Abstract
Study design
Retrospective review.
Objective
To describe clinical presentation, surgical management, long-term results, and complications in patients with segmental spinal dysgenesis (SSD). In addition, we sought to emphasize early surgery for this complex congenital abnormality.
Summary
SSD is a rare congenital malformation characterized by focal stenosis, spinal subluxation, kyphosis, and absence of the nerve roots. Neurologic function ranges from normal to complete paraplegia. Progression of the deformity and neurologic deterioration is the rule.
Methods
An independent spinal surgeon reviewed the complete records of 19 patients with SSD, between 1998 and 2015 at a single institution. Mean follow-up was 10 years and 6 months (2–14 years).
Results
We evaluated 11 males and 8 females, with a mean age of 2 years and 9 months (5 months–15 years). The dysgenetic segment involved an average of 2.9 vertebrae (1–5); the upper thoracic region was most commonly involved in ten cases. Fifteen patients had severe spinal stenosis. 14 patients presented neurological deficits and 15 patients had associated organ and musculoskeletal anomalies.Twenty-seven surgeries were performed, a mean of 1.76 procedures (1–5) to obtain solid fusion. Neurologic function improved in four, deteriorated in three, and remained unchanged in 12 patients Seven complications were recorded.
Conclusion
We strongly recommend decompression and fusion as soon as possible to preserve or prevent neurologic deterioration. Although challenging, it was possible to achieve a solid instrumented fusion in all cases; however, a high rate of patients may deteriorate or not recover neurological status after surgery.
Level of evidence
Level IV evidence
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Segmental spinal dysgenesis (SSD) is a rare congenital anomaly, usually located in the thoracolumbar or lumbar spine, and focally characterized by kyphosis or kyphoscoliosis, vertebral subluxation, spinal instability associated with a stenotic canal, narrowing of the thecal sac, and absence of nerve roots within the involved segments [1, 10]. Relative indemnity of the vertebrae and spinal-cord segments cephalad and caudal to the injury is typical. SSD is usually discovered on prenatal ultrasonography.
In 1988, Scott et al. [1] published the first series of three patients with dysgenesis of the lumbar and thoracolumbar spine characterized by focal abnormalities associated with spinal-cord and nerve-root compression. Faciszewski et al. [2] subsequently identified a series of 17 patients with SSD defining it as a separate entity different from lumbosacral agenesis due to spinal deformity, as progression, treatment, and outcome are distinct.
Typical features of the dysgenic spinal segment are the lack of development of vertebral bodies, congenital absence of the pedicle and posterior arches, an osseous ring that encircles the stenotic canal (absence of neurocentral cartilage at the dysgenic spine), a thin spinal cord, a band-like structure with absence of emerging nerve roots, and dural-sac compression. This formation failure of the intercalary segment of the spine (non-articular processes, discs, and ligaments) generates segmental instability manifested by anterior or lateral subluxation and stenosis of the spinal canal [2].
Neurological presentation is variable, ranging from a neurologically intact patient to paraplegia. Bladder or sphincter involvement is a common finding [1, 2]. The typical clinical course is progression of the deformity and related neurological impairment [3, 4].
SSD occurs in association with orthopedic abnormalities, such as clubfoot and hip dysplasia, as well as organic alterations, including congenital heart defects, renal anomalies, or facial and rib malformations.
The aim of this study was to describe the clinical presentation, diagnosis, surgical management, long-term results, and complications in patients with SSD. In addition, we sought to emphasize early surgery for this complex congenital abnormality.
Materials and methods
We retrospectively analyzed a series of 19 patients with complete records treated at the spinal unit of a single tertiary pediatric hospital between 1998 and 2015. The study had institutional review board (IRB) approval. The inclusion criteria were all patients met the diagnostic criteria for SSD [2] (characterized by focal kyphoscoliosis, vertebral subluxation, spinal instability associated with a stenotic canal, narrowing of the thecal sac, absence of nerve roots within the involved segments and relative indemnity of the vertebrae and spinal-cord segments cephalad and caudal to the injury is typical) and exclusion criteria were fundamentally all patients with congenital vertebral pathology who did not meet the strict criteria for segmental spinal dysgenesis.
Mean follow-up was 10 years and 6 months (range 2 years and 1 month–14 years). The study was conducted by a trained spine surgeon who performed the review of the clinical database as well as radiographs, computed tomography (CT) scans, and magnetic resonance imaging (MRI) studies before and after surgery.
