2020).
Impaired insulin secretion or action or both are also linked to imbalances in carbohydrate metabolism, leading to inadequate glucose utilization, eventually resulting in hyperglycemia. Therefore, to strengthen the finding of the current study regarding biochanin A efficacy, we determined the levels of the key carbohydrate-metabolizing enzymes in the treated and untreated control groups. Endogenous glucose production occurs mainly through two pathways: gluconeogenesis and glycogenolysis (McManus et al. 2005). The co-ordination between these two pathways and the activities of glycolytic and gluconeogenic enzymes are crucial for glucose homeostasis regulation (Sullivan and Forbes, 2019). Here, we assessed the levels of a glycolytic enzyme (hexokinase), two gluconeogenic enzymes (glucose-6-phosphatase and fructose-1,6-bisphosphatase), and glycogen metabolic enzymes in all groups. Hexokinase activity is impaired in DM patients possibly due to insulin insufficiency (Saravanan et al. 2009). On the contrary, DM patients exhibit increased activities of fructose-1,6-bisphosphatase and glucose phosphatase due to impaired insulin secretion or action. Fructose-1,6-bisphosphatase catalyzes an irreversible and rate-limiting step (fructose-1,6-bisphospate➔fructose) and maintains glucose homeostasis in the liver and kidney (Ramalingam et al. 2020). Studies have suggested that the application of fructose-1,6-bisphosphatase inhibitors can be used as a strategy to control blood sugar in DM (Kaur et al. 2017) because it affects gluconeogenesis without affecting glycolysis. The other enzyme glucose phosphatase, which hydrolyzes glucose-6-phosphate, stimulates glucose release from the tissues. The increased activity of these two enzymes in diabetes signifies that targeting these enzymes could be valuable in DM treatment (Saravanan et al. 2014). In this study, diabetic rats exhibited higher glucose phosphatase and fructose-1,6-bisphosphatase levels and lower levels of hexokinase than the normal control. Biochanin A treatment significantly restored the activities of these enzymes, which might be due to the inhibition of ATP-sensitive K+ channels and enhanced insulin release from remnant β-cells. Our results corroborated those of a previous study (Harini et al. 2012; Ramalingam et al. 2020).
Insulin not only plays a role in glucose uptake in tissues, but specifically stimulates glycogen synthesis by activating glycogen synthase and suppressing glycogen phosphorylase (Saravanan et al. 2014). Glycogen synthase removed non-oxidative glucose in the tissues by transferring glucose from UDP-glucose to glycogen. Glycogen phosphorylase separates glucose molecules by cleaving a (1–4) linkage of glycogen (McManus et al. 2005). In the current study, diabetic rats exhibited lower hepatic and muscle glycogen levels and higher renal glycogen levels. This finding may be attributed to altered activities of glycogen phosphorylase and synthase in muscle and liver of the diabetic rats, which, in turn, may be attributed to insulin deficiency, as they depend on insulin for influx of glucose. Furthermore, the elevated glycogen levels in the kidney of diabetic rats may be attributed to higher glucose influx during hyperglycemia, as renal tissue is independent of insulin action (Sullivan and Forbes, 2019). Earlier, Harini et al. (2012) reported in their study that biochanin A has a significant antihyperglycemic efficacy potential at a dose of 10 mg/kg bodyweight in STZ alone induced diabetic rats. In this study, the authors reported that biochanin A attenuates DM by enhancing glycolytic enzymes and controlling glucose metabolism (Harini et al. 2012). Similarly, our study also found the significant antidiabetic potential of biochanin A in STZ-HFD-induced diabetic rats. However, the difference between these two studies is that STZ alone can lead to type 1 DM but in addition to STZ, supplementation of HFD can help to develop type 2 DM characteristics such as insulin resistance, altered lipid profile, and other effects of hyperglycemia (Magalhães et al. 2019).Our results exemplified that biochanin A administration enhances insulin release from pancreatic β-cells and alters glycogen levels in diabetic rats by modulating the activities of glycogen metabolic enzymes. However, further animal studies are warranted to ascertain the potential of biochanin A in modulating the activity of carbohydrate-metabolizing enzymes and better understand its mechanism of action.
Conclusion
Overall, our results suggested that biochanin A supplementation restores the plasma levels of glucose and insulin, and the activities of key enzymes involved in carbohydrate metabolism in HFD-STZ-induced diabetic rats by altering insulin secretion from pancreatic beta cells. This leads to enhanced glycolysis and reduced gluconeogenesis, resulting in alleviation of hyperglycemia.
