Abstract
Lead-free NKLNTS ceramics with promising piezoelectric properties were fabricated using the solid phase method, which is a general ceramic manufacturing method. MnO2 was added to NKLNTS ceramics as a sintering aid to improve piezoelectric and dielectric properties. The added MnO2 content was adjusted to 0, 0.1, 0.3, 0.5, and 0.7 wt%, the powder was calcined at 900 °C for 2 h, and then the sintering temperature was changed from 1000 to 1100 °C to study the optimum temperature and composition that yields excellent piezoelectric properties and sinterability. Archimedes method and scanning electron microscope (SEM) were used to evaluate the sinterability, X-ray diffraction analysis (XRD) was performed to confirm the crystallinity of the sintered body, and piezoelectric and dielectric properties were evaluated using a d33-meter and an impedance analyzer. When 0.1 wt% of MnO2 was added, it was confirmed that density was the highest at the sintering temperature of 1050 °C and had excellent piezoelectric and dielectric properties. When 0.3 wt% or more of MnO2 was added, piezoelectric and dielectric properties were decreased due to the decreased density. When NKLNTS-0.1wt% MnO2 was sintered at a sintering temperature of 1050 ℃ for 2 h, it had a density of about 97%. Furthermore, lead-free piezoelectric ceramics with excellent piezoelectric and dielectric properties of d33 = 271 pC/N, kp = 0.40, εr = 1250, tan δ = 2.5%, and Tc = 348 °C were fabricated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
PZT-based ceramics have been applied to a wide range of fields such as ultrasonic devices, acoustic devices, communication devices, and measurement devices due to their excellent piezoelectric properties. The excellent piezoelectric and dielectric properties of PZT are observed in the morphotropic phase boundary (MPB) region where rhombohedral and tetragonal coexist. As a result, most studies on piezoelectric ceramics focus on MPB [1]. However, because lead-based materials such as PZT, which are widely used as piezoelectric ceramic materials, contain a large amount of Pb, which is harmful to environmental pollution and the human body, its use is restricted to developed countries in recent years. Therefore, the development of environmentally-friendly lead-free piezoelectric materials has been actively studied [2].
Among materials for lead-free piezoelectric ceramics, Bi-perovskite series and NKN series piezoelectric materials are widely used. In particular, NKN-based piezoelectric ceramics have excellent electrical and piezoelectric properties among lead-free piezoelectric ceramics, and have been studied to replace lead-based materials such as PZT due to their high phase transition temperature (Tc) of 420 °C [3, 4]. Among them, NKN series lead-free piezoelectric ceramics have poor sinterability due to the volatility of alkali metals such as Na and K during sintering and deliquescent property to absorb moisture in the air during specimen fabrication. As a result, it is difficult to fabricate high-quality ceramics [5]. Therefore, hot isostatic pressing method [6] and spark plasma sintering process method [7] are used to fabricate high-density piezoelectric ceramics, but it is not suitable for mass production due to high cost. Therefore, solid solutions such as NKN–BaTiO3 [8], NKN–LiNbO3 [9], NKN–SrTiO3 [10], NKN–LiTaO3 [11] and NKN–Li (Nb,Ta,Sb) O3 [12], or sintering aids such as CuO [13], ZnO [14] and MnO2 [15] in pure NKN-based piezoelectric ceramics has been studied to improve the sinterability and piezoelectric properties. In particular, Mn has been reported to enhance the densification of pure NKN piezoelectric ceramics [16]. In this study, a powder was prepared by controlling the MnO2 content added to NKN-based piezoelectric ceramics, and then a high-density sintered body with excellent piezoelectric and dielectric properties was fabricated by controlling the heat treatment temperature. Sinterability was evaluated using an Archimedes method and a scanning electron microscope (SEM). X-ray diffraction analysis (XRD) was performed to evaluate the crystallinity of the sintered compact. Electrical characteristics were evaluated using a d33-meter and an impedance analyzer.
