Abstract
A total of 79 bacteria and 101 actinobacteria strains were isolated from the sediment samples of the different points of Baratang mud volcano viz., point of the eruption (M), middle of the volcano (MD), and the dried part of the mud volcano (E). Based on the biochemical and molecular characterization, the isolates were categorized under the phyla Proteobacteria, Firmicutes and Proteobacteria included representatives of Classes Alphaproteobacteria, Gammaproteobacteria and Deltaproteobacteria of 29 genera with 38 distinct ribotypes. Thirty-eight bacterial strains from four different regions of mud volcano revealed excellent activity for indole-3-acetic acid (IAA) production. Excellent antagonistic property, plant growth promoting properties such as IAA production, phosphate, potassium and zinc solubilization were identified in Bacillus megaterium NIOT_MV 31 strain of 38 studied isolates. In this study, we investigated the optimization of IAA production by B. megaterium NIOT_MV 31 and its formulation as a plant growth promoter to improve economic and agricultural development. Maximum IAA yield was achieved using optimal conditions (42.63 mg/mL) in the presence of optimized tryptophan after 4 days of incubation. Twofold increase in the plant growth parameters were observed to that of control plants. Optimization of culture conditions resulted in a fourfold increase in IAA production by B. megaterium NIOT_MV 31 cells. The results clearly demonstrated that, B. megaterium NIOT_MV 31 holds great potential as a source for IAA production and may be useful for commercial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mud volcanoes are a natural phenomenon and it is a land form created by the eruption of mud or slurries, water and gases. The term “mud volcano” is coined to describe the set of structures associated with a constructional edifice (mud volcano) and feeder complex that connects the volcano to its source stratigraphic unit. Geological process caused by the formation of mud volcanoes. They are not true volcanoes and do not produce lava and magmatic activity. Mud volcano is triggered by high pressure gas, fault activity sedimentation. It increases the sea bed slope gradient thus trigger subsea slope instability. The volcanic activity is due to the ongoing subduction of the Indian plate beneath the Andaman Island arc, which forces magma to rise in this location of the Burma Plate. Due to these reasons; the Andaman Islands have volcanic eruptions and impacts of other geological processes, such as mud volcanoes [3]. Methanotrophic bacteria are predominantly found in these habitats [23]. A few novel bacterial species reported from these habitats include Altererythrobacter epoxidivorans [19], Cupriavidus pinatubonensis [30], Cupriavidus laharis, Hallobacillus profundi, Hallobacillus kuroshimensis [14] and Marinobacter alkaliphilus [36].
Actinobacteria are Gram positive bacteria, with high guanine (G) and cytosine (C) in their DNA (> 55 mol%), which are phylogenetically related from the evidence of 16S ribosomal cataloguing and DNA: rRNA pairing studies [13]. Terrestrial soil has been widely exploited for isolation of actinobacteria, wherein they perform significant biogeochemical roles contributing to the turnover of complex biopolymers [31]. Actinobacteria have been isolated from a diverse range of marine samples, including marine samples from deep sea [7] and also vicinity of hydrothermal vents [22]. Marine actinobacteria play an important ecological role, similar to their saprophytic relatives in soil, perhaps substantially impacting the cycling of complex carbon substrates in benthic ocean habitats [20]. However, a well-defined biodiversity and taxonomic study of actinobacteria is important to understand their diversity in marine environment [9]. Only less than 1% of the actinobacteria have been identified, investigated and documented [35]. Research in actinobacteria has gained prominence in recent years because of their potential in producing antibiotics [17]. Streptomycin, gentamycin, rapamycin are some of the antibiotics which are being in use produced from actinobacteria. Actinobacteria play an important role in agriculture also. Previous study showed that actinobacteria isolated from Malaysian soil have the potential to inhibit the growth of several plant pathogens [15]. Secondary metabolites of actinobacteria are therapeutically important compounds, especially antiviral, anti-cancerous, antibacterial compounds and around 70% of the antibiotics used in the world were identified and extracted from actinobacteria [32]. Bacterial isolates from mud volcano revealed excellent production of plant growth promoting hormones such as IAA, phosphate solubilisation, zinc solubilisation and potassium solubilisation properties. Plant growth promoting strains resulted in increased percentage of seedling emergence, cell division, root length and stem growth. Plant growth promoting bacteria has an important role in developing promising method for crop management [34].
Andaman and Nicobar Islands marine ecosystem are mostly unexplored, and provides a rich source of microorganism producing novel and efficient antimicrobial compounds [4]. A total 1,100 mud volcanoes were identified in the world from land and shallow water, in which 30 mud volcano is present in Andaman Islands [3]. Only limited studies on eubacteria and actinobacteria from mud volcano in Andaman and Nicobar Islands has been reported. Present study reveals the diversity of mud volcano associated actinobacteria, bacteria and their potential for IAA production. It plays a crucial role in many aspects of the regulation of plant growth and development, including cell elongation and establishment of apical basal polarity in whole plant. No studies have been reported on the biodiversity of mud volcano associated bacteria and actinobacteria from Baratang of Andaman Islands. Hence there is a possibility to identify and characterize the mud volcano associated bacteria and actinobacteria for the production of IAA for sustainable and organic agricultural system.
Materials and methods
Study area and sample collection
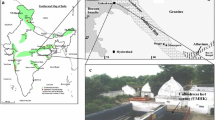
In Andaman and Nicobar Islands there are about 11 mud volcano site which are situated in Baratang Island and Diglipur. The study area is situated in Baratang Island, an elevation of 40 m above sea level, falls in North and Middle Andaman district. Erupted volcanic mud and small pebbles spread over an area of 0.62 hectares of land mass and the area is almost barren. Surrounding area of the site is mixed tropical forest. Nearby area is developed as habitation mask with agriculture field (Fig. 1). The samples were collected from 03 mud volcano locations viz., point of the eruption (M), middle of the volcano (MD), dried part of the mud volcano (E). The samples were stored in ice boxes and transported immediately to the laboratory for further processing.
Isolation of mud volcano associated bacteria and actinobacteria
Isolation of associated bacteria was performed by the following methodology with the medium composition: peptone (5 g), yeast extract (1 g), ferric citrate (0.1 g), sodium chloride (19.45 g), magnesium chloride (8.80 g), sodium sulphate (3.24 g), calcium chloride (1.8 g), potassium chloride (0.55 g), sodium bicarbonate (0.16 g), potassium bromide (0.08 g), strontium chloride (0.034 g), boric acid (0.022 g) sodium silicate (0.004 g) sodium fluorate (0.0024 g), ammonium nitrate (0.0016 g), disodium phosphate (0.008 g), agar (15 g) and final pH (at 25 °C) 7.6 ± 0.2 was dissolved in 100 ml of distilled water. All plates were incubated for 2 days for fast growing bacteria and 4 days for slow growing bacteria at room temperature (24 ± 2 °C). According to the morphological features, colonies were randomly picked and purified using the streak plate method.
