Abstract
The magnetic, electronic, structural, thermoelectric properties and magnetocaloric effect of Pr0.75Ba0.25MnO3 perovskite using, experimental, DFT calculation and Monte Carlo simulations were investigated. The ground state has half-metallic character. Our calculations also show that the Pr0.75Ba0.25MnO3 has a ferromagnetic behavior. Pr0.75Ba0.25MnO3 exhibits p-type behavior with dominant holes as the primary carriers, as indicated by its thermoelectric properties. This system exhibits a ferromagnetic–Paramagnetic transition. We have successfully obtained several properties, including magnetization, specific heat, variation of specific heat, magnetic entropy changes, relative cooling power, and the magnetic hysteresis cycle. For a magnetic field change of 5 T, the maximum value of the magnetic entropy change (∣ΔSmax∣) was approximately 12 J/kg.K, while the relative cooling power (RCP) reached 126 J/kg. The promising potential of the present system for magnetic refrigeration is evident due to its relatively large values of ∣ΔSmax∣ and RCP. Finally, the thermoelectric properties were given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Manganite-based perovskites are attractive functional materials, because they possess a wide range of reported inclusion physical properties, such as the magnetocaloric effect and thermoelectric properties [1,2,3,4,5,6]. These physical properties make these materials intended for several applications [7,8,9,10,11,12,13,14]. Experimentally, the magnetic and structural properties of Pr0.7Ba0.2Ca0.1MnO3 were investigated [15]. A study was conducted to investigate the impact of compaction pressure on the enhancement of ferromagnetic properties in La0.7Ba0.3MnO3 [16]. Pr0.75Sr0.25MnO3 exhibited a semiconductor–metal transition [17]. Pr0.75Sr0.25MnO3 has a half-metallic character with a huge band gap of 2.8 eV in the minority band [18]. This property of the half metals make them potential candidates for application in spintronic devises and magnetic sensors.
In the present work, we have prepared Pr0.7Ba0.3MnO3 using solid-state reaction method (more detailed is given in Refs. [19, 20]. The structural and morphological of the prepared samples were investigated. Furthermore, the electronic and thermoelectric properties of Pr0.75Ba0.25MnO3 are investigated using DFT calculation [21,22,23,24,25,26]. In addition, The Monte Carlo simulations (MCSs) calculations are used to shed light on the magnetic and magnetocaloric properties of Pr0.75Ba0.25MnO3. The T dependence of the magnetization is given. The thermal specific heat, variation of heat specific, magnetic entropy changes, relative cooling power, and magnetic hysteresis cycle are obtained.
2 Model and simulations method
The Hamiltonian of this system
The J1, J2, J3 and J4 are the first, second, third, and fourth exchange interactions between Mn–Mn in Pr0.75Ba0.25MnO3 with S(Mn4+) = 3/2.
The obtained results by DFT were used to calculate the exchange interaction between the magnetic atoms, J1 = 15.3, J2 = 12.3, J3 = 12.1, J4 = 9.5 K. Magnetocaloric effect Pr0.7Ba0.3MnO3 has been studied.
The magnetic and magnetocaloric properties of Pr0.75Ba0.25MnO3 were investigated using Monte Carlo simulations (MCSs) in conjunction with the Metropolis algorithm. Equation (1) was employed for this purpose.
The magnetization of Mn4+ in this perovskite
with N = 2465 spins.
The specific heat of this perovskite
where \(\beta = \frac{1}{{k_{B} T}}\).
The magnetic entropy changes
The RCP
3 Crystallographic structure and computational details
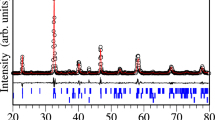
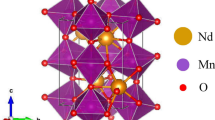
The structure de Pr0.7 Ba 0.3MnO3 dans le plan (b, c) and in [111] plane is presented in Figs. 1 and 2, respectively. The lattice parameters of our system are given in Table 1.
In Table 2, we have presented the crystallographic parameters Pr0.7 Ba0.3MnO3, and n is percentage occupancy.
The numbers in parentheses represent the error and the results in braces are for the Pr0.7 Ba 0.3MnO3 compound.