2 Experimental procedures
NKN series ceramics, which is (Na0.475K0.475Li0.05) (Nb0.9Ta0.05Sb0.05)O3-x wt% MnO2 (NKLNTS-x wt% MnO2, 0 ≤ x ≤ 0.7) with MnO2 added, were fabricated by a general mixed oxide method using metal oxide or carbonate powder. The raw materials including Na2CO3, K2CO3, Li2CO3, Nb2O5, Ta2O5, Sb2O5 and MnO2 (all from High purity Chemicals, > 99%, Japan) were weighed and then mixed under ethanol solvent using zirconia balls for 24 h. After calcining at 900 °C for 2 h, the mixture was ball milled with ethanol solvent for 24 h. The produced powder was compressed into a disk of 10 mm diameter and then cold isostatic pressed for 10 min under 200 MPa. The resulting pellets were then heated in an alumina crucible at 1050–1100 °C at the rate of the temperature of 5 °C per minute for 2 h in the air. To measure the electrical properties, after the thickness of the sintered body was polished to about 1 mm, the silver paste was applied to both sides of the specimen, and then fired at 650 °C for 30 min. To measure the piezoelectric properties, the ceramic samples were polarized under a dc field of 3.5 kV/mm at 120 °C for 30 min in a silicone oil bath. The crystal structure of the sintered samples was examined using X-ray diffraction (XRD) analysis with CuKα radiation (D2 PHASER, Bruker Corporation, Germany). The microstructure was observed using a scanning electron microscope (SEM) (Cambridge Instrument, Cambridge, UK). Density (ρ) was measured using the Archimedes method. The dielectric constant (εr) and loss factor (tan δ) were measured using an impedance analyzer (E4990A, Keysight Technologies, US). The piezoelectric constant (d33) was measured using a d33 meter (ZJ-30, H. C. Materials Corporation, China), and electromechanical coupling coefficients (Kp) and mechanical quality factors (Qm) in the planar mode were measured by the resonance-antiresonance method using an impedance analyzer.
3 Results and discussion
3.1 X-ray diffraction analysis of NKLNTS ceramic
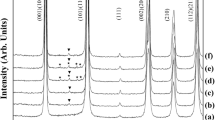
The results of X-ray diffraction analysis of NKLNTS-xMnO2 ceramics that were sintered at 1050 °C for 2 h according to the added MnO2 content are shown in Fig. 1 When MnO2 is not added, orthorhombic and tetragonal perovskite crystal structures are present. When MnO2 was added, two peaks of (002) and (200) exist near the diffraction angle (2θ) of 45°, and (200) peak was larger than (002) peak, from which It can be found that the tetragonal perovskite crystal structure does not have a second phase.
3.2 NKLNTS ceramic sintering characteristics
Changes in density of NKLNTS ceramics according to the sintering temperature and the added Mn content are shown in Fig. 2 is shown. The density increased when MnO2 was added at all sintering temperatures. It was found that the sintered density increased as MnO2 was added. The density increased when 0.1 wt% of MnO2 was added and the density decreased when 0.3 wt% or more of MnO2 was added. When NKLNTS ceramics containing 0.1 wt% MnO2 were sintered at 1050 °C, the density was the highest at 4.45 g/cm3, which was 97% higher than the theoretical density of 4.6 g/cm3.
The SEM photographs of NKLNTS ceramics according to the change of the added MnO2 content at sintering temperature of 1050 ℃ are shown in Fig. 3 is shown. Figure 3a shows the surface of NKLNTS ceramics without MnO2 added. It can be found that there are many pores on the surface and the average particle size is about 0.3 μm, which is very small. It can be found that the sintering was not completed due to the lack of grain growth. Figure 3b shows the surface of NKLNTS ceramics, in which 0.1 wt% MnO2 was added. When 0.1 wt% of MnO2 was added, the average particle size was 1.85 um, and some large abnormal grains of more than 4 μm were identified. Also, the pores decreased and the density increased. This is because the liquid phase is formed at the initial stage of sintering to promote sintering when MnO 2 is added, which promotes grain growth. After that, the grain size increased as the added MnO2 content increased.