Enumeration and isolation of actinobacteria was performed as described previously by Ellaiah et al. [10] using starch casein agar (SCA) medium contained soluble starch 10 g, vitamin free casein-0.3 g, KNO3-2 g, NaCl-2 g, K2HPO4-2 g, MgSO4.7H2O-0.05 g, CaCO3-0.02 g, FeSO4.7H2O-0.01 g, agar-20 g, pH-7.0 ± 0.2, Bennet’s agar (yeast extract-1.0 g, beef extract-4.0 g, casein enzyme hydrolysate-2.0 g, dextrose-10.0 g, agar-15.0 g, pH-7.3 ± 0.2) and Emerson agar (yeast extract-1.0 g, beef extract-1.0 g, peptic digest of animal tissue-2.0 g, dextrose-10.0 g, sodium chloride-2.5 g, agar-15.0 g pH-7.0 ± 0.2), with 50% aged sea water was dissolved in 100 ml of distilled water. The medium was supplemented with nalidixic acid 50 μg/mL (HiMedia, India) and Nystatin 25 μg/mL (HiMedia, India) to inhibit the fungal and fast growing Gram negative bacteria. The plates were incubated at room temperature (28 ± 2 °C) for 21 days. The appearance and growth of marine actinobacteria were monitored regularly. After incubation, morphologically diverse actinobacterial colonies were picked and further subcultured onto respective isolation medium. The colonies were purified using SCA and ISP2 medium, once the pure colonies were obtained, each colony was further identified on the basis of its earthy smell, colony morphology, colour of hyphae and the presence or absence of aerial and substrate mycelium. The selected and identified colonies of actinobacteria were sub cultured in SCA slants for further studies. The pure cultures were also preserved in 20% glycerol vials and stored at -80 °C for long term preservation.
Screening of isolates for plant growth-promoting properties
Screening of Indole Acetic Acid producers
Production of IAA was determined following the standard method [6]. Briefly, overnight grown single colony was streaked onto LB agar amended with 5 mM L-tryptophan. Plates were overlaid with sterile Whatman no. 1 filter paper (82-mm diameter), and bacterial strain was allowed to grow for 3 days at 28 °C. After incubation, the paper was removed and treated with Salkowski’s reagent (2% of 0.5 M ferric chloride in 35% perchloric acid) at room temperature for 60 min. In a Petri plate, the filter papers were saturated in Salkowski’s reagent and the production of IAA was identified by the formation of a red halo on the paper immediately surrounding the colony.
Phosphate solubilisation
All bacterial isolates were screened for inorganic phosphate solubilisation according to Verma et al. [39]. A loopful of fresh bacterial culture was streaked onto Pikovskaya’s medium amended with inorganic phosphate, and plates were incubated at 28 °C for 3–4 days. A clear halo around the bacterial colony indicated solubilisation of mineral phosphate.
Estimation of auxins
Quantitative estimation of auxins was done by colorimetric method with slight modifications, i.e., 2 to 3 drops of orthophosphoric acid was added to 2 ml supernatant and 4 ml of Salper reagent (1 ml of 0.5 M FeCI3 in 50 ml of 30% HCIO4). This mixture was incubated for 60 min in dark and the absorbance was measured at 535 nm. Concentration of auxins was estimated by preparing calibration curve using IAA as standard (10–100 μg/ml).
Optimization of IAA production
Effect of pH, temperature and batch time on IAA production
The potential strain was inoculated in production medium and incubated at 30ºC in shaker incubator with 125 rpm. The culture broth was analysed every 24 h of incubation for IAA production. Batch time ranging from 0 to 7 days was used to determine the effect of batch time on growth and active metabolite production. Impact of pH on the production of IAA was examined by culturing the strain in production medium adjusted to various pH levels ranging from 05 to 11. To determine the optimum temperature for IAA production, the strain was cultured in production medium at different temperatures (20–80°C) for 7 days.
Effect of NaCl concentration on IAA production
Impact of NaCl on the production of IAA was examined by culturing the strain in production medium adjusted to various NaCl concentrations ranging from 0 to 10%. The maximum production was observed between 2% and 3% NaCl concentration, the experiment was repeated with the various ranges of NaCl concentration between 2% and 3.5% (2, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3.0, 3.1, 3.2, 3.3, 3.4 and 3.5).
Effect of carbon and nitrogen sources on IAA production
To investigate the effect of carbon sources on IAA production by the potential strain, production medium was supplemented with different carbon sources such as glucose, starch, maltose, fructose, lactose and sucrose at the concentration of 1% (w/v) and impact of best carbon source (0.5–2.5% w/v) on IAA production of the strain was studied. Different nitrogen sources, namely, malt extract, peptone, yeast extract, soya peptone was added at the rate of 1–5% (w/v) and ammonium chloride, ammonium nitrate and ammonium sulphate were added at the rate of 0.5–2.5% in production medium to obtain an optimal amount of superior nitrogen source.
Effect of substrate concentration on IAA production
Impact of substrate concentration on IAA production was examined by culturing the strain in production medium adjusted to various L-Tryptophan concentrations ranging from 0% to 5%. The maximum production was observed between 2% and 3% of L-Tryptophan concentration, the experiment was repeated with the various ranges of L-Tryptophan concentration between 0, 1.0, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0 and 3.2%.
Production of IAA
Ten ml of nutrient broth was prepared and inoculated with one loopful of potent bacterial strain and incubated at 28 °C for 24 h and used as inoculums for IAA production. About 5 mL of bacterial inoculums was transferred to 100 mL of optimized nutrient broth production medium supplemented with 2.8% of L-Tryptophan as a sole nitrogen source and incubated at 28 °C for 4 days at 170 rpm. The IAA assay and growth parameters were regularly monitored once in 24 h up to 4 days/up to the product recovery.
Standardization of solvent for extraction of IAA
To determine the best solvent for the extraction of IAA from the microbial fermentation broth/production medium, the potential strain NIOT_MV 31 was inoculated in to the 500 mL of optimized production medium for the production of IAA. After 4 days of incubation the culture broth was centrifuged and the supernatant was extracted with 4 different solvents viz., ethyl acetate, diethyl ether, chloroform and petroleum ether. The solvent extracts were completely dried and were dissolved in methanol and subjected for the IAA assay to conclude the best solvent for the extraction of IAA.
IAA extraction
Auxins were extracted and separated from supernatant with ethyl acetate and detected for homogeneity by thin layer chromatography (TLC). The isolates viz., An-1-kul and An-13-kul were grown in nutrient broth for 72 h at 28 °C under shaking condition (100 rpm) and the supernatant was harvested by centrifugation at 10,000 rpm for 20 min.
Thin layer chromatography
TLC of auxins extracts was carried out on silica gel G pre-coated aluminium plates. 100 ml of the extracted auxins dissolved in methanol were spotted on silica gel G along with IAA standard. Plates were kept in solvent isopropanol–water (30:20 v/v) for 12–14 h and sprayed with Salper reagent. Plates were observed for presence of pink color spot and Rf value was also calculated using following equation:
Partial purification of extracted auxins by column chromatography
Ethyl acetate extracted sample from NIOT_MV 31were used for Sephadex G25 column chromatography.10 g of Sephadex G-25 was soaked in distilled water for overnight and was boiled for 4 h for complete swelling of the gel. Gel was washed and equilibrated with (0.2 M, pH 7.2) tris–HCl buffer and column (50 cm × 1.5 cm) was packed and equilibrated with tris–HCl buffer. 5 ml sample was applied to column and eluted with tris–HCl buffer with flow rate 3 ml/12 min. 3 ml fractions were collected and observance was detected at different wavelengths (220 nm, 240 nm, 260 nm, 280 nm, 300 nm, 320 nm). Estimation of auxins was done in each fraction and fraction showing auxin production was pooled for further analysis.
