Abstract
Implementation of Pharmacovigilance (PV) systems in resource-limited countries is a real challenge. The objective of this paper is to describe the implementation of an effective national PV system in Lebanon in the context of COVID-19, within a limited resources setting and with the absence of a guaranteed funding. In 2018, the PV center hosted at the Lebanese University, Faculty of Pharmacy under the supervision of the Quality Assurance of Pharmaceutical Products Program within the Lebanese Ministry of Public Health became an associate member of the World Health Organization (WHO) Program of International Drug Monitoring and recognized as Full member in 2021.This analysis highlights the requirements of the WHO that were met in Lebanon to create an effective PV system. The Lebanese experience shows that it is not only possible, but also crucial to implement a PV system in low to middle-income countries with limited resources and with the absence of a guaranteed funding in order to be able to promote patients’ safety. Support from organizations like WHO and World Bank, skilled leadership, hard work and dedicated staff with efficient training, and finally mass awareness initiatives were all considered as key elements to implement a successful PV System. In the midst of a turbulent political, economic and health context, Lebanon has been able to develop one of the most active and rapidly evolving PV systems in the Middle East.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicines and vaccines have transformed the manner in which diseases are prevented and handled. Despite their benefits, the use of medical products may sometimes be associated with unexpected effects. These effects may be unfavorable, ranging in severity, seriousness, and frequency within the intended population [1]. Although medicines and vaccines are assessed in controlled clinical trials and reviewed by regulatory authorities, some Adverse Events (AEs) remain unknown until the product is authorized by regulators and are used by a larger population in ‘real world conditions’, including special populations such as children, pregnant women and elderly [2]. It is therefore crucial that medicinal products continue to be observed for their effectiveness and safety post-marketing. In effect, this means having in place a well-functioning Pharmacovigilance (PV) system [2].
Drug safety and PV remain a vital clinical and scientific research area. PV systems monitor the safe and effective use of medicines, vaccines and other pharmaceutical products and devices. According to the World Health Organization (WHO) definition, PV is the “science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problems” [3]. On the report of the Council for International Organizations of Medical Sciences (CIOMS)/WHO Working Group on Vaccine PV, Vaccine PV is defined as “the science and activities relating to the detection, assessment, understanding and communication of Adverse Events Following Immunization (AEFI) and other vaccine- or immunization- related issues, and to the prevention of untoward effects of the vaccine or immunization” [3].
A well-shaped PV system aims to detect, assess, understand, and prevent AEs/AEFIs or any other possible medicinal product-related problem through generating signals, communicating risks and supporting decision making to minimize risk at various levels of social healthcare environment [3]. PV systems have been implemented by numerous countries worldwide. These systems may differ from fundamental to well established, with several countries just in the process of developing their PV systems. In the USA, the PV system is coordinated by the Food and Drug Administration: it was the first country to have determined drug safety regulations and has one of the most diligent legislative regimes on the safety of medicinal products [4]. In addition, The USA’s PV system is based on passive and active vigilance activities [4]. In the European Union (EU), the European Medicines Agency (EMA) regulates the European PV system and issues information on the safe and productive use of medicines and vaccines. Each member state of the EU has its own national regulatory agency which establishes its PV standards at national level within the framework of the EMA and European law. EMA also controls and supports EudraVigilance, the European system for managing, evaluating and investigating information on suspected Adverse Drug Reactions (ADR) associated with medicinal product authorized in the European Economic Area [5].
