Abstract
Ammonium nitrate is a chemical mostly used in agriculture and munitions to produce fertilizers and explosives, respectively. Its annual production and consumption exceed ten million tons. Despite is diverse uses, large production and consumption, and occupational risk, information on the toxicity that results from oral exposure to ammonium nitrate is limited. In this study, the safety of ammonium nitrate was therefore evaluated by observing its subchronic toxicity in rats. Ammonium nitrate (0, 100, 300 and 1000 mg/kg/day) was orally administered by gavage to rats at 5 times/week for 13 weeks. Reversibility of the effects of 1000 mg/kg/day was assessed in rats after 2 weeks. Mortality, clinical signs, body weight, and food consumption were monitored. Hematology, serum chemistry, urinalysis, organ weight, necropsy, and histopathology were performed. Salivation was intermittently observed in both sexes receiving 300 and 1000 mg/kg/day ammonium nitrate, which normalized 2 weeks post-treatment. Urine volume increased in both sexes receiving 1000 mg/kg/day ammonium nitrate. Urine pH decreased in both sexes of all dosing groups when compared with the concurrent control group. Urinary changes normalized 2 weeks post-treatment. Blood urea nitrogen levels increased in males receiving 1000 mg/kg/day, but normalized 2 weeks later. Potassium level in males and sodium and chloride levels in both sexes receiving 1000 mg/kg/day ammonium nitrate decreased, but normalized 2 weeks later. Hypertrophy of zona glomerulosa in the adrenals was observed in both sexes receiving 1000 mg/kg/day and in females receiving 300 mg/kg/day ammonium nitrate. After a 2-week recovery period, the same lesion was observed in one female receiving 1000 mg/kg/day ammonium nitrate. Our results indicate that ammonium nitrate induces reversible salivation, increases BUN levels, induces acidic diuresis with decreases in sodium, potassium, and chloride levels, and induces ZG hypertrophy. These results shed light on the toxicity profile of ammonium nitrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ammonium nitrate is a chemical formed from a reaction between ammonium and nitrate. It is highly water soluble; hence, it easily dissociates in water. Ammonium nitrate is a component of commercial fertilizers, explosives munitions, and oxidizing agents in instant cold packs. It is also used for the production of nitrous oxide, pyrotechnics, herbicides, and insecticides [1, 2]. In 2002, 13,608,000/13,487,000 and 686,000/1,149,000 tons were produced/consumed globally and in Asia, respectively [3].
Previous studies showed that direct exposure of humans to ammonium nitrate is usually accidental, and occurs through consumption of contaminated water or food [3, 4]. In addition, ammonium nitrate exposure is more common and frequent in occupational environments. However, the ammonium nitrate safety data sheet provides limited information on its toxicity; acute toxicity and irritation.
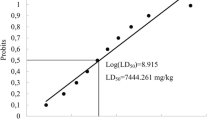
Ammonia has been reported to induce ulcer, edema, and/or erythema in the gastrointestinal mucosa (tongue and lips) when ingested [5]. Nitrate can induce hypertension and/or methemoglobinemia when ingested [1]. However, the toxicity information for ammonia and nitrate are derived from studies using ammonium chloride, potassium nitrate, or sodium nitrate, not ammonium nitrate. The present information on ammonium nitrate insufficiently illuminates its toxicity. The 50% lethal dose (LD50) of ammonium nitrate is reportedly 2217 mg/kg in rats dosed via oral gavage [2]. Methemoglobinemia-carboxyhemoglobinemia and body weight loss or decreased weight gain were also reported in rats dosed with 10 mg/kg ammonium nitrate via oral gavage in an acute toxicity study [2]. Changes in total protein (TP), bilirubin, cholesterol, and transaminases were noted in rats orally administered 65 mg/kg ammonium nitrate in a 26-week repeat-dose study [2].
Despite its wide range of applications, very high production/consumption, and occupational risks, current ammonium nitrate toxicity information is inadequate. Therefore, this study aimed at evaluating the safety of ammonium nitrate by examining its toxicity and reversibility in rats, following a 13-week repeated oral dosing and a 2-week recovery period.