Characterization/field experiments on purified IAA
Plant growth promotion ability of mud volcano site bacteria on flat beans
The flat beans seeds were sterilized with 70% ethanol for 2 min and in 2% sodium hypochlorite for 2 min, followed by washing ten times in sterile water. For this experiment, pure cultures were grown in nutrient broth at 28 °C and diluted to a final concentration of 108 colony forming units (CFU)/ml in sterile saline water (0.85%). The surface sterilized seeds were immersed in appropriate PGP, i.e., commercially available IAA (HiMedia, India), partially purified IAA from the mud volcano isolate (μg/ml) and cell free supernatant of the isolate for 1 h, air-dried and sown immediately. The following treatments with three replicates were investigated with two individual experiments: For (1) control (without treatment), (2) C-IAA (3) CFS (iv) B-IAA. Pots were sterilized with 20% sodium hypochlorite solution and filled with sterile loam soil. The flat beans seeds (10 seeds in each pot) were sown in plastic pots filled with 1 kg sterile field soil. The pots were arranged in a completely randomized factorial design. The seedlings were grown in a nursery at a temperature of 28–32 °C and 85% relative humidity in a day–night cycle of 13–14 h natural light. The pots were watered to 50% water-holding capacity and were maintained at this moisture content by watering to weight every day. The plants from the experimental set up were harvested 15 days after the emergence of seedlings, washed and morphological characteristics viz., root length, shoot length, dry and wet weight of stem and root of each plant was recorded.
Assessment of brinjal seed germination rate
In this assay, brinjal seeds were first disinfected by soaking in 80% ethanol for 3–5 min, followed by 0.2% sodium hypochlorite for 3 min, and washed thoroughly with sterilized distilled water three times. Samples were then dried under laminar flow for the next step. Sterilized seeds were soaked in four treatments; (distilled water, production medium only, Cell free supernatant purified IAA and commercial available IAA) for 2 h. Ten seeds from each treatment (with three replicates) were then placed in a Petri dish containing sterile wet tissue paper and kept under semi-dark conditions at 26 ± 1 °C for 1 week, after which germination time rates, shoot and root lengths were measured.
Molecular identification of mud volcano associated bacteria and actinobacteria
PCR amplification of 16S rDNA
The genomic DNA of the mud volcano associated bacteria and actinobacteria was isolated by the method of Kutchma et al. [18]. PCR amplification of 16S rDNA of the ascidian associated actinobacteria was performed using universal primers 16Sf (AGAGTTTGATCCTGGCTCAG) and 16Sr (GGTTACCTTGTTACGACTT) and to amplify the 16S rDNA of the ascidian associated eubacteria the universal primers 16S rDF (CGCTGGCGGCAGGCTTAACA); 16S rDR (CCAGCCGCAGGTTCCCCT) were used. Final volume of reaction was 50 μl, which comprised Taq buffer (1 ×), dNTP’s (200 μM) (MBI Fermentas, USA), forward and reverse primer (0.5 μM), MgCl2 (1.0 mM), Taq DNA polymerase (1.25 U; MBI Fermentas), template (1 μl) and remaining autoclaved Milli Q water. PCR was performed with the initial denaturation at 98 °C for 3 min, followed by 30 cycles of reaction with denaturation at 94 °C for 1 min; annealing at 53 °C and 55 °C for 1 min; extension at 72 °C and final extension at 72 °C for 10 min. PCR amplified products were analyzed on 1.5% agarose gel along with DNA molecular weight marker (MBI Fermentas). Positive amplicons as judged by size were purified using QIAquick PCR purification kit (Qiagen, Germany) and sequenced on an ABI PRISM 377 genetic analyzer (Applied Biosystems, USA).
Phylogenetic analysis
16S rDNA sequences of the associated bacteria and actinobacteria was aligned manually in GenBank database with BLAST [2] and the sequences with 98–100% homology were considered for molecular taxonomy analysis. Multiple alignments of 16S rDNA sequences in this study and sequences in GenBank database was performed with CLUSTAL X program. Phylogenetic trees were constructed by neighbor-joining and maximum-parsimony tree making methods in Molecular Evolutionary Genetic Analysis (MEGA version 5.0) and bootstrap values based on 1,000 replications.
Results
Population density of mud volcano associated bacterial community
To evaluate the diversity of mud volcano associated bacterial community, 12 sediment samples were collected from the 4 spots of 2 selected mud volcano stations in Baratang Islands. The sediment samples were collected from the different points of mud volcano viz., point of the eruption (M), middle of the volcano (MD), dried part of the mud volcano (E). Aseptically serially diluted samples were processed in nutrient agar to enumerate the mud volcano associated bacteria and inoculated in to the starch casein agar to enumerate the mud volcano associated actinobacteria (Figs. 2, 3, 4, 5). The maximum population density (2.67 × 103 CFU/g) was recorded in the Sta-1_sp-1-M (Station 1) and Sta-2_sp-1,-M, Sta-1_sp-2-M, Sta-1_sp-2-E, Sta-1_sp-2-MD, Sta-1_sp-1-E, Sta-1_sp-1-MD, Sta-2_sp-2-M, Sta-2_sp-1-MD, Sta-2_sp-2-MD, Sta-2_sp-1-E and Sta-2_sp-2-Ewere recorded the assorted population density of 2.04 × 103 CFU/g, 1.83 × 103 CFU/g, 1.74 × 103 CFU/g, 1.26 × 103 CFU/g, 5.70 × 102 CFU/g, 2.70 × 102 CFU/g, 2.40 × 102 CFU/g, 1.20 × 102 CFU/g, 1.20 × 102 CFU/g, 6.00 × 101 CFU/g and 3.00 × 101 CFU/g, respectively.
A total of 84 morphologically distinct mud volcano associated bacteria were from the 12 sediment samples, namely, Sta-1_sp-1-M, Sta-1_sp-1-MD, Sta-1_sp-1-E, Sta-1_sp-2-M, Sta-1_sp-2-MD, Sta-1_sp-2-E, Sta-2_sp-1-M, Sta-2_sp-1-MD, Sta-2_sp-1-E, Sta-2_sp-2-M, Sta-2_sp-2-MD andSta-2_sp-2-E. All the sampling spots the maximum population diversity was recorded from the mouth region samples than the middle and the end dried samples. Of 84 mud volcano associated bacteria, maximum number was recorded from Sta-1 sp-1, 35(41.66) %) than other samples: Sta-1_sp-2, 34 (40.47%), Sta-2_sp-2, 8(9.52%) and Sta-2_sp-2, 7(8.33%)). Of 84 isolates 79 purified isolates were selected for the further characterization studies.
Screening of indole acetic acid producers
Primary screening of IAA producers was determined following the standard method of Brick et al. [6]. Briefly, overnight grown single colony was streaked onto nutrient agar amended with 5 mM L-tryptophan. Plates were overlaid with sterile Whatman no. 1 filter paper (82 mm diameter), and bacterial strain was allowed to grow for 03 days at 28 °C. After incubation, the paper was removed and treated with Salkowski’s reagent (2% of 05 M ferric chloride in 35% perchloric acid) at room temperature for 60 min. In a Petri plate, the filter papers were saturated in Salkowski’s reagent and the production of IAA was identified by the formation of a red halo on the paper immediately surrounding the colony. Out of 79 isolates from the mud volcano samples, 64 isolates were produced red halo on the filter paper were confirmed as IAA producers, these isolates were selected for the further characterization studies to determine the best IAA producing strain.
Screening of salt, temperature and pH resistance for the IAA producers
To study the sodium chloride tolerance range among the IAA potential mud volcano isolates, 06 concentrations of sodium chloride was supplemented in the test medium along with the control medium with only distilled water and neither seawater nor sodium chloride was added. Of 38 isolates, 60.00% exhibited good growth at 0% NaCl concentration. With 0.5% and 1.0% sodium chloride concentration, of 38 isolates, all isolates exhibited good growth (Fig. 6). It was also observed that, 70.00% isolates revealed good growth at 22.21% sodium chloride, whereas 31.00% disclosed negative result in the medium. In 30.0% NaCl concentration, 26.00% revealed good growth, 13.00% exhibited moderate growth and 44.00% revealed negative result. In 35.00% NaCl concentration, 12.00% showed good growth, 32.00% exhibited moderate growth and 76.00% gave negative result.