In this work, DFT calculations were perfumed using approximate XC functional GGA executed via operating the PBE method as implemented in the Wien2k package [27,28,29,30,31,32]. In the electronic properties part, we take a concentration of 0.25 instead of 0.3. To avoid supercells, which give us too many atoms in the structure and which can spoil the convergence of the calculation. A cut-off parameter is RMT × Kmax = 8 and the Fourier expansion parameter Gmax = 12.0. The transport properties of Pr0.75Ba0.25MnO3 are obtained using the semi-classical Boltzmann theory as implemented in the BoltzTraP code [33]. Furthermore, in the BoltzTraP code, the semi-classical transport equations of Bloch–Boltzmann are solved in the constant relaxation time approximation [33]. The transport coefficients using the constant relaxation time approximation are τ = 10−14 s [34].
4 Results and discussion
The spin-polarized total and partial electronic density of states (DOS) for Pr0.75Ba0.25MnO3 are depicted in Figs. 3 and 4, respectively. The asymmetrical nature observed between the spin-up and spin-down DOS confirms the magnetic properties of the material. The magnetization is attributed to the transition metal Mn and the rare-earth element Pr. Specifically, the spin moment values are determined to be 1.89023 μB for Pr and 3.37955 μB for Mn. At the Fermi level (EF), the majority band exhibits conducting behavior, while the minority band displays insulating characteristics. This configuration allows for 100% spin polarization and exhibits a half-metallicity feature, with a band gap of 2.274 eV in the minority band. This half-metallicity property positions Pr0.75Ba0.25MnO3 as a promising candidate for applications in spintronic devices and magnetic sensors.
The partial DOS shows that Mn-3d and O-2p have an important electron density contribution in region [– 6, – 0.25 eV]. In the energy range of [– 0.25, + 2 eV], which encompasses the conduction band, the orbital hybridization is primarily influenced by the spin-up states of Pr-4f, Mn-3d, and O-2p orbitals. Within this range, an exchange splitting can be observed between the spin-down and spin-up partial states of Pr-4f and Mn-3d orbitals. This exchange splitting plays a significant role in contributing to the majority portion of the total spin magnetic moments of the unit cell in Pr0.75Ba0.25MnO3 compound.
Figure 5 illustrates the relationship between magnetization and temperature (magnetization vs. temperature curve) and magnetic susceptibility vs. temperature for Pr0.75Ba0.25MnO3. The data reveal a transition from a ferromagnetic phase to a paramagnetic phase, which occurs at a critical temperature (TC) of 156 K. Our value is near to that given by Ref. [20] (TC = 164 K), and this difference may be due to the atoms that we did not consider in our Pr, Br, and O calculation, because they are not magnetic. The value of saturation magnetization is equal 16 emu/g. This value is near to that obtained by the experiment results [20].
The magnetic entropy changes are given in Fig. 6. The value of ΔSmax is situated at the Tc = 156 K. For h = 5.0 T, the value of ΔSmax is 12 and 2.46 J/kg.K for 0.5 T. ΔS vs. temperature was given in Ref. [35]. In previous work [15] for Pr0.7Ba0.2Ca0.1MnO3, they found that 2.2 J/kg.K for 5 T.
The variation of specific heat CP vs. temperature is shown in Fig. 7. The maximum of CP is situated at 156 K. As the magnetic field values increase, there is a decrease in the maximum value of the specific heat capacity (CP). The large anomaly in the heat capacity of DyAl2 was observed due mainly to spin reorientation from [100] to [111][36].
The CP changes exhibit anomaly near to TC due to the ferromagnetic–paramagnetic transition. The thermal specific heat changes strongly changes from the negative value to the positive one as temperature increases (Fig. 8).
The relative cooling power vs. magnetic field is given in Fig. 9. The value of RCP obtained by Ref. [15] is 261 J/kg for 5 T and in this work for Pr0.7Ba0.3MnO3 is 126 J/kg for 5 T. The RCP vs. temperature is illustrated in Fig. 10. The RCP exhibits an anomalous around the TC = 156 K. The relative cooling power (RCP) increases with increasing temperature until it reaches a saturation point for each value of the magnetic field (h). Regarding the effect of the magnetic field (h), RCP increases as the value of h increases, as shown in Fig. 8. The magnetization versus magnetic field (h) is depicted in Fig. 11 for three different temperatures: 150 K, 130 K, and 170 K. It can be observed that the remanent magnetization and coercive field decrease with increasing temperature. Pr0.75Ba0.25MnO3 demonstrates a superparamagnetic behavior around the temperature TC.
To provide a more accurate response, I would need the complete relation for calculating the Seebeck coefficient. Could you please provide the full relation or equation for calculating the Seebeck coefficient
so, n is charge carrier concentration, m* is the effective mass, kB is Boltzmann constant, \(e = \, 1.67 \times 10^{ - 19} C\), and \(h = 6.626 \times 10^{ - 26} \;{\text{k.g.m}}^{2} \sec^{ - 1}\) ℎ.