Materials and methods
Test material
Ammonium nitrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in sterile water from Choongwae Pharma Co. (Seoul, Korea), to obtain the final test concentrations required (described below). The sterile water also served as the vehicle for the control group (dosing volume, 5 mL/kg/day).
Animals and experimental design
Five-week-old specific pathogen-free male and female Sprague–Dawley [Crl:CD(SD)] rats were purchased from Orientibio Inc. (Seongnam, Korea) and acclimatized for one week. Forty male and 40 female rats were randomly assigned to 4 groups, including one control and three dosing groups (100, 300 and 1000 mg/kg/day) for the main study. An additional 10 male and 10 female rats were also randomly assigned to the control and 1000 mg/kg/day groups, for the recovery study. Animals were orally dosed 5 times per week for 13 consecutive weeks using a gavage needle attached to a disposable syringe.
The initial rat body weights ranged from 187.5 to 208.8 g for males and 145.2–174.2 g for females. The rats were housed individually in stainless steel wire-bottom cages and supplied the commercial rodent diet from Harlan Laboratories Inc. (St. Louis, MO, USA), and filtered tap water ad libitum. Environmental conditions were controlled temperature, 21.1–23.9 °C; relative humidity, 34.9–64.5%; 12:12-h light:dark cycle; and fresh air ventilation, 10–15 times per hour. This experiment was conducted at Biotoxtech Co., Ltd. (Chungbuk, Korea) and approved by their animal experiment committee based on the Animal Protection Act (Approval No. 09305).
Animal observation
Body weights were individually measured for all animals prior to dosing, on the first dosing day, once a week thereafter, and on the day of necropsy. Body weight data on the day of necropsy were excluded during the evaluation of body weight because they were measured in a fasted state. Mortality was observed every day with a 6 h repeat interval before the dosing, and throughout the experimental period. Clinical signs were observed once a week. Daily food consumption was measured before dosing and once a week thereafter. Food consumption was calculated by subtracting the amount of leftover feed from the total feed provided.
Urinalysis
Urinalysis was performed for 5 rats in each group on week 13 for the animals in the main study, and on week 15 for the recovery study. Fresh urine (sampled within approximately 3 h from urine excretion) was collected to determine pH, protein, glucose, ketone body, bilirubin, and occult blood levels, using the Miditron® Junior II urine analyzer, or Combur 10 Test®M stick (test kits), both from Roche Diagnostics (Mannheim, Germany). Also, urine was visually observed for color and turbidity, and its sediment was analyzed for cast, epithelial cell, leukocyte, and erythrocyte counts using the Olympus BX50 microscope from Olympus Optical Co. (Tokyo, Japan). A cylinder was used to examine urine volume 24 h after excretion.
Hematology and serum chemistry
After the observation period, animals were allowed to fast overnight. On the following day, under isoflurane anesthesia, blood was collected from the abdominal aorta for hematological and serum biochemistry analyses.
Hematology measurements were taken using Advia 120 from Siemens Healthcare Diagnostics Inc. (Erlangen, Germany) and ACL 7000 from Instrumentation Laboratory (Bedford, MA, USA). They included total erythrocyte count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), platelet count (PLT), total leukocyte count (WBC), WBC differential count (neutrophil, lymphocyte, monocyte, eosinophil, and basophil ratio), reticulocyte count (Reti), prothrombin time (PT), and activated partial thromboplastin time (APTT).
Serum biochemistry parameters were measured using the Hitachi 7080 from Hitachi (Tokyo, Japan) and the Roche AVL9181 electrolyte analyzer from Roche (Mannheim, Germany). They included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), γ-glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), albumin/globulin (A/G) ratio, total cholesterol (T-Chol), triglyceride (TG), phosphorus (P), glucose (Glu), calcium (Ca), chloride (Cl), sodium (Na), and potassium (K) levels.