All isolates revealed acceptable pH tolerance growth at different ranges of pH 5.0 to pH 11.0 (Fig. 7). Of the total isolates, 70.00% revealed positive growth on all pH ranges. This depicts their tolerance to grow in acidic and alkaliphilic conditions. At pH 5.0, 3.1% isolates revealed positive result and all the isolates were revealed positive growth in the pH range of 7.0–9.0. The effect of temperature on growth is one of the most important factors used for the identification of actinobacteria. Different type of growth was recorded in mud volcano sediment associated IAA producers (Fig. 8), all isolates exhibited good growth in the temperature range of 30–50°C and 75.00% of the isolates were found grown at 60 °C. Of the total isolates, 30.00% were observed with moderate and fair growth at 70–80°C.
Estimation/secondary screening of IAA potential strains
To confirm the potential IAA producer, the positive isolates were screened by quantitative estimation of auxins by colorimetric method. Briefly, 2 ml of cell free supernatant and 4 ml of Salper reagent (1 ml of 0.5 M FeCI3 in 50 ml of 30% HCIO4). This mixture was incubated for 60 min in dark and the absorbance was measured at 535 nm. Concentration of auxins was estimated by preparing calibration curve using IAA as standard (10–100 μg/ml). The isolates were screened for the extracellular and intracellular IAA production assay to select the extracellular IAA producer. The purification, estimation and product recovery steps are complicated as well as expensive for the intracellular microbial producers. To overcome this issue, screening study was carried out to select the extracellular IAA producer. The positive isolates were inoculated in to the 5 ml nutrient broth supplemented with 5 mM L-Tryptophan and incubated in shaker incubator at 175 rpm, 28 °C for 4 days. The cell free supernatant and the cell pellet were separated after the incubation period, the quantitative estimation for the CFS and cell pellet were carried out separately for each isolate. After the quantitative estimation, out of 64 positive isolates were confirmed as the potential IAA producers (Figs. 9, 10, 11), out of the 38 potential isolates, the stain NIOT_MV 31 was confirmed as the excellent IAA producer (9.21 mg/mL) and the strain NIOT_MV 31 was also confirmed as the extracellular IAA producer.
Solubilisation assay for the IAA positive strains
All the IAA positive isolates were subjected for the solubilisation of zinc potassium and phosphate to find out the best producer. Of 38 IAA potential strains, 18.42% of the isolates were positive for zinc solubilisation, 15.79% of the isolates were positive for phosphate solubilisation and 10.53% of isolates revealed positive for potassium solubilisation (Fig. 12). Based on the above characteristics, the potential strain NIOT_MV 31 was selected for the optimization studies.
Optimization of IAA production
Effect of pH, temperature and batch time on IAA production
The production medium was inoculated with NIOT_MV 31 and culture broth was analysed every 24 h of incubation for IAA estimation assay. IAA production was observed on the first day of incubation and it increased as time progressed to reach a maximum activity on the 4th day (9.21 mg/mL) and reduced from 6th day. Maximum IAA production was related with dry weight of biomass and total protein (extracellular protein) of CFS (Fig. 13).
The effect of pH on IAA production medium was determined with pH 06–11. Acid pH had a negative effect on growth and IAA production. The IAA production was maximum (12.10 mg/mL) at pH 8. The IAA production were nil at pH 6, pH 6.5 and pH 11.0. However, pH 7.5 and 8.0 disclosed very nearer IAA production (Fig. 14). To narrowing the pH for IAA production, the assay was carried out with pH range of 7.4, 7.6, 7.8, 8.0, 8.2, 8.4 and 8.6 for isolate NIOT_MV 31. Production medium was inoculated with equal volume of inoculum and after incubation, the CFS was subjected for IAA estimation assay and production of IAA was maximum in pH 7.8 (14.45 mg/mL). The effect of temperature on the IAA production was studied by nurturing the strain at different temperature ranges between 10 and 40 °C. Optimum temperature for IAA production (11.26 mg/mL) was at 35 °C (Fig. 15). Extreme pH and temperature did not goodwill for cell growth as well as IAA production for NIOT_MV 31 mud volcano associated strain.
Effect of carbon and nitrogen sources on IAA production
To maximize the production of IAA, experiments were performed using various carbon sources. The maximum IAA production was found to be 15.68 mg/mL using starch (1%) and 9.15 mg/mL using glucose (1%) as sole source of carbon from NIOT_MV 31 isolate. The production of IAA was marginally favourable with low cost substrates viz., starch and glucose, there is no significant effect of fructose, sucrose and lactose. The optimal levels of starch and glucose need to be explored to maximize the production. Of 0.5–2.5% concentrations of starch, 1.5% revealed maximum (19.22 mg/mL) IAA production. Final pH of the fermentation broth is an important criterion on IAA production. Comparatively other carbon sources became acidic while fermentation period that may lead to decline in productivity of IAA, acidity of fermentation medium could inhibit the production of IAA. There was no activity of IAA detected acidic pH (< 6.0) and optimal activity was detected at pH (7.5–8.0). This study authenticated starch and glucose as an exclusive source for IAA production.
IAA production varied with different nitrogen compounds. Among them, culture medium supplemented with yeast extract favoured maximum IAA production (14.12 mg/mL) by the isolate followed by potassium nitrate (13.49 mg/mL). Influences of different yeast extract concentration on IAA production were performed (1.0–5.0%), a high level of IAA production (18.17 mg/mL) was observed when yeast extract 02% was used as nitrogen source. yeast extract stimulates the production of IAA, yeast extract is essential nitrogen source for cell growth of marine bacteria and IAA production but higher concentrations inhibit the production in NIOT_MV 31 strain. Yeast extract at 02% concentration supported good growth of the isolate reflected by its biomass. With increasing concentrations of yeast extract from 04 to 05%, there was a decline in IAA production. This might be due to the presence of high substrate concentration and induction of other proteolytic enzymes.
Effect of NaCl and substrate concentration on IAA production
Sodium chloride concentration on the production medium plays an important role for the production of secondary metabolites by bacteria. To select the most favourable NaCl concentration in the production medium to maximize the IAA production, experiments were performed using various concentration of NaCl. The maximum IAA production was found to be 13.23 mg/mL and 12.16 mg/mL for the NaCl concentrations 02 and 03%.
To select the most appropriate and optimal concentration(s) of substrate(s) for maximum production of IAA, the experiment was carried out different concentrations of L-Tryptophan and combination of L-Tryptophan, glucose and starch, respectively. The results obtained at different initial concentrations of starch or glucose or L-Tryptophan has been considered as control experiments. The effect of initial concentrations of glucose (above 1.0%) on the production of agarase from strain NIOT_MV 31 was found to be insignificant. The pH was decreased, when glucose concentration was increased (> 1.0%) in the culture broth, which might have influenced on the IAA production. However, observations have been made on the enhancement of IAA synthesis by different concentrations of L-Tryptophan in the culture medium. This study was carried out based on the earlier reports with L-Tryptophan as a nitrogen source stimulated more IAA production than natural. Thus, medium containing different concentrations (0–4.0%) of L-Tryptophan were used. Maximum IAA production was found to be 16.37 mg/mL at 2.0% of L-Tryptophan without glucose concentration. Then the medium optimization was carried out with different concentrations of glucose and starch with fixed L-Tryptophan (2.0%) concentration from 0.5 to 2.5%, the maximum IAA activity of 19.44 mg/mL was achieved at 1.8% of starch and L-Tryptophan (2.00%) concentration.
