Abstract
Chronic alcohol consumption has been implicated in male infertility, whereas Carica papaya (CP) ripe fruit possesses antioxidant activity. This study investigated histomorphological and hormonal effects of ripened CP in alcohol experimental model. Thirty Wistar rats were divided into six groups of five animals each as follows; groups 1, 2 and 3 received distilled water 2 ml, 40% ethanol 5 ml, and 40% ethanol 5 ml + 50 mg Clomiphene citrate/kg body weight, respectively, while groups 4, 5 and 6 received 40% ethanol 5 ml + CP 500, 1000 and 1500 mg/kg body weight, respectively. Sperm counts and motility were significantly decreased (p < 0.05) in group 2 compared to group 1. Testosterone significantly increased (p < 0.05) in CP-treated groups, and luteinizing hormone was significantly reduced (p < 0.05) compared to the control. Group 2 showed spermatogenic cell distortions which were ameliorated in the CP-treated groups. CP exerted testicular protective potential against ethanol-induced testicular toxicity plausibly via its antioxidant mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of alcohol represents the third largest risk factor for disease burden in high-income countries, behind smoking and hypertension, both of which are also associated with alcohol misuse [1, 2]. Alcohol consumption has been associated with a reduction in seminiferous tubular diameter and germinal epithelium, sperm concentration, percentage of spermatozoa with normal morphology and motility, in addition, to a decrease in serum levels of testosterone [3].
World Health Organization defines infertility in women as the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse [4], while infertility can also be the inability of a sexually active, non-contraceptive couple to achieve spontaneous pregnancy in 1 year [5]. It is undeniable that good quality semen is essential for reproductive success.
The testis is a male gland important for both reproductive (exocrine) and secretory (endocrine) functions [6]. They produce male gametes or sperms and they secrete hormones, primarily testosterone, which is responsible for the growth of male genitals and sperm production [6]. It is a paired, ovoid organ that sits in the scrotum, and has an average volume of 25 ml with 3.5–5 cm length by 2.5–3 cm in both widths [7]. Microscopically, testicular tissue contains interstitial connective tissues and lobule of glandular tubules interspersed with reproductive tissue cells [8, 9].
Carica papaya (CP) is a giant herbaceous plant resembling a tree, but not woody, in the family Caricaceae, found in Africa, believed to originate from South Mexico and/or in Central America [10]. Studies have linked papaya leaf extract with health benefits, such as the healing of wounds [11], reducing cardiovascular disease risk [12], anti-inflammatory activity [13], anti-tumor activity, alleviating allergic disorders and serving as an immune-adjuvant for vaccine therapy [14]. C. papaya have been shown to contain many active components that can increase the total antioxidant power in blood and reduce lipid peroxidation level, such as papain, chymopapain, cystatin, α-tocopherol, ascorbic acid, flavonoids, cyanogenic glucosides and glucosinolates [15]. It has been used to treat intestinal worms as well as warts, sinusitis, eczema, cutaneous tubercules and hardness of the skin [16]. Papain ointment is commonly made from fermented papaya flesh and is applied as a gel-like paste as documented in an anti-ulcer study [17]. Papaya seeds have been reported to possess possible female contraceptive effect on human [18].
Clomiphene citrate (CC) available as Clomid 50 mg, is an orally administered, nonsteroidal ovulatory drug which has estrogenic and anti-estrogenic properties and increases steroidogenesis [19]. It is a medication that is commonly used for the treatment of infertility both in females and in males [20]. This selective oestrogen receptor modulator increases gonadotropin levels, and ultimately stimulates testosterone and sperm production [21].
This study investigated the ameliorative potential of ethanolic extract of C. papaya ripe fruit on some male reproductive parameters and testis histomorphology in an alcohol toxicity model in Wistar rats.
Materials and methods
Plant collection, authentication and extract preparation
Five Cuban species of ripe C. papaya fruit weighing 2.5–3.2 kg were collected from a local farm in Nduetong Oku, within Uyo metropolis in the month of April, 2017, and authenticated by the Department of Botany, University of Uyo. A specimen voucher number of C. papaya (herbarium number UUPH18a) was obtained from Faculty of Pharmacy herbarium with taxonomic identified. Fresh fruits were washed and epicarp peeled off, seeds removed, and the flesh blended with a fruit blender to pulp as reported by Bhardwaj and Pandey [22]. The freshly blended fruit was wet macerated in 70% ethanol and kept for 72 h, and then filtered initially with cheese cloth and later with Whatman No. 4 filter paper. The extract was then concentrated by evaporating to dryness in a water bath at 45–60 °C, and crude extract obtained (330.05 g) was closed in a glass container, and refrigerated at 2–8 °C until use.