Necropsy, organ weight, and histopathological evaluation
All rats underwent complete necropsy, which included examination of the external surfaces of the body, all orifices, and the cranial, thoracic, and abdominal cavities and their contents. The brain, liver, kidneys, adrenals, testes, and ovaries were collected and weighed. Organ-to-body weight ratios were calculated for each of these organs. The brain, liver, kidney, spleen, and adrenals were preserved in 10% neutral buffered formalin. The preserved organ/tissues from rats receiving 0 and 1000 mg/kg/day ammonium nitrate and adrenals from rats receiving 100 and 300 mg/kg/day ammonium nitrate were paraffin-embedded, sectioned, hematoxylin and eosin (H&E) stained, and then microscopically examined.
Statistical analyses
Statistical analyses were performed for the body weight, food consumption, urine volume, hematology and serum biochemistry parameters, organ weights, and organ-body weight data using SAS version 9.1.3 from SAS Institute Inc. (Cary, NC, USA).
For the control and ammonium nitrate-treated groups of the main study, homogeneity of the variance in numerical data was determined using Bartlett’s test (p = 5%, two-tailed). For homogenous variance, groups were compared using one-way analysis of variance (ANOVA), while for heterogeneous variance, group comparisons were performed using the Kruskal–Wallis test. If statistical significance was observed, a Dunnett’s test (homogeneous data, p = 1% and 5%, two-tailed) or Steel test (heterogeneous data, p = 1% and 5%, two-tailed) was used for multiple comparisons of the control group with each dosed group.
For the recovery study, homogeneity of the variance between the control and ammonium nitrate groups was determined using the Fold-F test (p = 5%, two-tailed). For homogeneous variance data, groups were compared using the Student t test (p = 5%, two-tailed). For heterogeneous variance data, groups were compared using the Aspin-Welch t test (p = 5%, two-tailed).
Results
Clinical signs, body weight and food consumption
There were no dead or moribund animals. Salivation was observed intermittently during the dosing period and immediately after dosing in both sexes receiving 300 and 1000 mg/kg/day ammonium nitrate, in the main study (Table 1). It was not observed in the recovery study. No statistically significant difference was observed in the body weight and food consumption of animals of the main and recovery studies.
Urinalysis
There was a significant increase (p < 0.01) in the urine volume of males receiving 1000 mg/kg/day ammonium nitrate, when compared with that of males in the concurrent control in the main study. The tendency of urine volume to increase was mostly observed in females receiving 1000 mg/kg/day ammonium nitrate, when compared with that in females of the concurrent control in the main study. Changes in the urine volume were not observed in the animals of the recovery study (Table 2). Urine pH generally decreased in both sexes of all dosing groups when compared with the concurrent control group, in the main study. This was not observed after the 2-week recovery period (Table 2).
Hematology
MCHC significantly increased (p < 0.01) in males receiving 1000 mg/kg/day ammonium nitrate in the main study. This was not observed in the recovery study. A significant decrease in HGB, HCT, and lymphocyte (LYM) levels (HGB: p < 0.05, HCT: p < 0.05, LYM: p < 0.05) and increase in NEU levels (p < 0.05) were observed in females receiving 1000 mg/kg/day in the recovery study.
Serum chemistry
BUN and K levels significantly increased (p < 0.01) and decreased (p < 0.01) respectively, in males receiving 1000 mg/kg/day ammonium nitrate in the main study (Table 3). Na and Cl levels significantly decreased (Na: p < 0.01, Cl: p < 0.01) in both sexes receiving 1000 mg/kg/day ammonium nitrate in the main study (Table 3). The level of TG significantly increased (p < 0.05) in females receiving 1000 mg/kg/day ammonium nitrate in the main study (Table 3). ALT levels significantly decreased (p < 0.05) in females receiving 100 mg/kg/day ammonium nitrate in the main study. After the 2-week recovery period, TG levels significantly increased (p < 0.05) in both sexes receiving 1000 mg/kg/day ammonium nitrate (Table 3). A significant increase in the levels of T-Chol (p < 0.05) and Alb (p < 0.05) and a decrease in Crea (p < 0.05) and P (p < 0.05) levels, were observed in females receiving 1000 mg/kg/day ammonium nitrate in the recovery study.