Figure 12 illustrates the variation of the Seebeck coefficient (S) for Pr0.75Ba0.25MnO3. The data reveal that the Seebeck coefficient increases as the temperature increases. At 800 K, it reaches a value of 8 × 10–15 V/K. The positive value of the Seebeck coefficient for Pr0.75Ba0.25MnO3 indicates that the dominant charge carriers are holes. This positive Seebeck coefficient aligns with the p-type behavior exhibited by the material.
The electrical conductivity (σ) of Pr0.75Ba0.25MnO3 is depicted in Fig. 13. It is generally observed that the electrical conductivity is directly proportional to the charge carrier concentration (n) and the mobility (μ). At 800 K, Pr0.75Ba0.25MnO3 exhibits a high electrical conductivity with a value of \(9.35.10^{18} \Omega^{ - 1} .m^{ - 1} .s^{ - 1}\). On the other hand, the thermal conductivity (κ) reflects a material's ability to conduct and transmit heat. Figure 14 displays the variation of thermal conductivity with temperature. As depicted, the thermal conductivity increases as the temperature rises. The maximum value of thermal conductivity observed in the figure is \(2.75.10^{14} W.K^{ - 1} .m^{ - 1} .s^{ - 1}\).
The figure of merit ZT is calculated by the following relation:
with k = kl + ke, kl defines the lattice thermal conductivity and ke denotes electronic thermal energy. The lattice component of thermal conductivity kl caused by the phonon scattering is not considered in our calculations. As an estimate of Pr0.75Ba0.25MnO3 efficiency.
In Fig. 15, the variation of the ZT coefficient for Pr0.75Ba0.25MnO3 is displayed. The plot demonstrates that the ZT value increases as the temperature rises. The highest ZT value obtained is 0.19 at 800 K. Given that the thermal conductivity due to phonons is included in the calculations, this value of 0.19 is considered reasonable. It suggests that the thermal conductivity contribution from the lattice component (kl) is significant.
5 Conclusions
In our study, we employed a combination of experimental techniques, density functional theory (DFT), and Monte Carlo simulations (MCSs) to investigate the electronic, thermoelectric, magnetic properties, and magnetocaloric effect of the Pr0.75Ba0.25MnO3 perovskite system. One of the key findings is that the Pr0.75Ba0.25MnO3 perovskite exhibits a half-metallic character, with a band gap of 2.274 eV in the minority band. This indicates that it can selectively conduct one spin direction while behaving as an insulator for the opposite spin direction. We also determined the transition temperature, which was found to be in close agreement with experimental results, validating the accuracy of our approach. By analyzing the magnetization as a function of temperature, we were able to deduce the magnetic entropy change (∆S). It was observed that ∆Smax increases with the applied magnetic field (h). This suggests that the ferromagnetic order in the samples exhibits long-range characteristics. Furthermore, we observed a significant magnetocaloric effect at a magnetic field of 5 T, accompanied by large values of ∆Smax and relative cooling power (RCP). These findings indicate that the Pr0.75Ba0.25MnO3 perovskite system holds promise for magnetic refrigeration applications. In terms of thermoelectric properties, our theoretical investigation revealed that Pr0.75Ba0.25MnO3 exhibits p-type behavior, with holes being the dominant charge carriers. This characterization of the material's electronic behavior provides valuable insights into its thermoelectric performance. Overall, the combination of the observed large magnetocaloric effect, relatively high RCP, and high magnetization makes the Pr0.75Ba0.25MnO3 perovskite system a promising candidate for applications in magnetic refrigeration and spintronics.
Data availability
Not applicable.