Of the 19 patients evaluated, two patients were lost to follow-up; the first after 5 years of follow-up and the second 26 months after surgery. Both were included in this series because their clinical and radiological records were complete and follow-up was longer than 2 years.
All patients were studied with posteroanterior and lateral radiographs of the entire spine, complete spinal MRI and 2D- and 3D-reconstruction CT scans to better understand the pathomorphology of the spinal segment.
The spinal-canal diameter was evaluated to define as severe narrowing when obliteration was greater than 50% and moderate when obliteration was less than 50%.
Results
The study cohort included 11 boys and eight girls with a mean age at diagnosis of 2 years and 5 months (range 3 months–14 years and 9 months). Mean age at the time of surgery was 2 years and 9 months (range 5 months–15 years and 1 month).
The most common spinal deformity was kyphosis in 15 cases, kyphoscoliosis in three cases, and scoliosis associated with anterior spinal dislocation in only one case. (Table1).
The average number of vertebrae involved was 2.94 (range 1–5). The proximal thoracic spine was the most commonly affected segment in ten patients, followed by the thoracolumbar spine in five cases, the lumbar spine in two, and the cervicothoracic spine in two other patients.
Fifteen patients had severe spinal-canal stenosis (greater than 50%), while four had moderate stenosis (less than 50%) on anteroposterior and sagittal MRI. The dimension of the stenosis in the dysgenic segment was measured on sagittal and axial MRI and compared with the normal cephalad and caudal segments.
Mean stenosis rate was 72% (range 58–92%) in the severe stenosis group and 42% (range 35–48%) in the moderate stenosis group. On sagittal MRI the offset between the cephalad and caudal spine at the dysgenic level was measured. Fifteen patients had anterior subluxation with the cephalad segment lying anteriorly to the caudal spine. Mean offset was 82% (range 68–100%). Four patients presented tethering of the spinal cord. All of them underwent untethering surgery; one patient before deformity correction and the other three simultaneously with correction and fusion.
Mean preoperative Cobb values were: kyphosis 55° (range 38°–93°), kyphoscoliosis [kyphosis 72° (range 47°–91°)—scoliosis 62° (range 31°–82°)], and scoliosis 44° (range 15°–77°). After surgical correction, mean postoperative Cobb angles were: kyphosis 16° (range 6°–28°), kyphoscoliosis [kyphosis 18° (range 12°–22°)—scoliosis 20° (range 10°–24°)], and scoliosis 20° (range 13°–30°).
At the last follow-up control, angle values were: kyphosis 22° (range 8°–30°), kyphoscoliosis [kyphosis 16° (range 12°–29°)—scoliosis 18° (range 13°–25°)] and scoliosis 21° (range 15°–35°). Table 2
At the time of surgery, nine patients presented paraparesis, four paraplegia and one case with quadriparesis; while, the five remaining cases had a normal neurological examination. Six patients of 19 had a neurogenic bladder.
Fifteen (79%) of 19 patients had associated anomalies; nine patients presented musculoskeletal deformities (Klippel Feil syndrome in two, bilateral equinocavovarus feet in four, cavovarus feet in one, pterygium of the knee in one, and costal plastron in one) and six patients with organic malformations (of the kidney in three, of the heart in two, and a craniofacial malformation, hairy patch, and blindness in one).
Twelve patients had severe bilateral muscle hypotrophies and six of them presented with deformation of the lower limbs
None of the patients had open dysraphism; whereas, ten patients presented with closed–occult (muscle- and skin-covered) spinal dysraphism.
A total of 27 surgeries were performed in 19 patients, with a mean of 1.76 procedures (range 1–5) until solid fusion was achieved.
Of 19 patients, five underwent surgery using a simultaneous double approach on a single day or on staged days depending on surgical time. In all cases the surgery was started with anterior decompression followed by posterior fusion. Five patients underwent decompression by vertebral column resection (VCR), four patients posterior spinal instrumentation (PSI) and fusion, four underwent just anterior decompression and interbody fusion and one more patient with posterior spinal fusion alone. (Table 3).
Spinal-cord decompression was performed in 15 patients, in four of them by a single anterior approach, in five through a double approach (Fig. 1), five by vertebral column resection (VCR), (Fig. 2) and only one patient through a posterolateral approach.