Organ weight
There was a significant increase in the relative brain, liver, and kidney weights (brain and liver: p < 0.05, kidney: p < 0.01) in males receiving 1000 mg/kg/day ammonium nitrate in the main study. The absolute and relative kidney weights increased significantly (absolute: p < 0.05, relative: p < 0.01) in females receiving 1000 mg/kg/day ammonium nitrate in the main study. Statistically significant differences were not observed in the recovery study.
Necropsy and histopathological evaluations
At necropsy, a hepatodiaphragmatic nodule in the liver and crust in the tail were observed in one male receiving 1000 mg/kg/day ammonium nitrate in the main study.
Microscopic observations revealed hypertrophy of zona glomerulosa (ZG) in the adrenals of both sexes receiving 1000 mg/kg/day ammonium nitrate, and in one female receiving 300 mg/kg/day ammonium nitrate (Fig. 1). After the 2-week recovery period, the same lesion was noted in one female receiving 1000 mg/kg/day ammonium nitrate. Likewise, some spontaneous histopathological alterations were observed in the heart, kidney, liver, and spleen.
Hypertrophy of adrenal zona glomerulosa (ZG) in rats administered 1000 mg/kg/day ammonium nitrate (d–f). The ZG layer is widened and its cells are pale, as compared to the intact ZG layer in the control group rats (a–c). a and d 50 × magnification, b and e: 100 × magnification, c and f 200 × magnification. The hematoxylin and eosin stain was used
Discussion
We evaluated the potential toxicity of ammonium nitrate in rats via a 13-week repeated administration and 2-week recovery period oral dosing subchronic toxicity study.
Intermittent saliva release was observed in males and females receiving 300 and 1000 mg/kg/day ammonium nitrate in the main study. This was not observed after 2 weeks of recovery. Salivation was considered to be related to ammonium nitrate. A ammonium nitrate is categorized as an acid with a pH of 5.43, and a sour taste [2, 6]. This salivation can be attributed to the sour taste of ammonium nitrate, since the change was only observed directly after and/or before dosing, and not accompanied by any severe changes such as other pathological signs or abnormal neurological behavior.
Urine volume significantly increased in both sexes receiving 1000 mg/kg/day ammonium nitrate in the main study. Urine pH decreased in both sexes of all dosing groups when compared with that in the concurrent control in the main study. Ammonium nitrate is known to induce weak acidotic diuresis [7]. Therefore, the changes in urine volume and pH can be attributed to ammonium nitrate. These urinary changes were also observed in the animals of the recovery study, 2 weeks later.
Changes in MCHC values in the main study, and HGB, HCT, LYM, and neutrophil (NEU) levels in the recovery study were not attributed to ammonium nitrate because a majority of individual values were comparable with those in the concurrent control group. Furthermore, the changes observed did not lead to changes in related parameters or changes in the recovery study.
BUN levels increased in males receiving 1000 mg/kg/day ammonium nitrate in the main study and recovered after 2 weeks. Based on their mechanisms of action, causes of increased urea nitrogen can be categorized as prerenal, renal, or postrenal. Prerenal causes include increased urea synthesis and decreased renal blood flow; high-protein diets, protein catabolism, dehydration, or cardiovascular disease. Renal causes relate to renal injury and are generally accompanied by histopathological changes. Postrenal causes result from obstructing urine outflow by urinary calculi [8]. In this study, there was no evidence of protein catabolism, decreased renal blood flow, renal injury, or obstruction. However, ammonium nitrate is water-soluble, and readily dissociates into ammonium cation and nitrate anion [9]. The ammonium cation is then metabolized to urea in the liver of mammals [5]. Therefore, the change in BUN was considered to be related to nitrogen metabolism.