In the last 20 years, several low- and middle-income countries have created national PV systems and joined the WHO’s global PV network who has a critical role in supporting and coordinating these developments. WHO and its Program for International Drug Monitoring (PIDM) has played a key role in triggering and supporting PV in low middle-income countries [6, 7]. While policy, strategic development and coordination of the program are managed by WHO headquarters in Geneva, since 1978, the everyday technical operations have been hold by the WHO collaborating center for International Drug Monitoring in Uppsala, Sweden (UMC) [8]. UMC maintains the global Individual Case Safety Report (ICSR) database, VigiBase, that has at present 30 million ADR reports from 172 countries (149 Full Members that share ADR reports and 23 Associate Members) [6]. The UMC has built standardized reporting by all National Centers and has enabled communication between countries to endorse rapid identification of signals. The national centers are assigned by the governments of each of the countries participating in the WHO program. These centers are responsible for collecting spontaneous may be created gradually based on priorities and accessible resources of the country [9]. The Arab World is undergoing a remarkable activity with regards to PV [10]. However, like other developing countries, some of these countries differ significantly in their PV systems’ development level, ranging from those having either frail or non-existent systems to others having systems as advanced as those in developed countries [11]. In Lebanon, one of the core functions of the Ministry of Public Health (MoPH) is to safeguard the quality, safety and efficacy of medical products at the national level. The quality and safety tasks are carried out by the Quality Assurance of Pharmaceutical Products Program (QAPPP) at the MoPH. In the previous years, Lebanese national legislative bodies and national regulatory authorities had issued a significant amount of legislation and guidance to provide a legal foundation and practical implementation guidance for a Lebanese national PV system, appointing the Lebanese University as National PV Center. Despite all, the system was not active. In 2018, the QAPPP took the initiative to implement a sound and safe PV system.
Objective
The objective of this paper is to describe the implementation of an effective national PV system in Lebanon in the context of COVID-19, within a limited resources setting and with the absence of a guaranteed funding.
Evidence Acquisition
The Road Map for Implementing a PV Program
Given its commitment to strengthen PV in Lebanon, the QAPPP requested the WHO country office in Beirut and WHO Geneva Headquarters’ assistance for the technical assessment of the national PV activities. The WHO in turn responded by organizing an onsite visit to Beirut on 2019. The purpose of the visit was to conduct technical assessment with the view to advocating the importance of PV to senior government officials and decision makers, and to assess the current PV system in order to be able to propose a PV development plan. The technical assessment was conducted at the Faculty of Pharmacy at the Lebanese University, where the PV center is located, and at the Ministry of Public Health where the QAPPP is established. This separation between a center location at an educational facility and remote management by the MoPH has been supported by Sten Olsson et al., believed that because of a lack of political stability and good governance in some low middle-income countries it may be favorable to locate the coordination of a PV system away from Ministry of Health or equivalent and run the PV center, for example, in a university or hospital environment where effects of political fluctuations are often less dramatic [8].
The WHO delegates shared an overall view of the assessment and provided suggestions to draft a plan of action that could be developed by the QAPPP within the Lebanese MoPH. The plan of action worked on five main axes namely: Advocacy for PV, national policy, legal provisions, strengthening reporting processes and supporting PV infrastructure. Accordingly, the QAPPP succeeded in organizing the PV system infrastructure and designed the steps described below.
First, the Lebanese MoPH decided to review its already established regulations in order to be able to identify the gap and reinforce the PV legislation at a later stage. Consequently, the QAPPP contributed to the issuance of regulation including: Resolution No.1445 (2019) that appoints the head of QAPPP as PV coordinator between the MoPH, WHO and Lebanese National Pharmacovigilance Center (LNPVC) [12], Resolution No. 427/1 (2020) that describes the procedure for Reporting Adverse Drug Reactions related to COVID-19 treatments [13], Resolution No. 556/1 (2020) that states the procedure for reporting AEs related to the COVID-19 treatment by the responsible parties of Pharmaceutical Products and Drug Distributors [14], resolution No.180/1 (2021) that states the procedure for Spontaneous Reporting of Drugs AEs and AEFIs by the responsible parties [15] and finally resolution No.181/1 (2021) that states the procedure for Spontaneous Reporting of Drugs AEs and AEFIs by the Marketing Authorization Holders (MAHs) [16] (Fig. 1).