Partial purification of IAA
To determine the perfect solvent for the extraction of IAA from the microbial fermentation broth/production medium, the potential strain NIOT_MV 31 was inoculated in to the 500 mL of optimized production medium. After 4 days of incubation, the culture flasks were centrifuged and the supernatant was extracted with 4 different solvents viz., ethyl acetate, diethyl ether, chloroform and petroleum ether. The concentrated extracts were completely dried and weighed; the dried products were dissolved in the methanol and subjected for the IAA assay to conclude the best solvent for the extraction of IAA from the microbial fermentation broth. Of the tested solvents, ethyl acetate recovered maximum amount of IAA (22.49 mg/mL) from the fermentation broth, diethyl ether was the second best solvent to extract the IAA from the production medium, the solvents; petroleum ether and chloroform failed to recover the IAA from the production medium.
Ethyl acetate extracted sample from NIOT_MV 31 were used for Sephadex G25 column chromatography.10 g of Sephadex G-25 was soaked in distilled water for overnight and was boiled for 4 h for complete swelling of the gel. Gel was washed and equilibrated with (0.2 M, pH 7.2) tris–HCl buffer and column (50 cm × 1.5 cm) was packed and equilibrated with tris–HCl buffer. 5 ml sample was applied to column and eluted with tris–HCl buffer with flow rate of 3 ml/12 min. 03 ml fractions were collected and the OD was detected at different wavelengths (220 nm, 240 nm, 260 nm, 280 nm, 300 nm, 320 nm). Estimation of auxins was done in each fraction and fraction showing auxin production was pooled. TLC of auxins extracts was carried out on silica gel G pre-coated aluminium plates. 100 ml of the extracted auxins dissolved in methanol were spotted on silica gel G along with IAA standard. Plates were kept in solvent isopropanol: water (30:20 v/v) for 12–14 h and sprayed with Salper reagent. Results revealed that the standard IAA sample and putative IAA samples extracted from NIOT_MV 31 cells displayed the same retention factor (RF) value.
Characterization/field experiments of purified IAA
Plant growth promotion ability of mud volcano site bacteria on flat beans
The plant growth promoting potential of isolate was determined by pot assay in flat bean seeds. The higher germination percentage, root and shoot lengths were observed in the experimental seeds compared with control. The germination and growth rate were more than the seeds exposed with control and C-IAA, the maximum percentage of germination and growth rate was observed in the partially purified IAA extracted from the mud volcano isolates. A corresponding increase in the root and shoot biomass was also observed in partially purified IAA exposed seedlings. Root elongation assays and pot experiments were performed using flat bean seeds inoculated with the PGP from NIOT_MV 31 strain. Water, cell free filtrate and commercially available IAA (HiMedia, India) were significantly different from each other. In plant growth promotion assays, NIOT_MV 31 IAA/PGP significantly enhanced plant growth by increasing the root length, shoot length, fresh weight and dry weight of all plants. After 15 days of growth, NIOT_MV 31 IAA/PGP promoted root length to 2.69 ± 0.28 cm, shoot length to 48 ± 0.98 cm, fresh weight to 4.96 ± 0.41 g and dry weight to 1.12 ± 0.01. In filtrate-treated plants, root and shoot length were enhanced by 8 ± 0.41 cm and 41 ± 1.11 cm, respectively, while fresh weight and dry weight were increased to 3.53 ± 0.73 g and 0.94 ± 0.03 g, respectively. These plant growth parameters were significantly different from the other two treatments. Table 1 summarises the plant growth-promoting effects of this potent strain on flat beans plants (Fig. 16).
Assessment of brinjal seed germination rate
In germination assessment, commercial IAA and the IAA purified from the strain NIOT_MV 31 was exhibited 100% germination rates on brinjal seeds (Table 2). The effects of strain NIOT_MV 31 IAA on plant growth promotion, including root development and root elongation were assessed after six days of the inoculation. The morphological development stages of brinjal seeds from embryo to seedling were documented. The B-IAA treated seeds were started germination within 3 days of inoculation, on the other plates the germination started after 3 days of inoculation. After 6 days of growth, NIOT_MV 31 IAA/PGP promoted plant length to 2.3 ± 0.02 cm, root length to 1.6 ± 0.01 cm, shoot length to 0.7 ± 0.01 cm, and germination rate was 100%. In filtrate-treated plants, plant length, root and shoot length were enhanced by 1.9 ± 0.04 cm, 1.0 ± 0.01 cm and 0.9 ± 0.06 cm, respectively, but the germination rate was less than the purified IAA from the strain NIOT_MV 31. These plant growth parameters were significantly different from the other two treatments.
In vitro seed germination
To evaluate the influence of PGP extracted from mud volcano associated bacteria, the half strength of the MS medium was supplemented with cell free supernatant, commercially available IAA and purified IAA from the bacterial isolate NIOT_MV 31. The morphological development stages of flat bean seeds from embryo to seedling were documented. Seed germination of flat beans started with swelling embryo and rounding up at about 3 days after inoculation. Embryos were discharged from the testa and developed into a round, yellow form protocorm, a shoot apex became visible at one side of the protocorm When the size of the protocorm became bigger, the protocorm developed into elongated shape and the colour changed into green, followed by roots emerged from the seedling. Healthy growth rate was observed in the seeds supplemented with bacterial PGP than the commercial IAA and the contamination was observed in the control and the CFS plates. After 6 days of growth, NIOT_MV 31 IAA/PGP promoted wet weight of the seedlings in the plates to 3.76 ± 0.28 g, CFS, C-IAA and control seedlings wet weight were 3.20 ± 0.11 g, 3.07 ± 0.15 and 2.69 ± 0.28, respectively. The results showed that, the PGP extracted from the mud volcano associated bacteria is best for the in vitro seed germination, and also it enhances the growth faster than the commercially available product; moreover, the chances of contamination also absent when B-IAA used as the supplement.
Molecular and phylogenetic identification of mud volcano associated bacteria and actinobacteria
Phylogenetic relationships of the mud volcano associated bacteria were ascertained based on the 16S rDNA sequence similarity with reported strains using BLAST sequence similarity search. Upon analysis, it was established that the deduced 16S rDNA sequences of NIOT_MV 2 was highly homologous (96.34%) with reported sequences of Pseudomonas sp. (GenBank: LC547998.1). Sequence analysis also specified that 16S rDNA sequences of NIOT_MV 2 was closely related to the phylogenetic neighbors; Pseudomonas sp. KU681078.1 and Pseudomonas sp. EF119847.2 with sequence similarity of 91% and 88%, respectively. Phylogenetic analysis based on neighbor joining tree (Fig. 17) further revealed that strain NIOT_MV 2 formed a distinct branch with reported Pseudomonas sp. isolates. 16S rDNA sequences of NIOT_MV 6 were highly homologous (98%) with reported sequences of Rhizobium tropici (GenBank: MT539147.1). Sequence analysis also indicated that 16S rDNA sequence of NIOT_MV 6 was highly homologous to the phylogenetic neighbors; Rhizobium sp. LC498520.1 and Rhizobium miluonense JN896360.1 with sequence similarity of 90% and 89%. Neighbor-joining tree also disclosed that strain NIOT_MV 6 forms a single cluster with Rhizobium tropici (Fig. 18). The sequences of NIOT_MV 7 also established 93% homology with the previous report of Sulfurimonas denitrificans NR_118685.1. BLAST analysis also indicated that 16S rDNA sequences of NIOT_MV 7 was found extremely related to the phylogenetic neighbors; Sulfurimonas denitrificans NR_074133.1 and Sulfurimonas sp LC029406.1 with the similarity between 91 and 86%. Neighbor-joining tree also disclosed a distinct cluster between NIOT_MV 7 and Sulfurimonas denitrificans NR_118685.1. Upon analysis, it was established that the deduced 16S rDNA sequences of NIOT_MV 12 was highly homologous (91.24%) with reported sequences of Bacillus cereus (GenBank: MT052656.1). Sequence analysis also specified that 16S rDNA sequences of NIOT_MV 12 was closely related to the phylogenetic neighbors; Bacillus sp. MN456831.1 and Bacillus cereus AY138274.1 with sequence similarity of 85% and 81%, respectively. Phylogenetic analysis based on neighbour-joining tree further revealed that strain NIOT_MV 12 formed a distinct branch with Bacillus cereus MT052656.1.
