Abstract
This study proposes a novel waste permanent magnet (WPM) recycling technology by avoiding conventional technology that relies on strong acid and developing a feasible process using relatively inexpensive reagents, thereby reducing overall process cost to acceptable levels. Nd2Fe14B WPMs were transformed into a fluoride/oxide composite material through oxidation/fluorination heat treatment. The phase transition from FeF3-NdF3 to Fe2O3-NdF3 composition was performed via heat treatment. The Fe2O3-NdF3 composition is selectively leached using an oxalic acid. It was confirmed that Fe2O3 was selectively leached, and NdF3 was leached at less than 1 wt.% under various leaching conditions. The neodymium fluoride produced using this technology is expected to be applicable to related fields such as Nd smelting flux or catalysts, and this technology is expected to be applied to various materials containing Fe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nd permanent magnets are among the strongest permanent magnets with the highest magnetic field, in high demand in devices that require strong magnetic fields while remaining lightweight and compact, such as microphone speakers, computer hard drives, electric vehicle motors, wind turbine generators, and medical MRI machines. Nd recovery is essential to stabilize the supply and demand of the rare earth element, and various methods are being explored [1,2,3,4]. The average annual growth rate of Nd, which is used in various applications, exceeds 22.5%.
There are few commercial methods to produce Nd metal as molten salt or electrodeposition. Considering molten salt, NdF3-LiF flux or other halides/chlorides are used as electrolyte systems [5, 6]. Because Nd permanent magnets are very fragile, a large amount of scrap is generated during the magnet manufacturing process, and this process scrap is known to be more than 30%. Most of Nd, the key element of Nd permanent magnets, is buried in China. Therefore, Nd recycling technology is very important.
This study presents a novel method for Nd recovery as a compound from waste permanent magnets (WPMs) using NdF3 synthesis. Although little is known about NdF3 solubility, a chemically stable material was produced. The conventional Nd permanent magnet recycling process used strong acid solutions for Fe and Nd selective separation, however, due to environmental concerns, a relatively mild and weak acidic technology for Nd selective recovery is required.
2 Methods
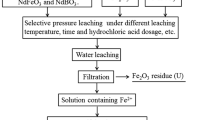
The fluorination heat treatment and selective leaching overall flowchart for WPMs is shown in Fig. 1.
2.1 Materials
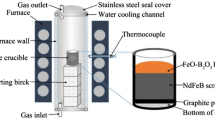
The WPM was obtained from the manufacturer and the powder was confirmed to be Nd2Fe14B permanent magnet through X-ray diffraction (XRD) (X-ray diffractometer, XRD-6100, Shimadzu, Japan) analysis in Fig. S1. The particle size was evaluated by particle size analyzer (PSA, Bluewave, Microtrac, Germany) in Fig. S2. The composition of WPM was analyzed by X-ray fluorescence (XRF) (X-ray fluorescence, XRF-1800, Shimadzu, Japan) in Table S1. The WPM powder was heat treated at 400 °C and 600 °C in air for oxidation from the metal phase, and XRD analysis was conducted on the powders, respectively, followed by phase transformation from oxide to fluorination. The oxidized powders were mixed with ammonium bifluoride (ABF). (ABF, NH4HF2, Sigma-aldrich, 98%) The ABF was mixed with mortar for 30 min.
2.1.1 Commercial powders used in fluorination heat treatment
Commercial powder was used to study the oxidized WPM material composition fluorination phase transformation. We experimented with fluorination reaction commercial Fe2O3 (Sigma-aldrich, 99%) and Nd2O3 (Sigma-aldrich, 99%), respectively. Considering Fe2O3, the Fe2O3 to FeF3 phase transition was observed through heat treatment at 350–600 °C at a molar ratio of 1:6.
Commercial Nd2O3 was also subjected to molar ratio heat treatment with ABF at a 1:4–1:6 ratio. The Nd2O3 fluorination heat treatment was performed at 600 °C.
2.1.2 WPM fluorination heat treatment
Fluorination heat treatment using commercial powders was performed on the WPM. The WPM powder was heat treated at 400 °C in air, thereafter, the ABF and oxidized WPM was mixed at a molar ratio of 1:6, and heat treatment was performed at 600 °C in an argon flow with low vacuum.