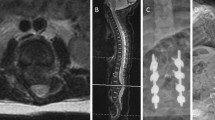
1 A neurologically intact 11-month-old patient with congenital spinal dislocation. 2 Lateral X-ray showing complete thoracolumbar dislocation. 3 Sagittal MRI showing complete spinal dislocation and stenotic canal. 4 2D CT lateral reconstruction: Thoracolumbar kyphosis plus complete dislocation of the cephalad spine. 5–6 3D CT reconstruction; frontal and lateral views showing 2 levels of dysgenic segment. 7–8 A 5-year-old neurologically intact patient with normal daily activities. 9 PA X-ray at the 5-year follow-up after 360 thoracolumbar instrumented fusion. 10 Lateral X-ray showing complete restoration of the thoracolumbar area
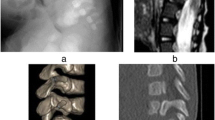
11–12 A 3-year-1-month-old neurologically intact patient with congenital dislocation, congenital stenosis, and segmental spinal dysgenesis. 13 Lateral X-ray: Thoracolumbar kyphosis T10–L3: 83 degrees. 14 Sagittal MRI showing complete spinal dislocation plus cord compression. 15–16 3D CT reconstruction; frontal and lateral views showing three levels of dysgenic segment. 17 Intraoperative reconstruction after 2-level vertebral column resection (VCR). 18–19 AP and lateral X-rays: immediate postop of VCR at the thoracolumbar level plus 360 instrumented fusion. 20–21 Clinical photos of immediate postop. 22–23 AP and lateral X-rays after 2 years of follow-up
Compared to the baseline neurological examination, four patients improved their neurological status (three cases with Frankel C showed an overall improvement, passing to Frankel D in two cases and Frankel E in the third). The remaining patient with Frankel E and sphincter involvement showed bladder continence in the long-term follow-up, while deterioration was observed in three, and 12 patients remained unchanged.
Seven complications were recorded. Three were surgery related, consisting of migration of the autologous fibular strut in one which required revision surgery and two cases with deep wound infection (Acinetobacter and Staphylococcus aureus) both resolved with debridement, irrigation plus intravenous and oral antibiotic; three were clinical complications (pleural effusion, ileus, and pneumonia). One patient died three years after surgery due to a cause unrelated to the surgical procedure.
Three patients showed progression of the deformity (kyphoscoliosis) more than 10 degrees at the last follow-up control requiring reoperation for fusion extension.
Thoracolumbosacral thermoplastic orthoses (TLSO) were prescribed in 11 patients for three–six months post-operatively. A halo vest was used in two children because of the cervicothoracic location of the dysgenesis.
Discussion
SSD is a complex disorder caused by defective embryogenesis of the neural tube and adjacent vertebral segments. SSD is a rare entity with a characteristic morphology defined by Faciszewski et al. [2, 11] in 1995 as including focal stenosis, congenital kyphosis with absence of the pedicles and transverse processes, hypoplasia or agenesis of lamina, a hypoplastic spinal cord, and root agenesis. Spina bifida may be associated and the vertebral segments cranial and caudal to the anomaly are relatively normal. The morphological characteristics of this malformation are far more florid than the description of the posterior hemivertebra by Shapiro and Herring in 1993 [8]. While Scott et al. [1] described the first series and coined the term spinal dysgenesis, one of these three cases does not correspond to the morphological features of typical spinal dysgenesis due to the finding of stenosis cephalad to the segment involved and associated lumbosacral malformations.
The etiology of SSD remains uncertain. Genetic, microvascular, infectious, or toxic insults during embryological development may play a role. Valdez Quintana et al. [17] documented abnormalities of the anterior spinal artery at the dysgenic segment proposing an ischemic environment as a possible cause. Tortori-Donati et al. [16] suggested a causal event may occur during the gastrulation period in which early embryonic chordamesodermal derangement disturbs the development of the somites. The spinal cord malformation may result from a genetically induced alteration in the process of embryonic axial patterning with elimination of wrongly specified cells by apoptosis.
Dias et al. [5] termed this malformation “congenital vertebral dislocation”, suggesting a disorder in embryogenesis from the sixth week of intrauterine life, which would develop at the expense of torsional and translational mechanisms of the vertebral segment prior to the time of vertebral chondrogenesis.
Dubousset et al. [6, 7] also defined the condition as congenital vertebral dislocation, considering it is produced by insufficient development of the anterior spine with translocation and anterior angulation of the vertebral body producing kinking and stenosis of the spinal canal; only, a few of his ten cases reported meet the definition of true dysgenesis.