References
Arjunan S, Thangaiyan R, Balaraman D (2020) Biochanin A, a soy isoflavone, diminishes insulin resistance by modulating insulin-signalling pathway in high-fat diet-induced diabetic mice. Arch Physiol Biochem:1–7. https://doi.org/10.1080/13813455.2020.1820525
Balaji M, Brahmanaidu P, Venkata Rao C (2017) Antiobesity potential of piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab 14:72. https://doi.org/10.1186/s12986-017-0228-9
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
Cornblath M, Randle PJ, Parmeggiani A, Morgan HE (1963) Regulation of glycogenolysis in muscle. Effects of glucagon and anoxia on lactate production, glycogen content, and phosphorylase activity in the perfused isolated rat heart. J Biol Chem 238:1592–1597
Gancedo JM, Gancedo C (1971) Fructose-1,6-bis-phosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting yeast. Arch Microbiol 76:132–138. https://doi.org/10.1007/BF00411787
Gothandam K, Ganesan VS, Ayyasamy T, Ramalingam S (2019) Protective effect of theaflavin on glycoprotein components and TCA cycle enzymes in high-fat diet and streptozotocin-induced diabetic rats. J Basic Appl Zool 80:43. https://doi.org/10.1186/s41936-019-0115-1
Harini R, Ezhumalai M, Pugalendi KV (2012) Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur J Pharmacol 676:89–94. https://doi.org/10.1016/j.ejphar.2011.11.051
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P (2018) Insulin regulation of gluconeogenesis. Ann N Y Acad Sci 1411:21–35. https://doi.org/10.1111/nyas.13435
Idu M, Obaseki O, Gabriel BO (2021) Anti-diabetic effect of Stachytarpheta jamaicensis on low dose streptozotocin induced diabetic rats fed on a high-fat diet. Clin Phytoscience 7:95–101. https://doi.org/10.1186/s40816-021-00326-9
Kaur R, Dahiya L, Kumar M (2017) Fructose-1,6-bisphosphatase inhibitors: a new valid approach for management of type 2 diabetes mellitus. Eur J Med Chem 141:473–505. https://doi.org/10.1016/j.ejmech.2017.09.029
Kinney LaPier TL, Rodnick KJ (2001) Effects of aerobic exercise on energy metabolism in the hypertensive rat heart. Phys Ther 81:1006–1017. https://doi.org/10.1093/ptj/81.4.1006
Ko WC, Lin LH, Shen HY, Lai CY, Chen CM, Shih CH (2011) Biochanin A, a phytoestrogenic isoflavone with selective inhibition of phosphodiesterase 4, suppresses ovalbumin-induced airway hyperresponsiveness. Evid Based Complement Alternat Med 2011:635058. https://doi.org/10.1155/2011/635058
Koida H, Oda T (1959) Pathological occurrence of glucose-6-phosphatase in liver disease. Clin Chim Acta 4:554–561. https://doi.org/10.1016/0009-8981(59)90165-2
Kotturu SK, Uddandrao VVS, Ghosh S, Parim B (2021) Bioactive compounds in diabetic cardiomyopathy: current approaches and potential diagnostic and therapeutic targets. Cardiovasc Hematol Agents Med Chem 19:118–130. https://doi.org/10.2174/1871525718666200421114801
Leloir L, Goldenber SH (1962) Glycogen synthase from rat liver. In: Kolowick SP, Kaplan OP (eds) Methods of Enzymology. Academic Press, New York, pp 145–148
Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos M, Kawashita NH, França SA (2019) High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad Bras Cienc 91:e20180314. https://doi.org/10.1590/0001-3765201920180314
McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24:1571–1583. https://doi.org/10.1038/sj.emboj.7600633
Ong KC, Khoo HE (2000) Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci 67:1695–1705. https://doi.org/10.1016/s0024-3205(00)00758-x
Oza MJ, Kulkarni YA (2018) Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed Pharmacother 107:1119–1127. https://doi.org/10.1016/j.biopha.2018.08.073
Parim BN, Ponmurugan P, Saravanan G (2015) Diosgenin reorganises hyperglycaemia and distorted tissue lipid profile in high-fat diet–streptozotocin-induced diabetic rats. J Sci Food Agric 95:3177–3182. https://doi.org/10.1002/jsfa.7057
Ramalingam S, Karuppiah M, Thiruppathi M, Palanivelu S, Panchanatham S (2020) Antioxidant potential of biflavonoid attenuates hyperglycemia by modulating the carbohydrate metabolic enzymes in high fat diet/streptozotocin induced diabetic rats. Redox Rep 25:1–10. https://doi.org/10.1080/13510002.2020.1722914