2.2 Leaching procedure
2.2.1 Commercial NdF3 solubility in various acid/base solutions
NdF3 solubility (Alfa-aesar, 99%) was evaluated using 10 M NH4OH, 4 M H2SO4, 4 M HNO3, 10 M HNO3, 4 M HCl, and 6 M HCl. The leaching tests were carried out at 25 °C, the solutions were maintained for more than 24 h, and the leached solution was analyzed by inductively coupled plasma (ICP) (iCAP Pro XP, ThermoFisher). The pulp density was set at 5 g/100 mL and RPM was fixed at 300.
2.2.2 Fluorinated WPM oxalic acid leaching study
WPM powders were heat treated for fluorination at 600 °C, the powder immersed in 0.01–0.6 M oxalic acid solution at 40–50 °C. The pulp density was set at 1%, and RPM was fixed at 300. The leaching procedure was conducted for 8 h, and the leached solution ICP analysis results and residual XRD analysis were conducted after reaction completion.
2.3 Thermodynamic calculations
All compositions were characterized by their Gibbs free energy of formation value, which quantify the energy released or consumed during transformation of a phase from its constituent elements in their standard state. The HSC Chemistry software 10.0, includes databases and various modules providing different types of chemical/thermodynamic calculations. The enthalpy and entropy values were available in their databases. We used the ‘Reaction equation’ module for calculation of changes of Gibbs free energy for various calcination temperatures” and “Equilibrium calculations” module for assumed that practical way to observe the effects of process variable, according to the calcination temperature and amounts of raw materials.
3 Results and discussion
3.1 Oxidation and fluorination heat treatment
The pulverized WPM powder XRD analysis established that it was an Nd2Fe14B permanent magnet. The particle size was confirmed to be approximately 1 μm. The WPM powder was subjected to oxidation heat treatment at 400 °C and 600 °C. The XRD analysis after heat treatment at 400 °C established that the main materials were Fe2O3 and Nd2O3 in Fig. 2.
XRD was used to identify the powder phases. The peaks observed at 2 theta = 24.14°, 33.15°, and 35.63° were identified as Fe2O3 (JCPDS #79–1741). Due to the relatively low amount of neodymium in the permanent magnet, the Nd2O3 minor peaks (JCPDS #21–0579) were observed at 2 theta = 27.85°, 46.30°, and 54.93°, detected via XRD. The 400 °C oxidation heat treatment results showed only Fe2O3 and Nd2O3, with no observation of the high-temperature FeNdO3 stable phase. In the 600 °C oxidation heat treatment, the observed FeNdO3 was identified at 2 theta = 22.84°, 32.54°, and 46.53° (FeNdO3, JCPDS #25–1149). Reactivity thermodynamic calculations with ABF were performed using HSC Chemistry 10 for the Nd2O3 and Fe2O3 produced through a 400 °C oxidation heat treatment, as well as for the NdFeO3 produced through a 600 °C oxidation heat treatment. The predicted reaction equations and their corresponding ΔG values are as follows:
(i) Fe2O3 + Nd2O3 + 6NH4HF2 = 2NdF3 + 2FeF3 + 6NH3(g) + 6H2O(g)
ΔG = – 785.46 kJ at 400 °C, ΔG = – 1,046.19 kJ at 600 °C in reaction (i) in Table S2.
(ii) 2NdFeO3 + 6NH4HF2 = 2NdF3 + 2FeF3 + 6NH3(g) + 6H2O(g)
ΔG = – 172.39 kJ at 400 °C, ΔG = – 237.302 kJ at 600 °C in reaction (ii) Table S2.
The thermodynamics calculation indicated that oxidation heat treatment at 400 °C would result in more spontaneous reactions, as well as a lower energy cost due to the facile phase transition at lower temperatures. We confirmed that both Fe2O3 and Nd2O3 were oxidized, respectively, whereafter we carried out a fluorination heat treatment study using commercial Fe2O3.
