Abstract
Carnosic acid possesses antioxidant and anti-inflammatory effects. Early evidence indicates that this phenolic diterpenoid protects the heart against myocardial infarction, although the mechanism is unknown. For this research, male rats were divided into four groups: the control group, the myocardial infarction group (85 mg/kg, isoproterenol on the 19th and 20th days of experiment), and myocardial infarction groups pretreated with 10 and 20 mg/kg/bw of carnosic acid for 21 days. Following animal sacrifice, serum cardiac markers, cardiac oxidative stress, inflammation, and histological and molecular analysis were conducted. Pretreatment of carnosic acid to myocardial infarction–induced rats showed to reduce the ratio of cardiac weight/body weight ratio as well as the size of the infarction. Furthermore, serum cardiac biomarkers and lipid peroxidation were significantly reduced, but antioxidant enzymes were significantly increased in myocardial infarction–induced rats pretreated with carnosic acid. Carnosic acid significantly lowers cardiac inflammatory markers as well as improved histological abnormalities in isoproterenol-injected rats. Carnosic acid has been shown to efficiently reverse isoproterenol-induced myocardial infarction by lowering infraction size and decreasing oxidative stress and inflammation by activating Nrf2/HO-1 and TLR4/NF-κB signaling pathways.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a common disease associated with a high morbidity and mortality rate. MI is killing more people every day, according to the World Health Organization (WHO), and both men and women are equally at risk (Alonso et al. 2021). MI is caused by hypertension, excessive cholesterol and triacylglyceride levels, alcohol use, overweight, diabetic complications, smoking, and autoimmune diseases. Consequently, MI is one of the most prevalent and deadly forms of cardiovascular disease. There have been many pathways identified in the pathophysiology of MI, but their roles are unclear. An imbalance of oxidants and antioxidants in the myocardium, as well as the resulting inflammation and reduced cell viability, all have an impact on heart function (Kones 2013; Devi et al. 2021).
Oxidative stress alters molecular and cellular lever signaling networks, which plays a key role in cardiac disease as a consequence of an imbalance between reactive oxygen species (ROS) and antioxidants (Byrne et al. 2021). The antioxidant and anti-inflammatory effects are mediated by the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) protein. Nrf2 is a cardioprotective factor because it protects against maladaptive remodeling and decreased heart function. Nrf2 separated from Keap1 in cytoplasm and translocated into the nucleus in response to potential oxidative stress stimuli, resulting in transcriptional activation of phase II enzymes/antioxidant genes such as HO-1, glutathione S-transferase (GST), and glutamate cysteine ligase (GCL) (Bellezza et al. 2018).
Because of oxidative stress, the production of pro-inflammatory cytokines is increased (Devi et al. 2021). Inflammation has been found to impair heart repair, induce ischemic damage, and reshape the heart after myocardial infarction (Neri et al. 2015). TLR4 is a member of the transmembrane recognition protein family, which can attach to MYD88 that results in the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, and interleukin-6 (IL-6) and interleukin-1β (IL-1β). Additionally, nuclear factor-κB (NF-κB) is activated with encoding of pro-inflammatory genes (Su et al. 2018).
A variety of medicines have been utilized throughout history to address acute cardiac problems. While synthetic medicines have been shown to be effective in the treatment of MI and its symptoms, they are linked to serious adverse effects. A variety of plant chemicals are available or are undergoing clinical trials to determine their effectiveness against this disease. The proposed antioxidant therapy mechanism for phytochemical protection on endothelial cells, low-density lipoprotein, inflammation, and thrombosis is included to control the oxidative burden due to excessive formation of free radicals which causes damage to endothelial cells and leads to the expression of vascular adhesion molecule which ultimately recruits monocytes and hinders the protective role of nitric oxide and the peroxidation of low-density lipoproteins, causing inflammation and altogether leading to accumulation of lipids in the arteries and, ultimately, to the development of cardiovascular disease (Devi et al. 2021).