The sequences of NIOT_MV 14 also established 94% homology with the previous report of Geobacillus kaustophilus (GenBank: MF965133.1). BLAST analysis also indicated that 16S rDNA sequences of NIOT_MV 14 was found extremely related to the phylogenetic neighbors; Geobacillus kaustophilus NR_114089.1 and Geobacillus kaustophilus EU652083.1 with the similarity between 78 and 72%. Neighbor-joining tree also disclosed a distinct cluster between NIOT_MV 14 and Geobacillus kaustophilus MF965133.1. 16S rDNA sequences of NIOT_MV 15 were highly homologous (99%) with reported sequences of Ruminococcus albus (GenBank: AF079847.1). Sequence analysis also indicated that 16S rDNA sequence of NIOT_MV 15 was highly homologous to the phylogenetic neighbors; Ruminococcus albus NR_115230.1 and Ruminococcus albus NR_113032.1 with sequence similarity of 76% and 68%. Neighbor-joining tree also disclosed that strain NIOT–NIOT_MV 15 forms a single cluster with Ruminococcus albus AF079847.1. The sequences of NIOT_MV 24 also established 91% homology with the previous report of Bacillus thuringiensis (GenBank: MT292101.1). BLAST analysis also indicated that 16S rDNA sequences of NIOT_MV 24 was found extremely related to the phylogenetic neighbors; Bacillus thuringiensis MT279583.1 and Bacillus thuringiensis MT279582.1 with the similarity between 77 and 72%. Neighbor-joining tree also disclosed a distinct cluster between NIOT_MV 24 and Bacillus thuringiensis MT292101.1. 16S rDNA sequences of NIOT_MV 25A was highly homologous (97%) with reported sequences of Streptococcus agalactiae (GenBank: KP294526.1). Sequence analysis also indicated that 16S rDNA sequence of NIOT_MV 25A was highly homologous to the phylogenetic neighbors; Streptococcus agalactiae AB297817.1 and Uncultured Streptococcus sp. JX841322.1 with sequence similarity of 64% and 59%. Neighbor-joining tree also disclosed that strain NIOT_MV 25A forms a single cluster with Streptococcus agalactiae KP294526.1.
The sequences of NIOT_MV 26 also established 96% homology with the previous report of Pseudomonas carboxydohydrogena (GenBank: NR_024703). BLAST analysis also indicated that 16S rDNA sequences of NIOT_MV 26 was found extremely related to the phylogenetic neighbors; Uncultured bacterium clone KJ600901.1 and Oligotropha carboxidovorans AB099659.1 with the similarity between 79 and 82%. Neighbor-joining tree also disclosed a distinct cluster between NIOT_MV 26 and Pseudomonas carboxydohydrogena NR_024703. 16S rDNA sequences of NIOT_MV 27 were highly homologous (97%) with reported sequences of Paracoccus denitrificans (GenBank: NR_114145.1). Sequence analysis also indicated that, 16S rDNA sequence of NIOT_MV 27 was highly homologous to the phylogenetic neighbors; Uncultured Paracoccus sp. clone MT491243.1 and Paracoccus sp. AJ012068.1 with sequence similarity of 84% and 79%. Neighbor-joining tree also disclosed that strain NIOT_MV 27 forms a single cluster with Paracoccus denitrificans NR_114145.1. Of 79 mud volcano associated bacteria, 38 isolates were confirmed as IAA potential strains. Based on the phylogenetic analysis the isolates were categorized under 29 genera, Bacillus, Alteromonas and Pseudomonas were the most dominant.
Identification of mud volcano associated IAA potential strain NIOT_MV 31
The isolate NIOT_MV 31 is a Gram-positive-rod, spore forming bacterium with a cell length of up to 4 μm and a diameter of 1.5 μm, Colonies were 1–2 mm, pale yellow, with morphologies of low convex to convex, entire, and were granular, or ground glass in appearance, entire, smooth, and very sticky or mucoid. The strain was catalase positive, gas production negative on TSI agar, indole negative and gelatin positive. The strain was negative for urease, indole, and positive for VP test. Based on the cultural and biochemical characteristics, the strain was categorised under the genus Bacillus.
Phylogenetic relationship of the stain NIOT_MV 31 was ascertained based on the 16S rDNA sequence similarity with reported strains using BLAST sequence similarity search. Upon analysis, it was established that the deduced 16S rDNA sequences of NIOT_MV 31 was highly homologous (97.14%) with reported sequences of Bacillus megaterium (GenBank: GU186111.1). Sequence analysis also specified that 16S rDNA sequences of NIOT_MV 31 was closely related to the phylogenetic neighbors; Bacillus megaterium, KX197921.1 and Bacillus megaterium KF010350.1 with sequence similarity of 94% and 86%, respectively. Phylogenetic analysis based on neighbour-joining tree (Fig. 19) further revealed that strain NIOT_MV 31 formed a distinct branch with reported Bacillus megaterium (GenBank: GU186111.1). Based on the phylogenetic analysis, the strain NIOT_MV 31 was confirmed as Bacillus megaterium NIOT_MV 31.
Discussion
Mud volcanoes of the Andaman Islands have received more attention, since they are one of the important features of this tectonic setting, located within an ocean basin that has one of the highest sedimentation rates in the world. The present study provides the first overview of the bacterial community in active mud volcano in Baratang Islands. Physical and chemical analyses from this study indicate that, this environment is an alkaline-moderate saline zone. Similar to the saline mud volcano at San Biagio-Belpasso and the Paclele Mari and Mici mud volcano in the Carpathian Mountains [1] nearly 40% of the bacterial 16S rDNA sequences from the Baratang mud volcano were related to clones or cultures from marine sediment and 36% of total acinobacterial 16S rDNA sequences (data not shown) were related to clones from submarine mud volcano and alkaline–saline lake sediments. Most of the microorganisms observed in this study are likely to be indigenous to this particular habitat.