K levels in males, and Na and Cl levels in both sexes decreased at 1000 mg/kg/day ammonium nitrate in the main study but were normalized after the 2-week recovery period. Decrease in renal diuresis is known to lead to decrease in serum Na, Cl, and K levels [8]. Therefore, these observations are considered to be ammonium nitrate-induced.
TG concentrations significantly increased in females receiving 1000 mg/kg/day ammonium nitrate in the main study, and in both sexes receiving 1000 mg/kg/day ammonium nitrate in the recovery study. TG concentration changes in males were only observed in the recovery group. The concentrations changed by 25-45 mg/dL TG, compared with concentrations in the concurrent control group. Hence, it was unclear if this change was ammonium nitrate-related or not. Small changes in serum TG concentrations (increases/decrease) are a relatively frequent finding in toxicology studies. The changes are generally believed to represent minor alterations in lipid metabolism that do not adversely affect the animal’s health [8].
Changes in ALT levels in the main study, and T-Chol, Alb, Crea, and P levels in the recovery study at 1000 mg/kg/day ammonium nitrate were not considered to be related to the toxicity of ammonium nitrate, because they were minor, unassociated to histopathologic alterations, or only observed in the recovery group.
Significant brain, liver, and kidney weight changes were observed at 1000 mg/kg/day ammonium nitrate in the main study. However, these were not attributed to ammonium nitrate, owing to the small magnitude of the changes, absence of related alterations, or their observation in only oneside sex.
Necropsy revealed a hepatodiaphragmatic nodule in the liver and crust in the tail of on male receiving 1000 mg/kg/day ammonium nitrate. These lesions in the liver and tail were correlated with a hepatodiaphragmatic nodule, and regeneration in the liver and tail, respectively. These were not considered ammonium nitrate-related changes because they were single cases and incidental.
Microscopic observations indicated hypertrophy of ZG in the adrenals of both sexes receiving 1000 mg/kg/day ammonium nitrate and females receiving 300 mg/kg/day ammonium nitrate in the main study. After the 2-week recovery period, the same lesion was noted in one female receiving 1000 mg/kg/day ammonium nitrate, but was considered to be partially recovered based on the reduced incidence. The hypertrophy of ZG was characterized by expanded width and weak stainability of the ZG layer. Hypertrophy of ZG in rats is usually detected after administration of some diuretics [10]. Cells of the ZG produce the mineralocorticoid hormone aldosterone under the control of angiotensin II [7]. High levels of angiotensin II stimulate rat adrenal glomerulosa cells, which promote cellular hypertrophy but not proliferation [11, 12]. Aldosterone stimulates the retention of Na+ in the kidney, the retention of water (as a consequence of Na+ reabsorption), and renal secretion of K+ and H+ [13], resulting in an antidiuretic effect. Angiotensin enhances the reabsorption of NaCl by nephrons, and antidiuretic hormone release [13], which also results in an antidiuretic effect. Therefore, hypertrophy of ZG may be a compensatory change following increased urine volume. All other microscopic changes observed in the heart, kidney, liver and spleen were not considered to be ammonium nitrate-related because they were incidental, spontaneous in nature, or showed similar frequency among control and test groups in both sexes.
In summary, the no-observed-effect-level was determined at 100 mg/kg/day. Ammonium nitrate induced reversible salivation, increase BUN levels, and reversible acidic diuresis with decreasing Na, K, and Cl levels, and ZG hypertrophy in rats receiving 1000 and/or 300 mg/kg/day ammonium nitrate.
Changes in BUN and K levels were only observed in the 1000 mg/kg/day dosed main study males. Although the underlying mechanism was not clarified, we revealed some novel changes induced by ammonium nitrate. These results can therefore contribute to the creation of an ammonium nitrate toxicity profile.