Rani AJI, Uddandrao VVS, Sangeethadevi G, Saravanan G, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Sethumathi PP, Vadivukkarasi S (2021) Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Arch Physiol Biochem:1–16. https://doi.org/10.1080/13813455.2021.1874017
Rathinasamy JIRA, Uddandrao VVS, Raveendran N, Sasikumar V (2020) Antiobesity effect of biochanin-A: effect on trace element metabolism in high fat diet-induced obesity in rats. Cardiovasc Hematol Agents Med Chem 18:21–30. https://doi.org/10.2174/1871524920666200207101920
Sangeethadevi G, VVS U, Jansy Isabella R, Saravanan G, Ponmurugan P, Chandrasekaran P, Sengottuvelu S, Vadivukkarasi S (2021) Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug Chem Toxicol:1–12. https://doi.org/10.1080/01480545.2021.1894707
Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B (2014) Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Can J Diabetes 38:409–414. https://doi.org/10.1016/j.jcjd.2014.02.004
Saravanan G, Ponmurugan P, Senthilkumar GP (2009) Modulatory effect of S-allylcysteine on glucose metabolism in streptozotocin induced diabetic rats. J Funct Foods 1:336–340. https://doi.org/10.1016/j.jff.2009.09.001
Sathibabu Uddandrao VV, Brahmanaidu P, Ravindarnaik R, Suresh P, Vadivukkarasi S, Saravanan G (2019) Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. Eur J Nutr 58:2425–2437. https://doi.org/10.1007/s00394-018-1795-x
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK (2016) Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights 11:95–104. https://doi.org/10.4137/BMI.S38440
Sullivan MA, Forbes JM (2019) Glucose and glycogen in the diabetic kidney: heroes or villains? EBioMedicine 47:590–597. https://doi.org/10.1016/j.ebiom.2019.07.067
Uddandrao V, Parim B, Singaravel S, Ponnusamy P, Ponnusamy C, Sasikumar V, Saravanan G (2022) Polyherbal formulation ameliorates diabetic cardiomyopathy through attenuation of cardiac inflammation and oxidative stress via NF-κB/Nrf-2/HO-1 pathway in diabetic rats. J Cardiovasc Pharmacol 79:e75–e86. https://doi.org/10.1097/FJC.0000000000001167
Uddandrao V, Rameshreddy P, Brahmanaidu P, Ponnusamy P, Balakrishnan S, Ramavat RN, Swapna K, Pothani S, Nemani H, Meriga B, Vadivukkarasi S, RN P, Ganapathy S (2020b) Antiobesity efficacy of asiatic acid: down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Arch Physiol Biochem 126:453–462. https://doi.org/10.1080/13813455.2018.1555668
Uddandrao VVS, Brahmanaidu P, Ganapathy S (2020a) Evaluation of the antioxidant and antidiabetic potential of the poly herbal formulation: identification of bioactive factors. Cardiovasc Hematol Agents Med Chem 18:111–123. https://doi.org/10.2174/1871525718666200207103238
Yu C, Zhang P, Lou L, Wang Y (2019) Perspectives regarding the role of biochanin A in humans. Front Pharmacol 10:793. https://doi.org/10.3389/fphar.2019.00793
Acknowledgements
The authors thank the management of K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, Namakkal District, Tamil Nadu, India, for providing necessary facilities to carry out this study.
Author information
Authors and Affiliations
Contributions
All authors have a significant contribution to the study design and experiments. SPP carried out animal experiments and biochemical analysis and drafted the manuscript. VVSU carried out the animal experiments and assay of biochemical parameters, analyzed the data, and drafted the manuscript. CP carried out the animal experiments. SS was involved in the animal experiments. TP carried out animal experiments. PP critically reviewed the manuscript. VS critically reviewed manuscript. MSB critically reviewed the manuscript. GS was responsible for the study design, supervised the experiments, and analyzed the data. All the authors are aware of the submission and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol of the study was approved by the Institute Animal Ethical Committee of Arulmigu Kalasalingam College of Pharmacy, Virudhunagar, Tamil Nadu, India (Approval No: AKCP/IAEC/83/20-21).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pudhupalayam, S.P., Uddandrao, V.V.S., Ponnusamy, C. et al. Biochanin A Attenuates Hyperglycemia in High-Fat Diet–Streptozotocin–Induced Diabetic Rats by Modulating the Activities of Carbohydrate-Metabolizing Enzymes in Vital Organs. Rev. Bras. Farmacogn. 32, 608–617 (2022). https://doi.org/10.1007/s43450-022-00280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00280-8