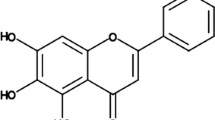
Carnosic acid (1) is a phenolic diterperne compound that is an abundant member of the mint family, Lamiaceae, such as sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.). Both plants are used in traditional medicine and culinary worldwide (Erkan et al. 2008). During the past decade, compound 1 has been found to have a wide range of bioactive properties, including antioxidant, anti-inflammatory, and anticancer activities (Lin et al. 2018). It was recently discovered that 1 functions as a regulator of Nrf2 (Cheng et al. 2021). Additionally, this phenolic diterpene decreased oxidative stress in acrylamide-induced damage (Albalawi et al. 2018). Compound 1 can also protect against ischemic/reperfusion damage in type 2 diabetes via regulating autophagy (Hu et al. 2019). Also, 1 in combination with carvedilol reduces doxorubicin-induced cardiotoxicity by lowering oxidative stress, inflammation, apoptosis, and autophagy (Zhang et al. 2019). However, few studies have been carried out to establish whether carnosic acid (1) has a therapeutic impact on cardiac protection. In the current study, a MI model was created via ISO injection to examine the effect of 1 on cardiac tissue and to determine whether Nrf2 activation might inhibit the TLR4/NF-B signaling pathway.

Materials and Methods
Experimental Animals
Male Wistar rats weighing 260 ± 20 g were kept in polyethylene cages, six to a cage, with free access to food and water ad libitum and maintained in cages with a 12-h light/dark cycle, a temperature of 22 ± 2 °C, and a moisture content of 60%.
Myocardial Infarction Induction
To produce MI, isoproterenol (ISO) was dissolved in normal saline and administered subcutaneously to rats (85 mg/kg bw) every 24 h for 2 days (Giribabu et al. 2016). Male Wistar rats were arbitrarily divided into four groups.
-
Group 1: NC (vehicle-normal saline)
-
Group 2: MI-induced rats (normal saline administered orally for 21 days; on day 19th and 20th, ISO was injected subcutaneously (100 mg/kg bw)
-
Groups 3 and 4: carnosic acid (1, CAS Number: 3650-09-7; purity ≥ 98%; ChemFaces, Wuhan Biochemical Co., Ltd., China) 10 and 20 mg/kg bw was given orally for 21 days, and on the 19th and 20th days, ISO (100 mg/kg bw) was injected subcutaneously.
After 24 h, following the second subcutaneous injection, all rats were anesthetized with thiopental sodium (30 mg/kg, i.p.). Blood was drawn into tubes with and without anticoagulant to obtain plasma and serum, respectively. Following sacrifice, the hearts were immediately excised and washed in ice-cold saline and they were weighed before being used in other studies. Triphenyltetrazolium chloride (TTC) was used to identify infarcted regions, and other portions were used for histopathological and molecular research.
Biochemical Assays
The serum cardiac markers such as lactate dehydrogenase (LDH; E-EL-R2547), troponin T (E-EL-R0151), and creatine kinase-MB (CK-MB; E-EL-R1327) were measured using ELISA kits (Elabscience, Wuhan, China). The heart homogenates were used to establish thiobarbituric acid responsive materials (TBARS) using the protocol described by Ohkawa et al. (1979); superoxide dismutase (SOD) was evaluated by using Misra and Fridovich (1972) procedures; catalase (CAT) was evaluated using the procedures described by Maehly and Chance (1954), and glutathione peroxidase (GPx) was evaluated with the Rotruck et al.’s (1973) methods.
Real-time RT-PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract the whole RNA from the heart tissue. A set of gene-specific primers (Table S1), a high-capacity RNA-to-cDNA kit, was used to make complementary DNA (cDNA) (Applied Biosystems, CA, USA), and SYBR™ Green PCR Master Mix (Vazyme, Biotech Co., Ltd., China) was used for PCR amplification. Real-time PCR instrument, iQ5 (Bio-Rad, Hercules, USA), was used for real-time analysis. The quantity of expression of messenger RNA (mRNA) was measured by using the delta-delta CT method. After normalization to the housekeeping gene, β-actin, the relative expression of each gene was determined. Three copies of each reaction were maintained to ensure that the findings could be replicated.
Histopathology and Immunohistochemistry
Cardiac Sects. (5 μm) were deparaffinized, rehydrated, and stained for 1 min with Mayer’s hematoxylin and eosin staining protocols. Immunohistochemical staining was performed according to previously described protocols (Adam et al. 2017).
Statistical Analysis
The data was expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used for the intergroup analysis, followed by Tukey’s post hoc tests which were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). When p < 0.05 was used, the results were considered statistically significant.