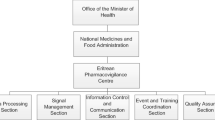
Once the legislation part was completed, the QAPPP established a PV framework to outline the relationship, role and responsibilities with other stakeholders in the system (Fig. 2).
Standardization, partnership and communication among different PV stakeholders helped to achieve the best possible health outcomes. The PV stakeholders mainly include policy makers (QAPPP from MoPH, Technical committee of Drugs from MoPH, PV committee from MoPH and WHO PIDM), the LNPVC which runs PV activities and operations in collaboration with UMC, and finally the reporters (multinational companies, local manufacture of drugs, importer of medicines, Healthcare Professionals (HCPSs), Academia, Public health programs, Primary healthcare centers, general public and patients).
As a second step, the QAPPP developed a Quality Management System and Guidelines on Good PV Practices at LNPVC based on an extensive literature review [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. All the PV activities at the center were precisely described and the related Standard of Operating Procedures of each activity were developed. Finally, setting up the PV System required qualified personnel. The WHO country office and the World Bank (WB) provided financial support for recruiting qualified and skilled staff members (Fig. 3).
Results
Once the legal framework, infrastructure and human resources were set in place, the QAPPP started developing reporting means for AEs/AEFIs. The National AEs/AEFIs Reporting Form was designed, piloted among selected HCPS, and adopted. The reporting forms were made available in both hard and soft copies in addition to an online reporting via the MoPH. Moreover, an existing hotline was made available for those wishing to report through the phone.
Furthermore, other means of reporting were developed, including but not limited to e-reporting (which is a standardized online ADR reporting form that was developed by the UMC in order to facilitate electronic reporting from patients and HCPS and XML files according to both ICH E2B (R2) and ICH E2B (R3) standards for MAHs to be able to import ICSR to the national database. In parallel, a safely stored, classified database which is retrievable and guarded by required degrees of confidentiality was adopted: VigiFlow [17]. All ADRs collected at the national level are analyzed, coded, assessed at the level of LNPVC/QAPPP and sent via VigiFlow to the Global database Vigibase. The proper training and continuous education of the LNPVC staff members were ensured by providing the needed learning material on data collection, cleaning, validation, processing entering, and coding of AEs/AEFIs based on Medical Dictionary for Regulatory Activities (MedDRA) [29]. Medicines and vaccines are coded according to WHO Drug Dictionary [27]. Training provided to the PV staff member covered different scopes and was conducted at introductory and intermediate levels. As an example; the WHO Drug basic introduction video [28], VigiFlow training [28], free Signal detection online course available on UMC website [29], and E-Learning Course on “Vaccines Safety Basics" [29] were all provided to the staff upon their enrollment. At a later Stage staff member joined an intermediate level training on « Covid-19 Vaccines Pharmacovigilance» organized by the Rabat Collaborating Center. Since the effectiveness of any national post-marketing surveillance program mainly relies on the active participation of HCPS, the last step was closing the loop with relevant national stakeholders. Consequently, a plan for training and initiation of collaboration was set. The PV System in Lebanon is comprehensive and includes many national and international stakeholders among which have patients and consumers.
HCPS are usually responsible for raising awareness about reporting of AEs/AEFIs among their patients, while patients or consumers may report any suspected AEs/AEFIs associated with the use of a medicinal product immediately to their HCPS or directly to LNPVC. Therefore, educating HCPS and MAHs on the importance of reporting was the next goal and for that reason, several capacity buildings were organized in this regard. These trainings targeted the HCPS, including medical doctors and pharmacists from different sectors, were provided through capacity buildings. The capacity buildings were initiated at an introductory level to introduce the PV system and its operation. At a later stage, an intermediated level training was delivered on “PV in the context of COVID-19 vaccines” targeting HCPS working in vaccination centers and health facilities with the aim to promote reporting. A more specific training was provided for medical doctors with the “Focus on AEFIs with COVID-19 Vaccines” [30]. Based on Sten Olsson et al., increased involvement of HCPS from public and private sectors, pharmaceutical companies, academic institutions and the public a large is necessary to assure a safe environment for drug and vaccine safety [8].