References
G. Kadim, R. Masrour, A. Jabar, E.K. Hlil, Room-temperature large magnetocaloric, electronic and magnetic properties in La0.75Sr0.25MnO3 manganite: Ab initio calculations and Monte Carlo simulations. Phys. A: Stat. Mech. Appl. 573, 125936 (2021)
G. Kadim, R. Masrour, A. Jabar, E.K. Hlil, First principal calculation and Monte Carlo simulations of the magnetocaloric effect, electronic and magnetic properties in perovskite oxide Pr0.65Sr0.35MnO3. IOP Conf. Ser.: Mater. Sci. Eng. 1160(1), 012010 (2021)
Y. Xu, J. Dhainaut, J.P. Dacquin, J.F. Lamonier, H. Zhang, S. Royer, On the role of cationic defects over the surface reactivity of manganite-based perovskites for low temperature catalytic oxidation of formaldehyde. Appl. Catal. B Environ. 342, 123400 (2023)
X. Yang, T. Wei, B. Chi, J. Pu, J. Li, Lanthanum manganite-based perovskite as a catalyst for co-production of ethylene and hydrogen by ethane dehydrogenation. J. Catal. 377, 629–637 (2019)
S. Taran, B.K. Chaudhuri, S. Chatterjee, H.D. Yang, S. Neeleshwar, Y.Y. Chen, Critical exponents of the La0.7Sr0.3MnO3, La0.7Ca0.3MnO3, and Pr0.7Ca0.3MnO3 systems showing correlation between transport and magnetic properties. J. Appl. Phys. 98(10), 103903 (2005)
F. Bern, M. Ziese, I. Vrejoiu, X. Li, P.A. van Aken, Magnetic and magnetotransport properties of ultrathin LaBaMnO3 epitaxial films embedded in SrRuO3. New J. Phys. 18(5), 053021 (2016)
E. Brück, O. Tegus, X. Li, F.R. De Boer, K.H.J. Buschow, Magnetic refrigeration towards room-temperature applications. Phys. B 327(2–4), 431–437 (2003)
A. Selmi, R. M’nassri, W. Cheikhrouhou-Koubaa, N.C. Boudjada, A. Cheikhrouhou, The effect of Co doping on the magnetic and magnetocaloric properties of Pr0.7Ca0.3Mn1−xCoxO3 manganites. Ceram. Int. 41(6), 7723–7728 (2015)
S.C. Maatar, R. M’nassri, W.C. Koubaa, M. Koubaa, A. Cheikhrouhou, Structural, magnetic and magnetocaloric properties of La0.8Ca0.2−xNaxMnO3 manganites (0≤ x ≤ 02). J. Solid State Chem. 225, 83–88 (2015)
M.H. Phan, S.C. Yu, Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 308(2), 325–340 (2007)
P. Lampen, A. Puri, M.H. Phan, H. Srikanth, Structure, magnetic, and magnetocaloric properties of amorphous and crystalline La0.4Ca0.6MnO3+δ nanoparticles. J. Alloys Compd. 512(1), 94–99 (2012)
S. Das, D. Dhak, M.S. Reis, V.S. Amaral, T.K. Dey, Room temperature giant magnetoimpedance in La0.7Ba0.15Sr0.15MnO3 compound. Mater. Chem. Phys. 120(23), 468–471 (2010)
M.D. Daivajna, N. Kumar, V.P.S. Awana, B. Gahtori, J.B. Christopher, S.O. Manjunath, A. Rao, Electrical, magnetic and thermal properties of Pr0.6–xBixSr0.4MnO3 manganites. J. Alloys Compd. 588, 406–412 (2014)
M. Oumezzine, S. Kallel, O. Pena, N. Kallel, T. Guizouarn, F. Gouttefangeas, M. Oumezzine, Correlation between structural, magnetic and electrical transport properties of barium vacancies in the La0.67Ba0.33−x□xMnO3 (x = 0, 0.05, and 0.1) manganite. J. Alloys Compd. 582, 640–646 (2014)
P. Bisht, A. Kumar, R.N. Mahato, Magnetic and magnetocaloric properties of the nanocrystalline Pr0.7Ba0.2Ca0.1MnO3 sample. AIP Adv. 11(1), 015239 (2021)
C.R. Duarte de Freitas, M. Marques de Góis, R. Bezerra da Silva, J.A. Pereira da Costa, J.M. Soares, Mater. Res. 21, 1–7 (2018)
W. Boujelben, M. Ellouze, A. Cheikh-Rouhou, J. Pierre, Q. Cai, W.B. Yelon, C. Dubourdieu, Neutron diffraction, NMR and magneto-transport properties in the Pr0.7Sr0.3MnO3 perovskite manganite. Phys. Status 191(1), 243–254 (2002)
M. Chakraborty, P. Pal, B.R. Sekhar, Solid State Commun. 145, 197–200 (2008)
M. Ellouze, W. Boujelben, H. Fuess, Rietveld refinement X-ray powder data of Pr0.7Ba0.3MnO3. Powder Diffr. 18(1), 29–31 (2003)
M. Ellouze, W. Boujelben, A. Cheikhrouhou, H. Fuess, R. Madar, Vacancy effects on the crystallographic and magnetic properties in lacunar Pr0.7Ba0.3−xMnO3 oxides. Solid State Commun. 124(4), 125–130 (2002)