Heat treatment was performed at a 1:6 molar ratio with ABF at 350–600 °C. After heat treatment, XRD powder analysis was performed as shown in Fig. 3. At 350 °C, we confirmed that Fe2O3 was phase transformed to single-phase FeF3ˑ0.33H2O (JCPDS #76–1262). It was identified at 2 theta = 13.79°, 23.62°, and 27.80°. The powders showed that FeF3ˑ0.33H2O remained at 385 °C, however, crystallinity was weaker than that at 350 °C. We observed phase transform to FeF3 with minor amounts of FeF2 at 400 °C. It was determined that 2 theta = 23.84°, 33.42°, and 40.18°. (JCPDS #88–2023). We conducted fluorination heat treatment at 600 °C, in which the powders transformed to single-phase Fe2O3. It appears that the powders re-oxidized to Fe2O3 at high temperatures of above 400 °C [7].
(iii) NH4FeF4 + H2O + NH3 + H2 < 350 °C
(iv) FeF3 0.33 H2O + evaporated gas < 400 °C
(v) FeF3 + dehydration gas (nH2O) →Fe2O3 + HF(g)
Based on the above-presumed reaction equations (iii) ~ (v), thermodynamic calculations were performed for FeF3 re-oxidation.
(vi) 2FeF3 + 3H2O(g) = Fe2O3 + 6HF(g)
Assuming a reaction of (vi), the Gibbs free energy has a negative value above 500 °C (ΔG = – 16.97 kJ) in Table S3, calculated as ΔG = – 49.41 kJ at 600 °C. The ΔG value increased proportionally as the temperature increased. The above reaction was calculated as a spontaneous reaction as the temperature increased [8, 9]. When FeF3 reacted with evaporated O2 was calculated, ΔG was also negative. FeF3 was not stable with the oxygen reaction in Table S4.
To elucidate the Nd2O3 reaction with ABF, commercial Nd2O3 was used with various ABF molar ratios. The reaction temperature was maintained at 600 °C. The XRD patterns are shown in Fig. S3. We confirmed that single-phase NdF3 was observed above a molar ratio of 1:6. The diffraction patterns matched NdF3 (JCPDS #09–0416) in Fig. S3., and minor NdF2 (JCPDS #33–0934) peaks are observed. Previously, the re-oxidation by hydration regarding FeF3 could be confirmed through thermodynamic calculation and actual experiments for the high-temperature reaction. Considering NdF3, the thermodynamic calculation was conducted as NdF3 reacted with oxygen and hydration. It was confirmed that NdF3 is stable against oxygen or hydration at high temperatures. The reaction calculation showed that when the NdF3 reacted with H2O(g), the ΔG value was 146.15 kJ at 1000 °C (Table S5). Additionally, when reacting with oxygen, the ΔG value calculated positive at 1000 °C (Table S6).
From the reaction results of commercial Fe2O3 and Nd2O3 with ABF, we confirmed that Fe2O3 transformed to FeF3 below 400 °C, however, it re-oxidized to Fe2O3 at 600 °C. To confirm the actual WPM powder reaction results, the WPM powder oxidized at 400 °C was subjected to fluorination heat treatment at 400 °C and 600 °C, respectively. Consequently, XRD results of the powder obtained after heat treatment by solid mixing with ABF at 400 °C confirmed FeF3 at 2 theta = 23.83° and 54.29°, respectively. (JCPDS #75–0451) The main phase was confirmed as NdF3 and some Fe2O3 was also observed in Fig. 4.
It was established that FeF3 completely disappeared during the fluorination heat treatment at 600 °C, and only two phases of Fe2O3 and NdF3 were observed. When the FeF3 reacts with ABF at high temperatures of 600 °C or above, it can be determined that both commercial and WPM powders phase transition from FeF3 to Fe2O3. Higher NdF3 and Fe2O3 crystallinity was confirmed in the 600 °C fluorination heat treatment. We performed thermodynamic calculations of the oxidized WPM powder fluorination. The total amount of each component generated can be calculated using Fig. S4(a). Correlation between thermodynamic calculations and the actual experiments of selective specific material such as Nd2O3, Fe2O3, NdF3, FeF3 and H2O(g) were confirmed as shown in Fig. S4(b).