Chellathurai et al. [15] recently proposed a classification of SSD divided into two types based on its embryological origin: Type I: presence of hypoplasia or dysgenesis of the spinal cord and roots, dysgenesis of the vertebral bodies, but absence of canal stenosis and kyphosis, presenting a bulging spinal cord at the distal level; its causal event occurs during the 3rd week of gestation (gastrulation period); Type II: presence of severe vertebral dislocation with stenosis of the spinal canal, and vertebral, medullary, and root dysgenesis; its causal event occurs during the 3rd and 6th intrauterine weeks (formation of the spine by somites).
Recently, Wang et al. [18] have described a new entity called junctional neural tube defect (JNTD) with clinical and radiological features that are similar to SSD. Whereas in SSD the bone component is mainly involved, in JNTD the emphasis is on the neural component. The authors suggest a common pathoembryologic error during junctional neurulation.
In our series, all patients had clinical and imaging characteristics of SSD as to the criteria by Faciszewski et al. and would correspond to type II dysgenesis described by Chellathurai et al. [15]. On the other hand, type I dysgenesis would correlate better with the new form of congenital spinal dysraphism—JNTD—proposed by Wang et al. [18] characterized by the upper and lower cords appearing to be connected by a non-functioning fibrotic structure.
We agree with the surgical strategy for type II SSD described by Chellathurai et al., as congenital stenosis, spinal instability, and progression of the deformity will lead to the development of neurological deterioration.
Fourteen patients of our series had neurological deficits at the time of diagnosis (paraparesis in nine, paraplegia in four, and quadriparesis in one). Neurological examination was normal in five. We want to emphasize that not all patients with SSD are paraplegic as mentioned in some reports [13, 14]
The degree of neurologic deficit appears to depend not only on how hypoplastic or aplastic the spinal cord is, but also on the degree of residual function. In agreement with Tortori-Donati et al. [16], we found a correlation between neurological status and the extent of spinal-cord involvement visualized on MRI. In addition, a correlation was observed between the level of dysgenesis and the neurological deficit: the more cephalad the dysgenic segment, the more severe the neurologic impairment. Two patients with cervicothoracic SSD presented with quadriparesis and paraplegia, respectively, and eight patients with upper thoracic SSD had paraplegia (in two cases) and paraparesis (in six cases). Performing decompression surgery in children presenting with paraplegia is controversial [13, 14]. Surgery may not be necessary because the hypothetical pathoembryogenesis (failure of the joining of the primary and secondary neural tube with formation of a thin fibrotic band) rather than mechanical compression is the probable cause of the poorly functional or non-functional cord. We believe surgery is useful in those with minimal evidence of motor function, as they are at an increased risk for aggravation of the neurologic deficit associated with spinal instability and focal stenosis. In addition, early detection of spinal-cord tethering or low-lying conus and surgery may be beneficial to prevent progression of kyphoscoliosis and neurologic deterioration.
After decompression and fusion surgery, neurological status improved in four patients, deteriorated in three, and remained unchanged in 12. Fifteen of the nineteen patients underwent direct spinal-cord decompression and only four indirect decompression and fusion via deformity correction.
At the last follow-up visit, progression of the deformity had arrested in 16 patients and neurological status had stabilized in all patients; five patients remained neurologically intact with no changes on the last assessment.
We, therefore, disagree with Faciszewski et al. [2] that the use of instrumentation is an aggravating factor for spinal stenosis and neurological deterioration with growth. In our series, 14 patients underwent fusion with instrumentation and only one of them showed neurological deterioration without development of stenosis. No correlation between deterioration and instrumentation was found; however, retethering of the cord may have played a role.
Associated orthopedic and thoracic abnormalities were found in nine patients (47%), and six had organic malformations and abnormalities (32%). This high prevalence of malformations associated with spinal dysgenesis may be explained by differences in the pathogenesis of the disease.
Similar to Dubousset [6] and Faciszewski et al. [2], we believe that the only treatment option for SSD is surgical resolution. The use of casts or braces only delays treatment and leads to the worst scenario in the management of the deformity and prevention of neurological deterioration. Flynn et al. [9] reported deterioration in three of seven patients after the use of orthoses. We disagree with Scott et al. [1] and Tortori-Donati et al. [16] who advocated surgery only for those deformities that progress in spite of the use of the corset; SSD should be considered as an instable deformity that results in additional cord damage if we do not treat aggressively [12, 19].