Discussion
Once the requirements for a functional PV system were met, the LNPVC was activated, the center started receiving ICSRs, as result Lebanon became a full Member of the WHO PIDM in February 2021. With the PV system put in place, the first aim was to enhance ADR reporting. Classical reporting forms were made available. These included hard copy of ADR reporting form, PV official email, and land line hotline. With the initiation of COVID -19 mass vaccination campaign in 2021, the challenge was an opportunity for the PV system to test its ability. Consequently, quick decisions were taken and actions were put in place to enhance AEFIs reporting. As a result, several new reporting means were created to facilitate the AEFIs reporting process. Vaccine recipients experiencing any AEFIs could report through one of the following newly developed means: 1214 Hotline call center, Impact Platform, Vaccination Sites/Hospital sites through a customized reporting tool (Kobo toolbox) and other sources including Preventive Medicine, Epidemiology Surveillance Program, Health Education and other departments from the MoPH. At the beginning of this reporting process, around 20 AEFI case reports were only received on weekly basis. This was acted upon by enhancing awareness on the importance of reporting among the consumers and HCPS. Awareness material was designed and distributed. Several capacity buildings focusing on how and why to enhance reporting were conducted. Direct contact with vaccination centers through WhatsApp group was created. Finally, on site field visits to random vaccination centers were performed. All of these efforts were successful in increasing the reporting of AEFI case reports to more than 100 reports on weekly basis within a period of couple of month. In parallel, the PV Program established a procedure for the management of reported AEFIs within the scope of the AEFI surveillance related to the available COVID-19 Vaccines in Lebanon. All received case reports were screened and validated for data completion. Incomplete or inconsistent case reports were followed up directly with the initial reporter. The case reports were classified as serious or non-serious cases based on WHO definition. The non-serious case reports were entered directly into VigiFlow. These reports were also followed up in conditions that may be related to serious underlying diseases. As for serious AEFI cases, these were also entered into VigiFlow after being thoroughly investigated by the PV team, analyzed, narrated with extensive literature search and assessed for causal relationship by both the PV team and the Serious AEFI Special Committee which was appointed in April 2021 through a Ministerial Decision #603/1 dated April 26, 2021 [31]. Until the date of this paper, more than 100 serious case reports have been thoroughly evaluated and assessed by the PV team. As a final step in strengthening AEFI surveillance system of the PV system, signal detection and validation was performed by adopting two sources for identifying signals that may be associated with AEFIs with Pfizer-BioNTech and AstraZeneca COVID-19 Vaccine. These two systems were “The French National Security Agency of Medicines and Health Products (ANSM)” and the “World Health Organization-Uppsala Monitoring Center (WHO-UMC) Classification” [32].
In Lebanon, at the time of this report, the highest number of case reports that were associated with confirmed signals for Pfizer-BioNTech and AstraZeneca COVID-19 vaccines (based on ANSM reports and/or the WHO-UMC Vigibase classifications) were: arterial hypertension and tinnitus for Pfizer-BioNTech. While flu like symptoms and tinnitus case reports were the highest with AstraZeneca COVID-19 vaccine [33, 34]. As a mean of communication, a monthly report summarizing all findings related to serious and non-serious AEFIs was published with an executive summary. Over one-year period, the PV team was successful in enhancing the content of this report to include necessarily information that may be of interest to all HCPS as well as consumers. Until the date of this paper; 9 reports were published at the PV web site [34]. This was also accompanied with a newsletter that was published on trimester basis. This was another way of communicating important messages related to AEFIs with COVID-19 vaccines to certain target audience [30]. Finally, more than 70 percent of onsite field visits to randomly selected vaccination centers was conducted. The aim was to assess their compliance with international operation standards. This activity was based on extensive literature search that aided in developing checklists that may be utilized by PV team. Site visits were conducted in the 8 Lebanese governorates (Mount Lebanon, Beirut, North Lebanon, Bekaa, South Lebanon, Nabatiyeh, Akkar, and Baalbeck-Hermel). Each visit was followed with a detailed report of observed process that included areas for improvement. As a follow to each site visit, a report including observations and recommendations for improvement was written and shared with corresponding site. Close monitoring for identified clusters and serious AEFIs is the core of AEFI surveillance system.