The most abundant bacterial group detected in the Baratang mud volcano was allocated to the Proteobacteria (79%). Of these, the Deltaproteobacteria were the predominant group in this phylum, followed by Betaproteobacteria and Alphaproteobacteria. The predominance of the Deltaproteobacteria has also been observed in other terrestrial mud volcanoes [1]. Non-SRB (sulfate-reducing bacteria) sequences of the Deltaproteobacteria were recovered in the present study. Specifically, phylotype NIOT_MV 40 was related with Nitrosomonas cryotolerans, an iron-reducing bacteria (IRB) that reduces Fe(III) using common fermentation products such as acetate, lactate, propionate, formate or hydrogen as electron donors [38]. IRB is believed to play an important role in iron cycling and alkalinity generation and, therefore, represents a potential bioremediation tool [24]. Phylotype of NIOT_MV 33 was identical to the selenate-respiring bacterium Pelobacter seleniigenes, which uses acetate as the carbon substrate and is able to grow fermentatively on short-chain organic acids such as lactate, citrate and pyruvate [28]. Phylotype of NIOT_MV 29 was related to Malonomonas rubra, a microaerotolerant facultative anaerobic bacterium found in mineral medium with malonate as the sole source of carbon and energy [5]. Finally, Phylotype of NIOT_MV 31 was related to Bacillus megaterium that is able to produce a variety of proteins and sources of bioremediation, plays important role in producing numerous proteins that are commonly used in the medical and agricultural field. Based on 16S rDNA sequence analysis, we propose that the actinobacterial community in the Baratang mud volcano was dominated by Streptomyces, which is similar to other terrestrial and submarine mud volcanoes [25].
Sustainable agriculture has evolved from three perspectives: system of production to achieve self-sufficiency in food, concept of stewardship and means of sustaining rural communities. The indiscriminate use of chemical pesticides not only causes pollution but also leads to uncalled losses of microbial diversity in the natural environment. In view of this, usage of bio-based fertilizers and pesticides are one of the promising ways to enhance crop productivity and to manage the plant diseases. In this view, use of plant growth promoting bacteria has an important role in developing promising method for crop management [34]. The PGPR mechanisms to promote plant growth are of diverse nature such as phosphorus solubilisation, production of plant hormones [27] and excretion of diverse compounds, such as antibiotics or proteolytic enzymes. Some plant–beneficial microorganisms are known to antagonize plant pathogens through competition for nutrients, parasitism by means of hydrolytic enzyme production; inhibition of the pathogens by anti-microbial compounds; induction of systemic resistance in host plants [8]. As a new initiative in the search of agriculturally important microorganisms from various sources, we have isolated the bacteria from mud volcano of the great Andaman archipelago. Though studies proving that the microorganisms isolated from the terrestrial mud volcano shows potentiality, such as hydrocarbon utilization and production of methane gas [1], the current study was carried out to find out their potential microbial source as plant growth promoters and its effective on in vitro and in vivo capability in the terms of field experiment as well as plant tissue culture.
Present study was carried out to isolate and identify the plant growth beneficial bacteria in the active mud volcano of Baratang Islands. Auxin and IAA produced by bacteria enhances plant cell elongation, cell division and better root growth [12]. It was interesting to note that about 87% of our isolates are IAA producers. Under field conditions precipitated phosphates should be solubilized to readily available ones for plant growth, which can be done by plant growth promoting bacteria [39]. The production medium optimisation method has been applied to IAA production, but optimisation of medium components for IAA production by Bacillus megaterium is yet to be reported. Bacillus megaterium species typically require organic carbon and inorganic nitrogen sources plus mineral salts for growth as well as secondary metabolite production [16]. The IAA-producing activity of PGPR varies among species and is greatly influenced by culture conditions, growth stage and substrate ability [21]. Optimisation of the fermentation medium is imperative for maximising the ability of microbes to produce secondary metabolites on an industrial scale. Although carbon nutrients are essential, nitrogen sources and other micronutrients should not be neglected when optimising production [37].
The results of medium optimization experiments showed that in addition to nutrients in the fermentation medium, tryptophan and incubation time played an important role in IAA production by NIOT_MV 31; IAA production was nil in the absence of tryptophan to 16.37 mg/ml in its presence, strongly indicating that tryptophan is a precursor for IAA production. Furthermore, NIOT_MV 31 appears to secrete IAA via a tryptophan-dependent biosynthetic pathway. This finding is consistent with the knowledge that actinobacteria possess the ability to produce the auxin phytohormone IAA in the presence of a suitable precursor such as L-tryptophan [11]. However, other pathways may be included in this mechanism because some bacteria possess more than one pathway [33]. We optimised the fermentation medium by tryptophan supplementation, NaCl concentration, carbon and nitrogen sources, pH and incubation time with various steps. NIOT_MV 31 produced only 9.21 mg/mL IAA in the original fermentation medium in the presence of tryptophan, but the titer was increased to 42.63 mg/mL after the optimisation, equating to a fourfold increase in IAA production. These findings are consistent with previous reports demonstrating the advantages of the medium optimizing approach [29]. TLC analysis of IAA standards and extracted samples revealed identical Rf values, consistent with previous studies [40].
In vivo plant growth promotion assays showed that, the plants treated with purified B-IAA exhibited the highest root and shoot lengths, as well as fresh and dry weights. Although plants treated with SP also displayed increased root elongation superior to that of plants treated with C-IAA and control (water), other characteristics were inferior to those of control plants for poor growth. Seeds treated with IAA from NIOT_MV 31 displayed significantly fast germination, increased growth, and those treated only with water (control) did not exhibit significant seed germination and plant growth promotion. Thus, the NIOT_MV 31 IAA promoted plant growth, which may be due to metabolites induced by NIOT_MV 31 IAA. However, at nearly every developmental stage (embryonic and postembryonic) and in every growth process (formation of lateral organs and growth of leaves), plants were affected by IAA directly or indirectly via secondary induced signalling molecules [26]. IAA compounds produced by NIOT_MV 31 played an essential role in plant growth promotion because the main function of auxins is to stimulate root elongation, and this improvement was obvious in NIOT_MV 31 treated plants.
In this study, we optimised IAA production by Bacillus megaterium NIOT_MV 31, the mud volcano associated bacteria which is best suited for commercial use in plant tissue culture and agriculture. A suitable medium for improved IAA production was successfully established, and IAA production was elevated by optimising the culture conditions. In pot assays and in vitro seed germination assays proven that IAA produced by Bacillus megaterium NIOT_MV 31 promote growth of plants, seed germination, rooting and root elongation. Finally, the results provide strong evidence that Bacillus megaterium NIOT_MV 31 is a promising and effective PGPR inoculant for plant growth promotion that may enhance the growth and crop yields.
Due to their unique geomorphic and prolonged biogenic succession, the Baratang mud volcanoes represent an unusual microbial ecosystem. More than 50% of the sequences of the bacterial 16S rDNA sequences retrieved in this study showed 90–96% similarity to sequences from isolated bacteria or reported clone sequences, and all the phylotypes of the actinobacterial sequences obtained in this study were affiliated with uncultured clones. These findings indicate that some novel microbial groups and potential indigenous species were likely present in this ecosystem. This work is a first step toward better understanding the role of microbial communities in mud volcanoes in biogeochemical cycles. Further researches including the development of appropriate culturing methodologies and activity assays are necessary to elucidate the microbial ecological roles in these systems.
Data availability
Phylogenetic data used in this research work are downloadable from NCBI (https://www.ncbi.nlm.nih.gov/). All other raw and processed data are available upon request to the corresponding author.
References
Alain K, Holler T, Musat F, Elvert M, Treude T, Kruger M. Microbiological investigation of methane- and hydrocarbon discharging mud volcanoes in the Carpathian Mountains, Romania. Environ Microbiol. 2006;8:574–90.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Balasubramanian A. Technical report. The Andaman’s Mud Volcanoes. 2008. p. 5–7.