Results and Discussion
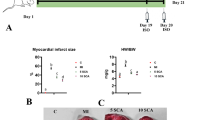
The development of novel therapeutic and preventive medications capable of effectively decreasing cardiomyocyte cellular damage is enabled by a greater understanding of the pathophysiology and biochemical processes underlying MI. In our present study, we looked at isoproterenol-mediated heart damage from a variety of perspectives, including pathologic and metabolic functions (Li et al. 2021). Isoproterenol-induced damage resulted in increased cellular infiltrations, increased water content, interstitial edema, and necrosis. Carnosic acid (1) suppressed cellular and interstitial processes that led in isoproterenol-induced alterations in heart mass. The ISO-treated rats showed a significantly (p < 0.01) higher heart weight to body weight ratio than the control group (Fig. 1A). Despite this, rats administered 10 and 20 mg/kg bw of 1 prior to MI induction exhibited a lower heart-to-body weight ratio than the MI control group. The infarction percentage of the normal control rats was negligible, while the infarction percentage of the ISO-injected MI-induced rats was significantly higher (p < 0.05) (Fig. 1B). When compared to the MI control group, pretreatment with 1 at 10 and 20 mg/kg bw significantly reduced (p < 0.05) the size of the myocardial infarction.
Effect of carnosic acid (1) on A heart/body weight ratio and B percentage of myocardial infarction. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
In the present study, the heart weight to body weight proportion increased significantly during ISO administration, which might be attributed to lymphocyte seepage and also water buildup, the formation of edematous intramuscular region, and cardiomyocyte fibrosis. Dizaji et al. (2021) discovered a similar pattern of results in ISO-injected rats by increasing heart/body weight ratio. Furthermore, pretreatment with 1 significantly reduced the ratio of heart weight/body weight in ISO-administered rats, indicating that this phenolic diterpene has a protective effect on myocardial damage in rats. Additionally, carnosic acid (1) protection was indicated by the decrease in the percentage of myocardial infarction in ISO-injected rats. The current findings are consistent with those of Hu et al. (2019) who discovered that CA protects type 2 diabetics (T2DM) against ischemic/reperfusion injury through an autophagy-mediated mechanism.
Figure 2 depicts serum cardiac markers such as CK-MB, troponins, and LDH in experimental rats. When compared to control rats, ISO-injected animals had significantly (p < 0.01) higher levels of CK-MB (Fig. 2A), troponins (Fig. 2B), and LDH (Fig. 2C). When ISO-injected rats were pretreated with 1 at 10 and 20 mg/kg bw, the serum cardiac markers were significantly reduced as compared with the MI control group. Myocardial necrosis occurs as a result of the release of cardiac marker enzymes such as CK-MB, LDH, and troponins, which is followed by changes in plasma membrane integrity, as seen in ISO-injected rats.
Effect of carnosic acid (1) on serum cardiac markers A CK-MB, B troponin, and C LDH. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
Hematoxylin and eosin staining of control rat heart sections showed normal histo-architecture and an intact myocardial cell membrane, whereas interstitial edema, occlusion, and inflammatory cell infiltration with broken myocardial fibers were seen histopathologically in the myocardium of ISP-injected rats. Carnosic acid (1) pretreated MI-induced rats at dosages of 10 and 20 mg/kg bw for 21 days revealed slightly separated myocardial fibers and a few disseminated inflammatory cells (Fig. 3). The deleterious effects of ISO were significantly reduced after pretreatment with 1; in addition, all marker enzymes were restored due to defense mechanism of 1 which may have a role in maintaining the cardiac membrane integrity and preventing heart enzyme leakage into the blood. The findings are consistent with earlier research investigations by Sahu et al. (2014) which show that compound 1 has a protective effect in reducing myocardial damage as shown by lower serum cardiac markers.
Effect of carnosic acid (1) on heart histopathological changes. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Magnification = 40 \(\times\); scale bar: 100 μm. Black arrow shows the structural changes in myocardial structure
mRNA levels of the nuclear factor erythroid 2-related factor (Nrf2; Fig. 4A), quinone acceptor oxidoreductase (NQO-1; Fig. 4C), and heme oxygenase-1 (HO-1; Fig. 4D) were downregulated significantly (p < 0.05) in MI-induced rat, while Kelch-like ECH-associated protein 1 (Keap-1; Fig. 4B) levels, a sensor for thiol-reactive chemopreventive compounds and oxidative stress, were upregulated as compared with normal control rats. However, rats were pretreated with 1 at 10 and 20 mg/kg bw, Nrf2, NQO-1, and HO-1 mRNA expression levels which were significantly increased with lower Keap-1 levels as compared to the MI control group. In rats with MI, lipid peroxidation products, e.g., malondialdehyde (MDA; Fig. 5A), were significantly elevated, while cardiac antioxidant enzymes such as superoxide dismutase (SOD; Fig. 5B), catalase (CAT; Fig. 5C), and glutathione peroxidase-1 (GPx; Fig. 5D) levels were significantly reduced (p < 0.05). However, rats pretreated with 1 at doses of 10 and 20 mg/kg bw for 21 days had shown reduction in MDA, as well as increase in the levels of cardiac antioxidants such as SOD, CAT, and GPx.