Phytochemical screening of Carica papaya
Phytochemical screening of the plant extract to test for alkaloids, saponins, phenol, cardiac glycosides, flavonoids, terpenes, phlobatannins, anthraquinones, tannins and carbohydrates was performed according to methods described by Sofowora [23] and Trease and Evans [24].
Determination of acute toxicity of Carica papaya fruit
Acute toxicity testing (LD50) of the fruit extract was carried out according to modified Lorke’s method [25]. Determination of the medial lethal dose (LD50) guides the selection of the low, middle and high dose (10, 20 and 30% of LD50) of the extract according to Okokon et al. [26].
Animal care and use
Thirty (30) adult male albino Wistar rats obtained from the Faculty of Basic Medical Sciences Animal House, University of Uyo, weighing 150–220 g were used for the study. The animals were allowed to acclimatize for 2 weeks and given humane care in accordance with the Principle of Laboratory Animal Care and Use [27]. The animals were randomly selected into six groups of five animals each, and housed in well ventilated wooden cages and kept under controlled environmental conditions of temperature 25 ± 5 °C and 12 h light/dark cycle. The rats were given standard chow, and water was provided ad libitum throughout the course of the study. All the studies conducted were approved by the Department of Anatomy and Faculty of Basic Medical Sciences Research and Ethics Committees.
Experimental design
The thirty experimental animals were weighed and then randomly divided into six experimental groups of five animals each as shown in the Table 1. The administrations were by oral gavage once daily at specific time between 7:00 and 8:00 am.
Body weights of the animals were recorded weekly and final body weight taken at the end of the treatment period, and then sacrificed 24 h after the last administration. Animals were anaesthetized with chloroform, and their thoraco-abdominal walls dissected to access the heart, epididymis and testis. Blood was aspirated from the heart through cardiac puncture, centrifuged and serum collected for hormonal assay. Immediately after, both testes were collected and gross morphometric assessments were performed. Caudal epididymis were dissected out and minced for semen analysis. The testes were thereafter fixed in 10% buffered formalin for 7 days, for tissue processing and histological studies.
Semen parameters: estimation of sperm motility
Caudal epididymis was incised to expose fluid. 5 µl of the epididymal fluid was collected with a micro pipette, and delivered onto a glass slide and covered with a 22 × 22 mm cover slip. Glass slide was taken to light microscope and viewed at magnification at ×400. Motility estimation was then carried out at room temperature between 24 and 28 °C. Microscopic field was scanned systematically and each spermatozoon encountered was assessed. Motility was recorded in percentage and classified as progressively motile, non-progressively motile and non motile. Procedure was repeated and average taken [28].
Estimation of sperm count and concentration
Harvested epididymis was cut with an anatomical scissors and minced in a petri dish. 50 μl of epididymal spermatozoa was diluted in 950 μl diluent. Well mixed solution was pipette into both chamber of hemocytometer. Hemocytometer was placed on the stage of the microscope. Objective of the microscope was adjusted to ×40 magnification. Hemocytometer was viewed and counting was done. Values of counts were recorded. Counting was repeated in each chamber and average count documented. Average number of cells, cell density, dilution factor, and cell concentration was then calculated for by applying the formula as described for a similar reproductive toxicity study [29].
Gross morphometry
Testicular weight and volume
Testis was extracted from the animal while the blood and connective tissue were cleaned off. Testis was then placed on the weighing balance with a setting to measure in grams. Weight of both testes was recorded, and average weight documented.
Fluid (water) was put into a measuring cylinder to a marked point on it scale. Testis was then introduced into the cylinder with fluid. Change in volume of the fluid in the cylinder was observed. Volume of testis was then recorded by the expression.
Volume of testis = final volume of fluid in cylinder − initial volume of fluid in cylinder.
Major testicular axis
Testis was extracted from the animal while the blood and connective tissue were cleaned off. Cleaned testis was placed in a petri dish. Jaws of the digital vennier caliper were extended to touch both pools of the right and left testis. Values were observed and recorded.
Testicular diameter
Testis was extracted from the animal while the blood and connective tissue were cleaned off. Cleaned testis was place in a petri dish. Jaws of the digital vennier caliper were extended to touch the circumference of both the right and left testis at the equatorial pool. Values were observed and recorded.