Conclusion
In patients with SSD, we recommend early surgical treatment based on spinal-cord decompression and circumferential fusion in order to prevent neurological deterioration or damage and to arrest progression of the kyphotic deformity. However, despite early treatment, in a high percentage of these patients, neurological status may not change or may even deteriorate after surgery as preoperative compromise is often significant.
Although challenging, achievement of spinal-cord decompression and solid instrumented fusion was possible in all cases. We strongly support early decompression surgery for cord preservation and kyphosis deformity correction with instrumentation to provide the best-case setting for the growing spine.
References
Scott RM, Wolpert SM, Bartoshesky LE et al (1988) Segmental spinal dysgenesis. Neurosurgery 22:739–744
Faciszewski T, Winter RB, Lonstein JE et al (1995) Segmental spinal dysgenesis. a disorder different from spinal agenesis. J Bone Joint Surg Am 7:530–537
Winter RB, Moe JH, Wang JF (1973) Congenital kyphosis. Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am 55:223–256
McMaster MJ, Singh H (1999) Natural history of congenital kyphosis and kyphoscoliosis. A study of one hundred and twelve patients. J Bone Joint Surg Am 81:1367–1383
Dias MS, Li V, Landi M, Schwend R, Graab P (1998) The embryogenesis of congenital vertebral dislocation; early embryonic buckling? Pediatric Neurosurg 29:281–289
Dubousset J, Duval Beaupere G, Anquez L (1973) Deformations vertebreles congenitas compliquees de troubles neurologiques. In: Rougerie J (ed) Compressions medullaries. Masson, Paris, pp 193–201
Dubousset J (1994) Congenital Kyphosis and lordosis. In: Weinstein SL (ed) The pediatric spine: principles and practice, vol 1. Raven Press, New York, pp 245–258
Shapiro J, Herring J (1993) Congenital vertebral displacement. J. Bone Joint Surg. 75-A:656–662
Flynn J, Segmental ON, Dysgenesis S (1997) Early neurologic deterioration and treatment. J Pediatr Orthop 17(1):100–104
Zana E, Chalard F, Mazda K et al (2005) An atypical case of segmental spinal dysgenesis. Pediatr Radiol 35:914–917
Ofiram E, Winter RB, Segmental LJE, Dysgenesis S (2006) Case report of a 50-year follow-up after surgery at age 3 years. Spine 31:E59–E61
Bristol RU, Theodore N, Rekate HL (2007) Segmental spinal dysgenesis: report of four cases and proposed management strategy. Childs Nerv Syst 23:359–364
Cacciola F, Lippa L (2017) Segmental spinal dysgenesis associated with occult dysraphism: considerations on management strategies. J Craniovertebr Junction Spine 8(2):144–148
Naik S, Bhoi SK, Panigrahi K (2019) Segmental spinal dysgenesis: a rare congenital spinal malformation. Indian J Radiol Imaging 29(4):480–481
Chellathurai A, Ayyamperumal B, Thirumaran R (2019) Segmental spinal dysgenesis “redefined “. Asian Spine J 13(2):188–197
Tortori-Donati P, Fondelli MP, Rossi A et al (1999) Segmental spinal dysgenesis: neuroradiologic findings with clinical and embryologic correlation. Am J Neuroradiol 20:445–456
Valdez QM, Jean MJ, Darine E-C (2016) Fetal segmental spinal dysgenesis and unusual segmental agenesis of the anterior spinal artery. Childs Nerv Syst 32:1537–1541
Wang KC, Lee JS, Kim k. (2020) Do junctional neural tube defect and segmental spinal dysgenesis have the same pathoembryological background? Childs Nerv Syst. 36(2):241–250
Ford EG, Jaufmann B, Kaste S (1996) successful staged surgical correction of congenital segmental spinal dysgenesis and complete rotary subluxation of the thoracolumbar spine in an infant. J Pediatric Surg 31(7):960–964
Funding
This study did not receive financial support, grants or money for its development
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission. Made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Drafted the work or revised it critically for important intellectual content. Approved of the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Hospital Nacional de Pediatria Juan P. Garrahan approved this study.
Informed consent
Informed consent was obtained from all parents and legal guardians included in the study. Parents and legal guardians signed informed consent regarding publishing
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Remondino, R.G., Tello, C.A., Bersusky, E.S. et al. Surgical treatment of segmental spinal dysgenesis: a report of 19 cases. Spine Deform 9, 539–547 (2021). https://doi.org/10.1007/s43390-020-00209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-020-00209-y