This is important in order to be able to strengthen both risk assessment and alert generation at the national level. At the time of publishing this paper, two Risk minimization initiatives were taken: first activity was “Management of Vaccine-induced Thrombotic Thrombocytopenia (VITT) Guidelines”, while the second on was “Raising awareness among vaccinated/ unvaccinated population”. The first activity targeted HCPS, mainly physician with the aim of providing guidance on how to manage VITT cases. While the second activity main target audience was general public with the aim of informing about the most common expected AEFIs with available vaccines in Lebanon. This will guide them on when to seek medical advice.
In summary, the PV program managed to establish a rigorous safety profile in regards to the COVID-19 Vaccines administered in Lebanon over a very short period of time. In the time period covering February 2021 to February 2022, a total of 6808 case reports and 24,837 AEFIs were received following the administration of 5,134,093 doses of COVID-19 vaccines (Pfizer-BioNTech, AstraZeneca, Sputnik V and Sinopharm) for an estimated population of 6,825,000 people. With a structured leadership, dedication, hard work, and limited financial support; Lebanon’s performance in the surveillance of COVID-19 vaccines AEFI management was praised by WHO and the rate of case report entry into Vigiflow was among the highest in the Middle East.
Conclusion
The implementation of PV systems in resource-limited countries is challenging. In the midst of a turbulent political, economic and health context, Lebanon has been able to develop one of the most active and rapidly evolving PV systems in the Middle East. Lack of financial resources was the first obstacle to a safe and sound PV System. Facing a degrading economical context, the Lebanese government was not able to allocate financial support from the annual total budget to PV Program. The WHO and WB supported in funding all the operations contributing to the first and most essential step in any system implementation. The second limitation was the lack of commitment from PV main stakeholders such as physicians due to several reasons among which lack of time to report and lack of motivation. The reporting culture still needs to be spread in Lebanon without fear of punitive actions. Finally, the pandemic context, despite playing a positive role in testing the newly introduced PV System, shifted the surveillance on vaccines at the detriment of medicines. Against all odds, the QAPPP succeeded in implementing an effective PV system.
Future Prospective
The QAPPP at MoPH aims for continuous improvement. In this regard, an assessment of the PV system is scheduled in 2022 in order to evaluate current practices and identify what would be the areas for improvement. Moreover, frequent visits to COVID-19 vaccination sites which started by end of 2021 will be pursued. This aims to improve and ameliorate the vaccination practices. Finally, the PV team at the QAPPP at MoPH is currently working on drafting the Guideline on Good Pharmacovigilance Practice for MAHs accompanied with a regulation for its implementation to be issued officially by the end of the year.
Abbreviations
- AEs:
-
Adverse Events
- AEFIs:
-
Adverse Events Following Immunization
- ADRs:
-
Adverse Drug Reactions
- ANSM:
-
Agency of Medicines and Health Products
- CIOMS:
-
Council for International Organizations of Medical Sciences
- EMA:
-
European Medicines Agency
- EU:
-
European Union
- HCPs:
-
Health Care Professionals
- ICSR:
-
Individual Case Safety Report
- LNPVC:
-
Lebanese National Pharmacovigilance Center
- MAHs:
-
Marketing Authorization Holders
- MoPH:
-
Ministry of Public Health
- PIDM:
-
Program for International Drug Monitoring
- PV:
-
Pharmacovigilance
- QAPPP:
-
Quality Assurance of Pharmaceutical Products Program
- UMC:
-
Uppsala Monitoring Center
- VITT:
-
Vaccine-Induced Thrombotic Thrombocytopenia
- WB:
-
World Bank
- WHO:
-
World Health Organization
- WHO-UMC:
-
World Health Organization-Uppsala Monitoring Center
References
European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) Module VI – Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev.2). EMA. 2017. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf. Accessed 24 Jan 2022.