H. Rached, D. Rached, M. Rabah, R. Khenata, A.H. Reshak, Full-potential calculation of the structural, elastic, electronic and magnetic properties of XFeO3 (X = Sr and Ba) perovskite. Phys. B 405(17), 3515–3519 (2010)
D. Rached, M. Hichour, M. Rabah, S. Benalia, H. Rached, R. Khenata, Prediction study of the structural, elastic, electronic and optical properties of the antiperovskite BiNBa3. Solid State Commun. 149(45–46), 2002–2006 (2009)
H. Benmhidi, H. Rached, D. Rached, M. Benkabou, Ab initio study of electronic structure, elastic and transport properties of fluoroperovskite LiBeF3. J. Electron. Mater. 46, 2205–2210 (2017)
I. Bourachid, M. Caid, O. Cheref, D. Rached, H. Heireche, B. Abidri, N. Benkhettou, Insight into the structural, electronic, mechanical and optical properties of inorganic lead bromide perovskite APbBr3 (A = Li, Na, K, Rb, and Cs). Comput. Condens. Matter 24, e00478 (2020)
H. Rached, S. Bendaoudia, D. Rached, Investigation of Iron-based double perovskite oxides on the magnetic phase stability, mechanical, electronic and optical properties via first-principles calculation. Mater. Chem. Phys. 193, 453–469 (2017)
S. Al-Qaisi, M. Mushtaq, S. Alomairy, T.V. Vu, H. Rached, B.U. Haq, M.S. Al-Buriahi, First-principles investigations of Na2CuMCl6 (M = Bi, Sb) double perovskite semiconductors: materials for green technology. Mater. Sci. Semicond. Process. 150, 106947 (2022)
K. Schwarz, P. Blaha, G.K. Madsen, Electronic structure calculations of solids using the WIEN2k package for material sciences. Comput. Phys. Commun. 147(1–2), 71–76 (2002)
G. Kadim, R. Masrour, A. Jabar, A comparative study between GGA, WC-GGA, TB-mBJ and GGA+ U approximations on magnetocaloric effect, electronic, optic and magnetic properties of BaMnS2 compound: DFT calculations and Monte Carlo simulations. Phys. Scr. 96(4), 045804 (2021)
G. Kadim, R. Masrour, Thermoelectric, magneto-optic properties and magnetocaloric effect of PbS doped with Mn2+ ions. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02677-x
G. Kadim, R. Masrour, Density functional theory and Monte Carlo simulation insights into electronic structure and magnetic properties in HoSi and CeSi. Mater. Today Commun. 37, 107176 (2023)
G. Kadim, R. Masrour, Effect of Zn-doping CdTe on the internal and external quantum efficiency: ab initio calculations. J. Korean Ceram. 60(6), 896–904 (2023)
A. Jabar, R. Masrour, G. Kadim, M. Hamedoun, A. Hourmatallah, N. Benzakour, J. Kharbach, Intrinsic ferromagnetism in CoBr2 nanolayers: a DFT+U and Monte Carlo study. Commun. Theor. Phys. 73(11), 115702 (2021)
G.K. Madsen, D.J. Singh, BoltzTraP: a code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175(1), 67–71 (2006)
G. Kadim, R. Masrour, A. Jabar, Magnetocaloric, electronic, magnetic, optical and thermoelectric properties in antiferromagnetic semiconductor GdCrO3: Monte Carlo simulation and density functional theory. J. Cryst. Growth 581, 126509 (2022)
V. Franco, J.S. Blázquez, A. Conde, Field dependence of the magnetocaloric effect in materials with a second order phase transition: a master curve for the magnetic entropy change. Appl. Phys. Lett. 89(22), 222512 (2006)
A.L. Lima, A.O. Tsokol, K.A. Gschneidner Jr., V.K. Pecharsky, T.A. Lograsso, D.L. Schlagel, Magnetic properties of single-crystal DyAl2. Phys. Rev. B 72(2), 024403 (2005)
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masrour, R., Kadim, G. & Ellouze, M. Magnetic, thermoelectric properties and magnetocaloric effect of Pr0.7Ba0.3MnO3 perovskite: experimental, DFT calculation and Monte Carlo simulations. J. Korean Ceram. Soc. 61, 411–418 (2024). https://doi.org/10.1007/s43207-023-00343-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-023-00343-z