Effect of carnosic acid (1) on Nrf2/Keap-1 signaling pathways in the heart. mRNA levels of A Nrf2, B Keap1, C NQO-1, and D HO-1. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
Effect of carnosic acid (1) on oxidative stress and antioxidant enzymes in the heart. Lipid peroxidation product levels: A malondialdehyde (MDA), B superoxide dismutase (SOD), C catalase (CAT), D glutathione peroxidase (GPx). NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
ISO induces oxidative stress by generating reactive oxygen species, which results in lipid peroxidation and irreversible cell membrane damage as well as damage to intracellular macromolecules (DNA, proteins, membrane lipids), which leads to cell death or apoptosis. Numerous studies have shown that the Nrf2 signaling pathway is strongly related to oxidative stress. Nrf2, a redox-sensitive transcription factor that may link with antioxidant reaction elements, controls the production of antioxidant genes as well as cleaning enzymes (cytoprotective stage II) (Chen and Maltagliati 2018). Severe oxidative stress consumes a large amount of Nrf2, interfering with homeostasis. In our present study, Nrf2, NQO-1, and HO-1 mRNA levels also decreased in ISO-injected rats. The equilibrium between complimentary radicals and chemical antioxidants is a critical process for the effective management of oxidative stress. According to recent research, carnosic acid (1) activates the Nrf2 transcription factor, causing the overexpression of cytoprotective stage I and II enzymes (Mimura et al. 2011). Compound 1 also protects the brain from cyanide-induced damage by activating the Nrf2 transcription factor (Zhang et al. 2015). Furthermore, sulforaphane and carnosic acid supplementation mitigates the effects of 4-hydroxy-2-nonenal-induced mitochondrial dysfunction. Moreover, free radical–scavenging antioxidant enzymes, including SOD, CAT, and GPx, are the first line of defense against oxidative damage by breaking down superoxide radicals (O2−) and hydrogen peroxidase (H2O2) prior to their interaction to create hydroxyl radical, which is very reactive. In our study, antioxidant enzymes were decreased in ISO-injected rats heart (Zhang et al. 2019).
As demonstrated in Fig. 6, mRNA levels of Toll-like receptor 4 (Tlr-4; Fig. 6A); nuclear factor kappa B subunit 1 (Nfkβ1; Fig. 6B); and the pro-inflammatory cytokine, interleukin 6 (Il-6; Fig. 6C) and Il-1β (Fig. 6D), significantly increased (p < 0.05) in MI-induced rats. After 21 days of pretreatment with carnosic acid (1) at dosages of 10 and 20 mg/kg bw, Tlr-4, Nfkb1, Il-6, and Il-1β mRNA levels were significantly reduced as compared to the MI control group. According to ELISA results, Tlr-4 (Fig. 7A), NFKβ-p65 (Fig. 7B), TNF-α (Fig. 7C), and IL-1β (Fig. 7D) levels were similarly significantly (p < 0.05) elevated in MI-induced rats compared to those in normal control rats. When compared to the MI control group, TLR-4, NFKβ-p65, TNF-α, and IL-1β levels were significantly lower following 21 days of pretreatment with 1 at dosages of 10 and 20 mg/kg bw. Immunohistochemistry results confirmed that Tlr4 (Fig. 8) and Nfkβ-p65 (Fig. 9) protein distribution was higher in MI-induced rats as compared to that in normal control rats. However, MI rats pretreated with CA at doses of 10 and 20 mg/kg bw for 21 days had lower Tlr4 and Nfkβ-p65 protein distribution than MI-induced rats. On the other hand, oxidative stress may cause inflammation. TLRs are often connected to the adaptor particle MyD88, which activates NF-κB and, in turn, increases inflammatory gene transcriptions (TNF-α, IL-1β, and IL-6) that are known to cause inflammation (Su et al. 2019). Furthermore, ISO may also induce a rise in the inflammatory and pro-inflammatory cytokines, which is consistent with previous results (Jin et al. 2020). Additionally, when rats received carnosic acid (1), an anti-inflammatory effect that could protect the cardiac cells from being damaged was observed. The levels of these cytokines were substantially decreased in the heart cell homogenate (Kocak et al. 2016).