World Health Organization. Safety of medicines: priming resource-limited countries for pharmacovigilance. WHO Drug Inf. 2017;31(4):575–80.
The Global Fund, World Health Organization. Minimum requirements for a functional Pharmacovigilance system. WHO. 2010. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/PV_Minimum_Requirements_2010_2.pdf.
Accessed 24 Jan 2022.
CDER Office of Surveillance and Epidemiology. 2018 https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cder-office-surveillance-and-epidemiology. Accessed 21 Dec 2021.
Pharmacovigilance: Overview | European Medicines Agency. Ema.europa.eu. 2018. https://www.ema.europa.eu/en/human-regulatory/overview/pharmacovigilance-overview. Accessed 21 Dec 2021.
Olsson S. The role of the WHO programme on international drug monitoring in coordinating worldwide drug safety efforts. Drug Saf. 1998;19(1–10):63.
Pal S, Dodoo A, Mantel A, Olsson S. The World Medicines Situation 2011: Pharmacovigilance and Safety of Medicines. Geneva: WHO; 2011.
Elshafie S, Zaghloul I, Marie A. Pharmacovigilance in developing countries (part I): importance and challenges Shaimaa. Int J Clin Pharm. 2018;40:758–63.
Alshammari TM, Mendi N, Alenzi KA, Alsowaida Y. Pharmacovigilance systems in Arab countries: overview of 22 Arab countries. Drug Saf. 2019;42(1):849–68.
Alshammari TM, Alenzi KA, Ata SI. National pharmacovigilance programs in Arab countries: a quantitative assessment study. Pharmacoepidemiol Drug Saf. 2020;29(9):1001–10.
Ministry of Public Health. Pharmacovigilance System in Lebanon. 2021. https://www.moph.gov.lb/en/Pages/4/44742/pharmacovigilance-system-lebanon Accessed 16 Mar 2021.
Ministerial Decision #407/1 dated 14 April 2020. Minister's Decision No.427 of 14/4/2020 https://moph.gov.lb/en/Pages/4/44742/pharmacovigilance-system-lebanon. Accessed 25 Mar 2021.
Ministerial Decision #556/1 dated 28 May 2020. Minister's Decision No.556 of 28/5/2020 https://moph.gov.lb/en/Pages/4/44742/pharmacovigilance-system-lebanon. Accessed 26 Mar 2021.
Ministerial Decision #180/1 dated 3 February 2021. Minister's Decision No.180/1 of 3/2/2021. https://moph.gov.lb/en/Pages/4/44742/pharmacovigilance-system-lebanon. Accessed 25 Mar 2021.
Ministerial Decision #181/1 dated 3 February 2021. Minister's Decision No.181/1of 3/2/2021. https://moph.gov.lb/en/Pages/4/44742/pharmacovigilance-system-lebanon. Accessed 26 Mar 2021.
WHO-UMC. WHO Programme for International Drug Monitoring. Uppsala Monitoring Centre: World Health Organization; 2020. https://www.who-umc.org/global-pharmacovigilance/who-programme-for-international-drug-monitoring. Accessed 8 Nov 2020.
Good Pharmacovigilance Practices for the Americas. Pan American Health Organization. (PANDRH Technical Document Nº 5). ISBN 978-92-75-13160-2. https://www.paho.org/hq/dmdocuments/2011/Series-Red-PARF---5-Eng.pdf. Accessed on Jan 2021.