As a result of the SEM analysis, the WPM powder was observed at approximately 1 μm as shown in Fig. S5(a). Smaller particles were observed, which are believed to be due to surface oxidation from heat-generated friction created during the metal powder crushing/grinding process. It was confirmed that plate-like particles were entangled around the surface with a surface size of several nanometers.
In the powder after the oxidation heat treatment process at 400 °C, some oxidized particles were observed in Fig. S5(b). The primary particles were nano-sized, however, secondary particles sizes were observed at a maximum of 10 μm or greater.
3.2 Selective leaching
Little is known about NdF3 solubility in acid or base solutions [10]. Several acid/base solutions were prepared, and each concentration was set and maintained for more than a day to observe the leaching rate. The final pH of 10 M NH4OH was measured as 12.31 and NdF3 did not dissolve at all. The final pH of 4 M H2SO4 was measured as -1.44, and the Nd concentration was analyzed in the solution at 79.5 ppm. The final pH of both the 4 M and 10 M nitric acids was measured as – 1.35, and the Nd concentration was 30.9 ppm. Regarding 4 M HCl, the final pH was measured as – 0.15, and Nd was confirmed to have similar behavior to sulfuric acid at 80.6 ppm, however, this leaching rate was negligible. When leaching was attempted using 6 M HCl, it was performed for a maximum of 2 d, and NdF3 solubility was approximately 0.2%. It was confirmed that NdF3 is highly stable in the commonly used acidic solution. However, FeF3 is known to exist in various phases such as a number of hydrates and it is known that it could be leached into water and acid when it consists of hydrate [11].
Based on the pure NdF3 leaching results, using the Fe2O3 and NdF3 composite phase from fluorination heat treatment at 600 °C, only Fe2O3 could be selectively leached and separated. Fe2O3 selective leaching was attempted using oxalic acid. Oxalic acid is known as a weak acid with an acidity of 1.25 or 4.14 depending on the dissolved ionic form. There has been scant research on highly selective leaching techniques using a weak acid [12, 13]. This technology uses a weak oxalic acid, in contrast to most techniques which use strong acids or expensive ionic liquids, which can reduce the environmental burden and process costs. Selective Fe2O3 leaching with oxalic acid is well known [14,15,16], also especially the oxalic acid is preferable and more effective leaching agents such as citric, ascorbic, gluconic or malic acid for leaching out of iron oxide [17, 18]. There are several oxalic acid-assisted Nd magnet recycling studies [19, 20].
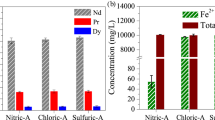
The overall Fe2O3 leaching rate in fluorinated-WPM powders under various leaching conditions is shown in Fig. 5, and that of rare earth material (Nd) in fluorinated-WPM powders under the same conditions is shown in Fig. 6.
During the after 2 h, the leaching rate exceeded 50% in most leaching conditions (except for 0.01 M oxalic acid). It was confirmed that the leaching rate increased continuously over time, and the 0.6 M Fe leaching rate approached 97.8% at 40 °C and 96.9% at 50 °C after 8 h. The 0.3 M leaching rate was approached below 80% even after 8 h. (71.8% after 4 h, 72.7% after 8 h, respectively) We considered the reaction time, it was expected that the leaching rate did not increase for 4 h. The reason for the negligible Fe leaching rate according to reaction time is that we considered to have occurred from re-precipitation as Fe oxalate during Fe2O3 leaching in oxalic acid. This was clearly confirmed when XRD analysis of the residue was performed as shown in Fig. 7. Optimal leaching conditions were confirmed by using a minimum amount of oxalic acid to minimize energy use and resources during leaching. Under experimental conditions, only Fe oxide could be selectively leached using a concentration of 0.08 M oxalic acid. Consequently, the leaching rate using 0.05 M oxalic acid was less than 60% within 8 h, and in the case of 0.01 M oxalic acid, the leaching rate was less than 20%. It can be expected that the oxalic acid concentration for leaching Fe2O3 in the powder was insufficient. This result contrasted from a Fe leaching rate of 70% or greater at a concentration of 0.08 M oxalic acid. As a result of selective leaching with oxalic acid through fluorination heat treatment, the rare earth (Nd) material leaching rate was suppressed to a maximum of 0.3%, confirming selective leaching behavior. A maximum Nd leaching rate of 0.3% was obtained using 0.6 M oxalic acid, where Fe2O3 was leached out very selectively, and it was confirmed that NdF3 material has a high chemical resistance to both weak acid/strong acid/base solutions. Rare earth leaching data for Nd, Dy and Pr are shown in Table 1.