Effect of carnosic acid (1) on TLR4/NFKβ p65 pathway in the heart. mRNA levels of A Tlr4, B Nfkb1, C Il-6, and D Il-1β. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
Effect of carnosic acid (1) on TLR4/NFKβ p65 pathway in the heart. A TLR4. B NFKβ p65. C TNF-α. D IL-1β. NC, normal control; ISO, MI-induced group; ISO + 10 CA: 10 mg/kg/bw of carnosic acid pretreated to MI-induced rats; ISO + 20 CA: 20 mg/kg/bw of carnosic acid pretreated to MI-induced rats. Data show the mean ± SD (n = 6). *p < 0.05: significant difference from control rats. #p < 0.05: significant difference from MI-induced rats
Effect of carnosic acid (1) on immunohistochemistry of TLR4 in the heart. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid pretreated to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid to MI-induced rats. Magnification = 40 \(\times\); scale bar: 100 μm. Black arrow shows the expression of protein in brown color
Effect of carnosic acid (1) on immunohistochemistry of NF-κB p65 in the heart. NC, normal control; ISO, MI-induced group; ISO + 10 mg/kg/bw of carnosic acid to MI-induced rats; ISO + 20 mg/kg/bw of carnosic acid pretreated to MI-induced rats. Magnification = 40 \(\times\); scale bar: 100 μm. Black arrow shows the expression of protein in brown color
Conclusion
Our findings showed that carnosic acid (1) protects against ISO-induced myocardial infarction in rats. The cardioprotective effect may be attributed to its capacity to relieve cardiac marker enzymes and antioxidants, and to preserve the architecture of the heart. Consequently, it is possible that carnosic acid might be used as an encouraging cardioprotective drug to treat myocardial infarction.
Protection of Human and Animal Subjects
The authors declare that no experiments were performed on humans. All animal experiments were approved and conducted in compliance with Institutional Animal Care Guidelines. The study protocol was approved by the Hongze District People’s Hospital Ethics Committee in Jiangsu, China (No. 2021006767).
Conflict of Interest
The authors declare no competing interests.
References
Adam SH, Giribabu N, Bakar NMA, Salleh N (2017) Marantodes pumilum (Kacip fatimah) enhances in-vitro glucose uptake in 3T3-L1 adipocyte cells and reduces pancreatic complications in streptozotocin-nicotinamide induced male diabetic rats. Biomed Pharmacother 96:716–726. https://doi.org/10.1016/j.biopha.2017.10.042
Albalawi A, Alhasani RHA, Biswas L, Reilly J, Akhtar S, Shu X (2018) Carnosic acid attenuates acrylamide-induced retinal toxicity in zebrafish embryos. Exp Eye Res 175:103–114. https://doi.org/10.1016/j.exer.2018.06.018
Alonso A, Aparicio FHJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson FAP, Chamberlain FAM, Kissela BM, Knutson FKL, Lee CD (2021) Heart disease and stroke statistics-2021 update. Circulation 143:e254–e743. https://doi.org/10.1161/CIR.0000000000000950
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 1865:721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Byrne NJ, Rajasekaran NS, Abel ED, Bugger H (2021) Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med 169:317–342. https://doi.org/10.1016/j.freeradbiomed.2021.03.046
Chen QM, Maltagliati AJ (2018) Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics 50:77–97. https://doi.org/10.1152/physiolgenomics.00041.2017
Cheng J, Xu T, Xun C, Guo H, Cao R, Gao S, Sheng W (2021) Carnosic acid protects against ferroptosis in PC12 cells exposed to erastin through activation of Nrf2 pathway. Life Sci 266:118905. https://doi.org/10.1016/j.lfs.2020.118905
Devi A, Dwibedi V, Khan ZA (2021) Natural antioxidants in new age-related diseases. Rev Bras Farmacogn 31:387–407. https://doi.org/10.1007/s43450-021-00175-0
Dizaji NM, Garjani A, Mousavi S, Mohammadi M, Vaez H (2021) Time-dependent influence of infliximab on hemodynamic responses and cardiac injuries of isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol 903:174122. https://doi.org/10.1016/j.ejphar.2021.174122
Erkan N, Ayranci G, Ayranci E (2008) Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110:76–82. https://doi.org/10.1016/j.foodchem.2008.01.058