Baskaran R, Vijayakumar R, Mohan PM. Enrichment method for the isolation of bioactive actinomycetes from mangrove sediments of Andaman Islands, India. Malays J Microbiol. 2011;7:22–8.
Bovre K, Holten E. Neisseria elongata sp. nov., a rod-shaped member of the genus Neisseria. Re-evaluation of cell shape as a criterion in classification. J Gen Microbiol. 1970;60:67–75.
Brick JM, Bostock RM, Silversone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilization on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57:535–8.
Colquhoun JA, Mexson J, Goodfellow M, Ward AC, Horikoshi K, Bull AT. Novel Rhodococci and other mycolataactinomycetes from the deep sea. Antonie Van Leeuwenhoek. 1998;74:27–40.
Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71:4951–9.
Das S, Lvla PS, Khan SA. Distribution and generic composition of culturable marine actinomycetes from the sediments of Indian continental slope of Bay of Bengal. Chin J Oceanol Limnol. 2008;26:166–77.
Ellaiah P, Kalyan D, Rao VS, Rao BV. Isolation and characterization of bioactive actinomycetes from marine sediments. Hindustan Antibiot Bull. 1996;38:48–52.
El-Tarabily KA, Sivasithamparam K. Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem. 2006;38:1505–20.
Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol. 1998;190:63–8.
Goodfellow M, Williams ST. Ecology of actinomycetes. Annu Rev Microbiol. 1983;37:189–216.
Hua NP, Kanekiyo A, Fujikura K, Yasuda H, Naganuma T. Halobacillus profundi sp. nov. and Halobacillus kuroshimensis sp. nov., moderately halophilic bacteria isolated from a deep-sea methane cold seep. Int J Syst Evol Microbiol. 2007;57:1243–9.
Jeffery LSH, Sahilah AM, Son R, Tosiah S. Isolation and screening of actinomycetes from Malaysian soil for their enzymatic and antimicrobial activities. J Trop Agric Food Sci. 2007;35:159–64.
Kontro M, Lignell U, Hirvonen MR, Nevalainen A. pH effects on 10 Streptomyces spp. growth and sporulation depend on nutrients. Lett Appl Microbiol. 2005;41:32–8.
Kumar KS, Sahu MK, Kathiresan K. Isolation and characterization of specromycetes producing antibiotics, from a mangrove environment. Asian J Microbiol Biotechnol Environ Sci. 2005;7:457–64.
Kutchma AJ, Roberts MA, Knaebel DB, Crawford DL. Small-scale isolation of genomic DNA from Streptomyces mycelia or spores. Biotechniques. 1998;24:452–6.
Kwon KK, Woo JH, Yang SH, Kang JH, Kang SG, Kim SJ, Sato T, Kato C. Altererythrobacter epoxidivorans gen. nov., sp. nov., an epoxide hydrolase-active, mesophilic marine bacterium isolated from cold-seep sediment, and reclassification of Erythrobacter luteolus Yoon et al. 2005 as Altererythrobacter luteolus comb. nov. Int J Syst Evol Microbiol. 2007;57:2207–11.
Mincer TJ, Jensen PR, Kauffman CA, Fenical W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol. 2002;68:5005–11.
Mirza MS, Ahmad W, Latif F, Haurat J, Bally R, Normand P, Malik KA. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil. 2001;237:47–54.
Murphy P, Hill RT. Marine visions become reality: drugs from the sea. Biofuture. 1998;179:34–7.
Niemann H. Novel microbial communities of the Haako Mosby mud volcano and their role as a methane sink. Nature. 2010;443:854–8.
Omoregie EO, Niemann H, Mastalerz V, de Lange GJ, Stadnitskala A, Mascle J, Foucher JP, Boetius A. Microbial methane oxidation and sulfate reduction at cold seeps of the deep Eastern Mediterranean Sea. Mar Geol. 2009;261:114–27.
Pachiadaki MG, Lykousis V, Stefanou EG, Kormas KA. Prokaryotic community structure and diversity in the sediments of an active submarine mud volcano (Kazan mud volcano, East Mediterranean Sea). FEMS Microbiol Ecol. 2010;72:429–44.
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004;135:279–86.
Reyes I, Bernier L, Antoun H. Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modified strains of Penicillium rugulosum. Microb Ecol. 2002;44:39–48.
Riviere D, Desvignes V, Pelletier E, Chaussonnerie S, Guermazi S, Weissenbach J, Li T, Camacho P, Sghir A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009;3:700–14.
Sasirekha B, Shivakumar S. Statistical optimization for improved indole- 3-acetic acid (IAA) production by Pseudomonas aeruginosa and demonstration of enhanced plant growth promotion. J Soil Sci Plant Nutr. 2012;12:863–73.
Sato Y, Nishihara H, Yoshida M, Watanabe M, Rondal JD, Concepcion RN, Ohta H. Cupriavidus pinatubonensis sp. nov. and Cupriaviduslaharis sp. nov., novel hydrogen-oxidizing, facultatively chemolithotrophic bacteria isolated from volcanic mudflow deposits from Mt. Pinatubo in the Philippines. Int J Syst Evol Microbiol. 2006;56:973–8.
Singh SL, Baruah I, Bora CT. Actinomycetes of Loktak habitat: Isolation and screening for antimicrobial activities. Biotechnology. 2006;5:217–21.
Sivakumar K, Sahu MK, Thangaradjou T, Kannan L. Research on marine actinobacteria in India. Indian J Microbiol. 2007;47:186–96.
Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31:425–48.
Sturz AV, Christie BR, Nowak J. Bacterial endophytes: potential role in developing sustainable system of crop production. Crit Rev Plant Sci. 2000;19:01–30.
Subramani R, Sipkema D. Marine rare actinomycetes: a promising source of structurally diverse and unique novel natural products. Mar Drugs. 2019;17:249.
Takai K, Moyer CL, Miyazaki M, Nogi Y, Hirayama H, Nealson KH, Horikoshi K. Marinobacter alkaliphilus sp. nov., a novel alkaliphilic bacterium isolated from subseafloor alkalineserpentine mud from ocean drilling program site 1200 at South Chamorro Seamount. Mariana Forearc Extremophiles. 2005;9:17–27.
Treichel H, de Oliveira D, Mazutti MA, Di Luccio M, Oliveira JV. A review on microbial lipases production. Food Bioprocess Technol. 2010;3:182–96.
Vandieken V, Mußmann M, Niemann H, Jørgensen BB. Desulfuromonas svalbardensis sp. nov. and Desulfuromusa ferrireducens sp. nov., psychrophilic, Fe(III)- reducing bacteria isolated from Arctic sediments, Svalbard. Int J Syst Evol Microbiol. 2006;56:1133–9.
Verma SC, Ladha JK, Tripathi AK. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;9:127–41.
Xie H, Pasternak J, Glick BR. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr Microbiol. 1996;32:67–71.
Acknowledgements
The author’s great fully acknowledge the financial support given by the Earth System Sciences Organization, Ministry of Earth Sciences, Government of India, New Delhi to conduct the survey and research. The authors are thankful to Dr. M. A. Atmanand, Director, ESSO-National Institute of Ocean Technology (NIOT), Chennai for providing the necessary facilities to perform this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The research concept and the experiments were executed by BM, LA and KA. NVV and GD analyzed the data and reviewed the manuscript. All of the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meena, B., Anburajan, L., Aryamol, K. et al. Studies on biodiversity and bioprospecting of active mud volcano associated bacteria and actinobacteria from Baratang, Andaman Islands, India. Syst Microbiol and Biomanuf 3, 339–357 (2023). https://doi.org/10.1007/s43393-022-00118-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00118-3