SEM images of NKLNTS ceramics at different sintering temperatures at 0.1 wt% MnO2 composition are shown in Fig. 4. Figure 4a shows the surface of the sample sintered at 1000 °C. At 1000 °C., it had an average particle size of 0.35 um, and due to the low sintering temperature, the pores increased and the density decreased. Figure 4b shows the surface of the sample sintered at 1050 °C. At 1050 °C., it has an average particle size of 1.85 μm, and due to sufficient sintering temperature, the pores decrease to have high density. Figure 4c shows the surface of the sample sintered at 1100 °C. At 1100 ℃, the average particle size was about 2.65 μm, and abnormal grain growth was active. Due to the volatility of alkali metals due to the high sintering temperature, it appears to have more pores and a lower density than the density at 1050 °C. Particle size was found to increase with increasing sintering temperature.
3.3 NKLNTS ceramic piezoelectric properties
The piezoelectric constants (d33) and electromechanical coupling coefficients (Kp) of NKLNTS ceramics according to the added MnO2 content are shown in Fig. 5. The piezoelectric constant d33 has the highest value at 1050 °C, and shows negligible change at 1000 °C even though the added MnO2 content was changed. Above 1050 °C, it increased when 0.1 wt% MnO2 was added, after which it decreased as the added MnO2 content increased. The electromechanical coefficient (Kp) also shows a similar trend as the piezoelectric constant. When a small amount of MnO2 is added, Mn acts as a sintering aid to reduce the pores and increase the density, thereby increasing the piezoelectric charge coefficient (d33). Subsequently, as the MnO2 content increases, Nb5+ (ion radius 0.64 Å) of the perovskite structure, which is an ionic bond to decrease d33 and Kp, is substituted with Mn3+ (ion radius 0.66 Å) ions having a similar ion radius, which causes oxygen vacancies and forms space charges inside to limit the movement of domains. d33 and Kp have the highest values of 271 pC/N and 0.4 in the composition with the sintering temperature of 1050 ℃ and x = 0.1.
The mechanical quality factor values of NKLNTS ceramics according to the sintering temperature and the added MnO2 content are shown in Fig. 6. At all sintering temperatures, Qm increased as the added MnO2 content increased, and showed a high value of 110 when the sintering temperature was 1000 ℃ and the added MnO2 content was x = 0.7. MnO2 acts as an acceptor as a stabilizing compound, inducing oxygen vacancies to form internal space charges, thereby limiting the movement of domains. As a result, the mechanical quality factor was increased due to the decrease of internal friction. Sintering temperature showed the highest mechanical quality factor at 1000 ℃ and relatively low mechanical quality factor at 1050 ℃ and 1100 ℃. As the grain size becomes smaller, when the piezoelectric ceramic vibrates, the propagation of the crack proceeds to the grain boundary, increasing the fracture toughness. Therefore, it has a high mechanical quality factor at 1000 ℃ with a relatively small grain size.