Abbreviations
- LD50:
-
50% lethal dose
- APTT:
-
Activated partial thromboplastin time
- ALT:
-
Alanine aminotransferase
- Alb:
-
Albumin
- A/G:
-
Albumin/globulin ratio
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- Ca:
-
Calcium
- Cl:
-
Chloride
- Crea:
-
Creatinine
- Glu:
-
Glucose
- HCT:
-
Hematocrit
- H&E:
-
Hematoxylin and eosin
- HGB:
-
Hemoglobin concentration
- LDH:
-
Lactate dehydrogenase
- LYM:
-
Lymphocyte
- MCH:
-
Mean cell hemoglobin
- MCHC:
-
Mean cell hemoglobin concentration
- MCV:
-
Mean cell volume
- NEU:
-
Neutrophil
- ANOVA:
-
One-way analysis of variance
- P:
-
Phosphorus
- PLT:
-
Platelet count
- K:
-
Potassium
- PT:
-
Prothrombin time
- Reti:
-
Reticulocyte count
- Na:
-
Sodium
- T-Bili:
-
Total bilirubin
- T-Chol:
-
Total cholesterol
- RBC:
-
Total erythrocyte count
- WBC:
-
Total leukocyte count
- TP:
-
Total protein
- TG:
-
Triglyceride
- ZG:
-
Zona glomerulosa
- GGT:
-
γ-Glutamyl transpeptidase
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2017) Toxicological profile for nitrate and nitrite. Department of Health and Human Service. https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=1452&tid=258. Accessed 22 Feb 2019
National Center for Biotechnology Information (NCBI) (2005) PubChem database. Ammonium nitrate, CID = 22985, https://pubchem.ncbi.nlm.nih.gov/compound/22985. Accessed 25 Mar 2019
International Agency for Research on Cancer (IARC) (2010) IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans, volume 94 Ingested nitrate and nitrite, and cyanobacterial peptide toxins. International Agency for Research on Cancer, World Health Organization, Lyon, France, pp 45–325
Jablonska S (1975) Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica 150:287–291
Agency for Toxic Substances and Disease Registry (ATSDR) (2004) Toxicological profile for ammonia. Department of Health and Human Service. https://www.atsdr.cdc.gov/phs/phs.asp?id=9&tid=2. Accessed 22 Mar 2019
Zumdahl SS, DeCoste JD (2010) Introductory chemistry, 7th edn. Cengage Learning, California, pp 179–180
Alagarsamy V (2010) Textbook of medicinal chemistry, vol 1. Elsevier, India, pp 587–589
Carrow RN, Waddington DV, Rieke PE (2001) Turfgrass soil fertility and chemical problems: assessment and management. Wiley, New Jersey, pp 309–310
Hall LR, Everd EN (2014) Principles of clinical pathology for toxicology studies. In: Hayes AW, Kruger LC (eds) Heyes’ principles and methods of toxicology, 6th edn. CRC Press, Florida, pp 1305–1344
Gopinath C, Prentice DE, Lewis DJ (1987) Atlas of experimental toxicological pathology. MTP Press Limited, Lancaster, p 104
Otis M, Campbell S, Payet MD, Gallo-Payet N (2005) Angiotensin II stimulates protein synthesis and inhibits proliferation in primary cultures of rat adrenal glomerulosa cells. Endocrinology 146:633–642
Chandra S, Hoenerhoff JM, Peterson R (2013) Endocrine glands. In: Sahota SP, Popp AJ, Hardisty FJ, Gopinath C (eds) Toxicologic pathology: nonclinical safety assessment. CRC Press, Florida, p 682
Kierszenbaum LA (2007) Histology and cell biology: an introduction to pathology, 2nd edn. Mosby Elsevier, Pennsylvania, p 551
Acknowledgements
This work was supported by the Korea Occupational Safety and Health Agency, Ministry of Labor, Republic of Korea, and a Grant-in-Aid for chemical hazard assessment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lee, M.J., Chung, Y.H., Choi, H.Y. et al. Evaluation of subchronic repeated administration toxicity of ammonium nitrate in rats. Toxicol Res. 36, 115–122 (2020). https://doi.org/10.1007/s43188-019-00022-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-019-00022-4