Giribabu N, Roslan J, Rekha SS, Salleh N (2016) Methanolic seed extract of Vitis vinifera ameliorates oxidative stress, inflammation and ATPase dysfunction in infarcted and non-infarcted heart of streptozotocin–nicotinamide induced male diabetic rats. Int J Cardiol 222:850–865. https://doi.org/10.1016/j.ijcard.2016.07.250
Hu M, Li T, Bo Z, Xiang F (2019) The protective role of carnosic acid in ischemic/reperfusion injury through regulation of autophagy under T2DM. Exp Biol Med 244:602–611. https://doi.org/10.1177/1535370219840987
Jin W, Zhang Y, Xue Y, Han X, Zhang X, Ma Z, Sun S, Chu X, Cheng J, Guan S (2020) Crocin attenuates isoprenaline-induced myocardial fibrosis by targeting TLR4/NF-κB signaling: connecting oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol 393:13–23. https://doi.org/10.1007/s00210-019-01704-4
Kocak C, Kocak FE, Akcilar R, Isiklar OO, Kocak H, Bayat Z, Simsek H, Taser F, Altuntas I (2016) Molecular and biochemical evidence on the protective effects of embelin and carnosic acid in isoproterenol-induced acute myocardial injury in rats. Life Sci 147:15–23. https://doi.org/10.1016/j.lfs.2016.01.038
Kones R (2013) Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag 9:617–670. https://doi.org/10.2147/VHRM.S37119
Li J, Thangaiyan R, Govindasamy K, Wei J (2021) Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum Exp Toxicol 4:915–927. https://doi.org/10.1177/0960327120975131
Lin KI, Lin CC, Kuo SM, Lai JC, Wang YQ, You HL, Hsu ML, Chen CH, Shiu LY (2018) Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Biosci Rep 38:BSR20180005. https://doi.org/10.1042/BSR20180005
Maehly A, Chance B (1954) Catalases and peroxidases. Methods Biochem Anal 1:357–424. https://doi.org/10.1002/9780470110171.ch14
Mimura J, Kosaka K, Maruyama A, Satoh T, Harada N, Yoshida H, Satoh K, Yamamoto M, Itoh K (2011) Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J Biochem 150:209–217. https://doi.org/10.1093/jb/mvr065
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D (2015) Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol 13:26–36. https://doi.org/10.2174/15701611113119990003
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Rotruck J, Pope A, Ganther HE, Swanson A, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Sahu BD, Putcha UK, Kuncha M, Rachamalla SS, Sistla R (2014) Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol Cell Biochem 394:163–176. https://doi.org/10.1007/s11010-014-2092-5
Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J (2018) Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem 47:1497–1508. https://doi.org/10.1159/000490866
Su S, Zhang P, Zhang Q, Yin Z (2019) GSK-3β inhibitor induces expression of the TLR4/MyD88/NF-κB signaling pathway to protect against renal ischemia-reperfusion injury during rat kidney transplantation. Inflammation 42:2105–2118. https://doi.org/10.1007/s10753-019-01074-2
Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, Sanz-Blasco S, Newmeyer T, Ambasudhan R, McKercher SR (2015) Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem 133:898–908. https://doi.org/10.1111/jnc.13074
Zhang QL, Yang JJ, Zhang HS (2019) Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed Pharmacother 109:71–83. https://doi.org/10.1016/j.biopha.2018.07.037
Acknowledgements
We are grateful to HOD of the Department Cardiology, Hongze District People’s Hospital, for help in technical and instrumental support.
Author information
Authors and Affiliations
Contributions
RN carried out the animal experiments and wrote the manuscript first draft. XD carried out the qPCR, histopathology, and statistical analysis. QW carried out immunohistochemistry and preparation of figures. YG carried out experimental design, funding, and editing the manuscript. All authors have contributed to the writing of the manuscript and approved the final submission.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ning, R., Deng, X., Wang, Q. et al. Carnosic Acid Protects Against Myocardial Infarction by Controlling Oxidative Stress and Inflammation in Rats. Rev. Bras. Farmacogn. 31, 794–804 (2021). https://doi.org/10.1007/s43450-021-00216-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-021-00216-8