3.4 NKLNTS ceramic dielectric properties
The relative dielectric constant of NKLNTS ceramics according to the sintering temperature and the added MnO2 content are shown in Fig. 7. At 1000 °C., due to insufficient sintering temperature, the sintering is not completely performed, and thus the porosity is high. The dielectric constant was increased when 0.1 wt% MnO2 was added, and then was decreased as the added MnO2 content was increased. When a small amount of MnO2 was added, it is likely that the low-temperature sintering aid forms a liquid phase at the initial stage of sintering to promote sintering, thereby growing grain and increasing the dielectric constant. The decrease in dielectric constant from 0.3 wt% composition to 0.7 wt% composition of MnO2 might be because Mn3+ ions are replaced by Nb5+ ions at the B site, and it acts as an acceptor, forming a space charge layer inside the material to limit the movement of domains. The sintering temperature is 1050 °C and a high dielectric constant value of 1250 at a composition of 0.1 wt% MnO2 was observed. Dielectric loss is the loss of power in a dielectric due to dielectric polarization when an alternating electric field is applied to the material. The sintering temperature is 1050 ℃ and the change of dielectric loss at 0.1 wt% composition of MnO2 is shown in Fig. 8. Specimens with MnO2 added had a low dielectric loss, and the addition of MnO2 decreased the dielectric loss. When MnO2 is added, it appears that MnO2 promotes sintering like a sintering aid, thereby reducing the dielectric loss.
Changes in the dielectric constant according to changes in temperature at the sintering temperature of 1050 ℃ and 0.1 wt% MnO2 composition are shown in Fig. 9. Unlike PZT piezoelectric ceramics, NKN-based piezoelectric ceramics have a first phase transition from orthorhombic to tetragonal phase and second phase transition from tetragonal to cubic phase. It is known to have excellent piezoelectric properties in the boundary region (T0−T) of the orthorhombic and tetragonal phases. The second phase transition temperature is 348 ℃ (Tc), which is a high Curie temperature.
The change of d33 at a polling temperature of 120 ℃ according to the electric field is shown in Fig. 10. A 1 mm sample was polled for 30 min while raising the polling voltage from 0 V to 5 kV in 0.5 kV increments, during which the change of d33 was measured. It was confirmed that the d33 value was saturated at 3.5 kV. This confirms that the minimum voltage for the polarization of NKLNTS ceramics is 3.5 kV/mm.
4 Conclusion
NKNNTS ceramics were fabricated by a general method of fabricating ceramics. MnO2 was added as a sintering aid to improve piezoelectric and dielectric properties. Powders were synthesized by controlling the added MnO2 content, and then were sintered at 1000–1100 °C to find optimum temperature and composition that yield excellent piezoelectric properties and sinterability. It was confirmed that it had the best piezoelectric properties at the sintering temperature of 1050 °C. When 0.1 wt% MnO2 was added, it showed the best density, piezoelectric properties, and dielectric properties, and when more MnO2 was added, the density, piezoelectric properties, and dielectric properties tended to decrease. When a small amount of MnO2 was added, it improved the sinterability as a sintering aid, thereby improving the piezoelectric and dielectric properties. Furthermore, when more MnO2 was added, it may be because Nb5+ (ion radius 0.64 Å) ions at the B site are replaced with Mn3+ (ion radius 0.66 Å) ions with similar ion radius and Mn3+ ions act as acceptor ions. When 0.1 wt% MnO2 was added and sintered at 1050 ℃, lead-free piezoelectric ceramics exhibited excellent piezoelectric and dielectric properties including density of about 97%, d33 = 271 pC/N, kp = 0.40, εr = 1250, tan δ = 2.5%, and Tc = 348° C.