It was confirmed that the main peaks were observed as Fe2O3 almost disappeared as the residue powder was heated up to 600 °C for fluorination before leaching as shown in Fig. 7. In particular, the Fe2O3 main peaks at 2 theta = 33.15°, and 35.63°, were not observed using 0.6 M at 50 °C. It was observed that Fe2O3 was completely leached by oxalic acid, and FeC2O4 · 2H2O (JCPDS #23–0293) was detected from XRD analysis of the residue after the Fe ions in the leachate were reduced for 8 h or more. It was shown that 2 theta = 18.46°, 18.86°, 29.71°, and 33.74° coincides with the main peaks, respectively. Fe oxalate dihydrate is composed of two hydrates, however, its solubility in water is known to be very low at 0.097 g/100 ml. Therefore, it was confirmed that it was not dissolved in an aqueous solution of 0.6 M oxalic acid and re-precipitated during the leaching process. It was confirmed that the amount of Fe oxalate decreased as the oxalic acid concentration for leaching decreased. Fe oxalate hydrate was hardly observed at 0.08 M and not observed at 0.05–0.01 M. This is expected to have insufficient driving force for Fe oxalate to be precipitated while Fe oxide is leached by oxalic acid. Therefore, it is considered that pure NdF3 can be recovered by selectively leaching sufficient Fe2O3 and separating it from NdF3 during multi-stage leaching at a concentration of 0.05 M.
4 Conclusion
To recycle WPMs, we developed a technology that can replace the existing strong acid-based technology such as sulfuric acid/hydrochloric acid. By inducing a solid-state reaction with solid ABF, a fluoride/oxide complex was prepared, and the prepared Fe oxide was leached with a weak acid, oxalic acid, to obtain an NdF3 and Fe ion solution. It could be recovered together in the form of Fe oxalate according to the reaction time control. It has the advantage of significantly lowering the process cost by eliminating the acid treatment burden due to the strong acid use, and not only does it have no elements harmful to the environment, the recovered NdF3 can be used as a flux to reduce Nd2O3, or used as a catalyst.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, its supplementary information file.
References
T. Itakura, R. Sasai, H. Itoh, Resource recovery from Nd-Fe-B sintered magnet by hydrothermal treatment. J. Alloys Compd. 408, 1382–1385 (2006). https://doi.org/10.1016/j.jallcom.2005.04.088
C.-H. Lee, Y.-J. Chen, C.-H. Liao, S. R. Popuri, S.-L. Tsai, C.-E. Hung, Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 44, 5825–5833 (2013). https://doi.org/10.1007/s11661-013-1924-3
T.H. Okabe, O. Takeda, K. Fukuda, Y. Umetsu, Direct extraction and recovery of neodymium metal from magnet scrap. Mater. Trans. 44(4), 798–801 (2003). https://doi.org/10.2320/matertrans.44.798
S. Li, Z. Cui, W. Li, D. Wang, Z. Wang, Technical actuality and prospect of NdFeB waste recycling. Mater. Rep 35, 3001–3009 (2021)
C. Huang, X. Liu, Y. Gao, S. Liu, B. Li, Cathodic processes of neodymium(III) in LiF-NdF3-Nd2O3 melts. Faraday Discuss. 190, 339–349 (2016). https://doi.org/10.1039/c6fd00014B
E. Stefanidaki, C. Hasiotis, C. Kontoyannis, Electrodeposition of neodymium from LiF-NdF–3-Nd2O3 melts. Electrochim. Acta 46(17), 2665–2670 (2001). https://doi.org/10.1016/S0013-4686(01)00489-3
J. Chun, C. Jo, S. Sahgong, M.G. Kim, E. Lim, D.H. Kim, J. Hwang, E. Kang, K.A. Ryu, Y.S. Jung, Y. Kim, J. Lee, Ammonium fluoride mediated synthesis of anhydrous metal fluoride-mesoporous carbon nanocomposites for high-performance lithium ion battery cathodes. ACS Appl. Mater. Interfaces 8(51), 35180–35190 (2016). https://doi.org/10.1021/acsami.6b10641