Guidance on the format of the risk management plan (RMP) in the EU – in integrated format. Human Medicines Evaluation. EMA/164014/2018 Rev.2.0.1 https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-format-risk-management-plan-rmp-eu-integrated-format-rev-201_en.pdf. Accessed Jan 2021.
Guideline on good pharmacovigilance practices (GVP). Annex I - Definitions (Rev 4) European Medicines Agency and Heads of Medicines Agencies, 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf. Accessed Jan 2021.
Palleria C, Di Paolo A, Giofrè C, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. 2013;18:601–10.
Rajinder KJ Chapter 30 - Risk Management in Pharmacovigilance. In: Vohora D, Singh G, editors. Pharmaceutical Medicine and Translational Clinical Research. Academic Press; 2018. https://doi.org/10.1016/B978-0-12-802103-3.00031-6 Accessed on 22 April 2021.
The Importance of Pharmacovigilance (Safety Monitoring of Medicinal Products). World Health Organization 2002ISBN 941590157. https://apps.who.int/iris/bitstream/handle/10665/42493/a75646.pdf?sequence=1&isAllowed=y. Accessed Jan 2021.
Tilson H, Hines LE, McEvoy G, et al. Recommendations for selecting drug-drug interactions for clinical decision support. Am J Health Syst Pharm. 2016;73:576–85.
WHO. Safety Surveillance Manual. Geneva: World Health Organization; 2020. https://www.who.int/vaccine_safety/committee/covid_vaccine_safety_manual/en/. Accessed 24 Jan 2022.
WHO. Standard Precautions: Injection Safety and Needle-Stick Injury Management. Geneva: World Health Organization; 2020. https://openwho.org/courses/IPC-IS-EN. Accessed 24 Jan 2022.
Medical Dictionary for Regulatory Activities. https://www.meddra.org/. Accessed 21 Jul 2021.
WHODrug Global. Uppsala Monitoring Centre. https://www.who-umc.org/whodrug/whodrug-portfolio/whodrug-global/. Accessed 24 Jan 2022.
Training and Guidance. 2022. https://www.who-umc.org/global-pharmacovigilance/vigiflow/training-materials/english/. Accessed 10 Jan 2022.
Welcome - WHO Vaccine Safety Basics. 2022. https://vaccine-safety-training.org/. Accessed 21 Mar 2021.
Pharmacovigilance System in Lebanon. 2022. https://www.moph.gov.lb/en/DynamicPages/index/4#/en/Pages/4/44742/pharmacovigilance-system-lebanon. Accessed 15 January 2021.
Ministerial Decision #603/1 dated April 26, 2021. https://www.MoPH.gov.lb/userfiles/files/Laws%26Regulations/Karar%20603-2021.pdf. Accessed 26 Mar 2021.
Actualité - Point de situation sur la surveillance des vaccins contre la Covid-19 – Période Du 28/01/2022 au 10/02/2022 - ANSM. Ansm.sante.fr. https://ansm.sante.fr/actualites/pointde-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-28-01-2022-au-10-02-2022. Accessed Feb 2022.
Analysis of Reports in the WHO Global Database of Individual Case Safety Reports, Vigibase: Tinnitus: https://vigilyze.who-umc.org/downloadfile/609b9c690a3bd8c6283506b7?activity=News.
Acknowledgements
We would like to acknowledge the technical support provided by the World Health Organization (WHO), World Bank (WB), and the ministry of Public Health (MoPH).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors confirm their contribution to the paper as follows: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and Drafting the work or revising it critically for important intellectual content; and Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing Interests
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbas, H., Zeitoun, A., Watfa, M. et al. Implementation of a Pharmacovigilance System in a Resources-Limited Country in the Context of COVID-19: Lebanon’s Success Story. Ther Innov Regul Sci 57, 178–185 (2023). https://doi.org/10.1007/s43441-022-00460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-022-00460-7