References
B. Jaffe, W.R. Cook, H. Jaffe, Piezoelectric Ceramics (Academic Press, London, 1997), p. 92
D. Gao, K.W. Kwok, D.M. Lin, H.L.W. Chan, Microstructure, electrical properties of CeO2-doped (K0.5Na0.5)NbO3 lead-free piezoelectric ceramics. J. Mater. Sci. 44, 2466–2470 (2009)
S.J. Park, H.Y. Park, K.H. Cho, S. Nahm, Effect of CuO on the sintering temperature and piezoelectric properties of lead-free 0.95(K0.5Na0.5)NbO3–0.05CaTiO3 ceramics. Mater. Res. Bull. 43, 3580–3586 (2008)
M.R. Yang, C.S. Hong, C.C. Tsai, S.Y. Chu, Effect of sintering temperature on the piezoelectric and ferroelectric characteristics of CuO doped 0.95(Na0.5K0.5)NbO3–0.05LiTaO3 ceramics. J. Alloys Compd. 488, 169–273 (2009)
D.M. Lin, K.W. Kwok, H.L.W. Chan, Piezoelectric and ferroelectric properties of KxNa1−xNbO3 lead-free ceramics with MnO2 and CuO doping. J. Alloys Compd. 461(1–2), 273–278 (2008)
R.E. Jaeger, L. Egerton, Hot pressing of potassium-sodium niobates. J. Am. Ceram. Soc. 45(5), 209–213 (1962)
J.F. Li, K. Wang, B.P. Zhang, L.M. Chang, Ferroelectric and piezoelectric properties of fine-grained Na0.5K0.5NbO3 lead-free piezoelectric ceramics prepared by spark plasma sintering. J. Am. Ceram. Soc. 89(2), 706–709 (2006)
C.W. Ahn, H.C. Song, S. Nahm, S.H. Park, K. Uchino, S. Priya, H.G. Lee, N.K. Kang, Effect of MnO2 on the piezoelectric properties of (1−x)(Na0.5K0.5)NbO3-xBaTiO3 ceramics. Jpn. J. Appl. Phys. 44(2), L1361–L1364 (2005)
Y. Guo, K. Kakimoto, H. Ohsato, Phase transitional behavior and piezoelectric properties of (Na0.5K0.5)NbO3-LiNbO3 ceramics. Appl. Phys. Lett. 85(18), 4121–4123 (2004)
R. Wang, R. Xie, K. Hanada, K. Matsusak, H. Bando, M. Itoh, Phase diagram and enhanced piezo-electricity in the strontium titanate doped potassium–sodium niobate solid solution. Phys. Status Solidi(a) 202(6), R57–R59 (2005)
Y. Guo, K. Kakimoto, H. Ohsato, (Na0.5K0.5)NbO3–LiTaO3 lead-free piezoelectric ceramics. Mater. Lett. 59, 241–244 (2005)
Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya, M. Nakamur, Lead-free piezo ceramics. Nature 432, 84–87 (2004)
Y. Lee, J. Yoo, K. Lee, I. Kim, J. Song, Y. Park, Dielectric and piezoelectric characteristics of the non-stoichiometric (Na, K)NbO3 ceramics doped with CuO. J. Alloys Compd. 506(2), 872–876 (2010)
S.H. Park, C.W. Ahn, S. Nahm, J.S. Song, Microstructure and piezoelectric properties of ZnO-added (Na0.5K0.5)NbO3 ceramics. Jpn. J. Appl. Phys. 43(2), L1072–L1074 (2004)
M. Matsubara, K. Kikuta, S. Hirano, Piezoelectric properties of (K0.5Na0.5)(Nb1−xTax)O3–K5.4CuTa10O29 ceramics. J. Appl. Phys. 97(11), 114105 (2005)
J.G. Hao, Z.J. Xu, R.Q. Chu, Y.J. Zhang, G.R. Li, Q.G. Yin, Effects of MnO2 on phase structure, microstructure and electrical properties of (K0.5Na0.5)0.94Li0.06NbO3 lead-free ceramics. Mater. Chem. Phys. 118(1), 229–233 (2009)
Acknowledgements
This study was funded by the Ministry of Trade, Industry and Energy (MOTIE) of Korea and conducted under “the Competency Development Program for Industry Specialists,” undertaken by Korean Institute for Advancement of Technology (KIAT). (No. P0002007, HRD program for 3D Printing based on 3D Printing Materials).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cha, J.M., Lee, J.W., Bae, B. et al. Synthesis and characterization of MnO2 added (Na0.475K0.475Li0.05)(Nb0.9Ta0.05Sb0.05)O3 lead-free piezoelectric ceramics. J. Korean Ceram. Soc. 57, 440–446 (2020). https://doi.org/10.1007/s43207-020-00042-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-020-00042-z