B. Kozekanan, A. Moradkhani, H. Baharvandi, Thermodynamic and phase analysis of SiC-nano/microB4C-C composites produced by pressureless sintering method. J. Korean Ceram. Soc. 59, 180–192 (2022). https://doi.org/10.1007/s43207-021-00173-x
H. Bae, Y. Shin, L. Mathur, Defect chemistry of p-type perovskite oxide La0.2Sr0.8FeO3-δ: a combined experimental and computational study. J. Korean Ceram. Soc. 59, 876–888 (2022). https://doi.org/10.1007/s43207-022-00237-6
E.P. Lokshin, O. Tareeva, Solubility of YF3, CeF3, PrF3, NdF3, and DyF3 in solutions containing sulfuric and phosphoric acids. Russ. J. Inorg. Chem. 52(12), 1830–1834 (2007). https://doi.org/10.1134/S0036023607120042
K.M. Österdahl, Å.C. Rasmuson, Solubility of β-FeF3·3H2O in mixtures of nitric and hydrofluoric acid. J. Chem. Eng. Data 51(1), 223–229 (2006). https://doi.org/10.1021/je050347n
M. Gergoric, A. Barrier, T. Retegan, Recovery of rare-earth elements from neodymium magnet waste using glycolic, maleic, and ascorbic acids followed by solvent extraction. J. Sustain. Metall. 5, 85–96 (2019). https://doi.org/10.1007/s40831-018-0200-6
G. Reisdörfer, D. Bertuol, E.H. Tanabe, Recovery of neodymium from the magnets of hard disk drives using organic acids. Miner. Eng. 143, 105938 (2019). https://doi.org/10.1016/j.mineng.2019.105938
S.O. Lee, T. Tran, B.H. Jung, S.J. Kim, M.J. Kim, Dissolution of iron oxide using oxalic acid. Hydrometallurgy 87(3–4), 91–99 (2007). https://doi.org/10.1016/j.hydromet.2007.02.005
C. Nwoye, Model for evaluation of the concentration of dissolved phosphorus during leaching of iron oxide ore in oxalic acid solution. JMMCE 8(3), 181–188 (2009). https://doi.org/10.4236/jmmce.2009.83016
R. Salmimies, M. Mannila, J. Kallas, A. Häkkinen, Acidic dissolution of hematite: Kinetic and thermodynamic investigations with oxalic acid. Int. J. Miner. Process. 110, 121–125 (2012). https://doi.org/10.1016/j.minpro.2012.04.001
V. Ambikadevi, M. Lalithambika, Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 16, 133–145 (2000). https://doi.org/10.1016/S0169-1317(99)00038-1
V. Arslan, A study on the dissolution kinetics of iron oxide leaching from clays by oxalic acid. Physicochem. Probl. Miner. Process, 57 (3), 97–111 (2021). https://doi.org/10.37190/ppmp/135749
F. Chen, F. Liu, L. Wang, J. Wang, Comparison of the preparation process of rare earth oxides from the water leaching solution of waste Nd-Fe-B magnets’ sulfate roasting products. Processes 10(11), 2310 (2022). https://doi.org/10.3390/pr10112310
Q. Liu, T. Tu, H. Guo, H. Cheng, X. Wang, High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching. J. Rare Earths 39(3), 323–330 (2021). https://doi.org/10.1016/j.jre.2020.04.020
Acknowledgements
This work was supported by the Korea Evaluation Institute of Industrial Technology (KEIT), which is funded by the Ministry of Trade, Industry, and Energy, Republic of Korea (Project No. 20015769). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03045059). We thank the Korea Basic Science Institute for the technical support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, YH., Kim, HS., Yang, JK. et al. Nd composite selective recovery from waste permanent magnet scrap powders by solid-fluorination reaction. J. Korean Ceram. Soc. 61, 97–103 (2024). https://doi.org/10.1007/s43207-023-00350-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-023-00350-0