Determination of reproductive hormones
The serum levels of Luteinizing hormone (LH), follicle stimulating hormone (FSH) and testosterone were determined using AccuBind ELISA Microwells kits (Monobind Inc., Lake Forest, CA, USA) with catalog numbers: 425–300, 625–300 and 3725–300 for FSH, LH and total testosterone, respectively, in accordance with manufacturer’s protocol.
Sacrifice and tissue processing
At day 25, rats were humanely sacrificed under chloroform inhalation and the testis dissected out, blotted dry, fixed, processed and stained using haematoxylin and eosin method [30]. The slides were viewed under light microscope for histological changes.
Statistical analysis
Data obtained from this study were analyzed using Graphpad 6 version II (San Diego, CA) system package. Results were expressed as mean ± standard error of mean. One way ANOVA and multiple comparism and T Test were employed with the significance level at p < 0.05.
Results
Median lethal dose of ethanolic extract of C. papaya ripe fruit
No mortality was observed with an increasing dose of the extract up to 5000 mg/kg body weight. At this point the process was discontinued and the medial lethal dose of C. papaya ripe fruit was estimated to be over 5000 mg/kg body weight.
Phytochemical analysis of ethanolic extract of C. papaya ripe fruit
Phytochemical analysis showed that C. papaya ripe fruit ethanolic extract has the presence of saponins (0.94 g), cardiac glycoside (0.0987 g), flavonoid (0.98 g), deoxy-sugar, terpenes and steroids, phlobatannins (0.46 g), alkaloids (0.76 g), anthraquinone and carbohydrates. However, tannins and phenols were observed to be absent, since there were no significant expression of their presence by testing (Table 2).
Effect of ethanolic extract of C. papaya ripe fruit on testicular morphometry
Analysis of the different gross morphometric parameters; testicular length (right and left), testicular volume (right and left), testicular weight (right and left) and testicular diameter (right and left) show no significant differences in all groups when compared to the control. However, there was significance decrease (p < 0.05) in the diameter of the right testis (TDR) of the group 4 and 5 (40% ethanol + C. papaya 500 mg/kg and 40% ethanol + C. papaya 1000 mg/kg) as compared to the group 2 administered 40% ethanol (Table 3).
Effect of ethanolic extract of C. papaya ripe fruit on semen parameters
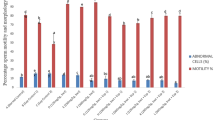
Analysis of the sperm activities showed a significant increase (p < 0.05) in sperm count in groups 5 and 6 administered with 40% ethanol + C. papaya (1000 and 1500 mg/kg) respectively compared to the 40% ethanol group, but significant decrease (p < 0.05) in sperm count in groups 2 (40% ethanol) and 3 (40% ethanol + clomiphene citrate) compared to the control group. There was also significant increase (p < 0.05) in sperm motility in the same groups 4, 5 and 6 compared to the group 2, but significant decrease (p < 0.05) in sperm motility in the groups 2 (40% ethanol) and 3 (40% ethanol + clomiphene citrate) compared to the control group (Table 4).
Effect of ethanolic extract of C. papaya ripe fruit on reproductive hormones
The result of hormonal assay showed a significant increase (p < 0.05) in testosterone in the ethanol + C. papaya (500 mg/kg) (group 4) compared to the animals that received 40% ethanol alone (group 2), and a significant decrease (p < 0.05) in the luteinizing hormone in the groups 3, 4, 5 and 6 administered with 40% ethanol + clomiphene citrate, 40% ethanol + C. papaya (500, 1000, and 1500 mg/kg) compared to the control. However, there were significant increase (p < 0.05) in the follicle stimulating hormone in the groups 2 and 4 compared to the control (Table 5).
Histological observations
The photomicrograph of transverse section of the testis from group 1, showed a normal histoarchitecture and arrangements of spermatogenic cells, and presence of interstitial cells of Leydig and basal germinal cells. No degeneration was observed in the seminiferous tubules, and dividing spermatocytes with numerous spermatids and migrating spermatozoa toward the central parts of the seminiferous lumen were observed and tubules are well distinct (Fig. 1G1).
G1–G6 The photomicrographs of transverse section of the testis of each group (1–6) at ×100 stained with H&E. Ict interstitial connective tissue, Lc leydig cells, Spc spermatogenic cells, sp spermatozoa, st sertoli cells, SL seminiferous lumen, sc spermatocytes, Ec eroded cells, Dc cellular degenerations, Dct interstitial tissue degenerations
The photomicrograph of transverse section of the testis from group 2, presented an abnormal histo-arrangement of the spermatogenic cells. Interstitial connective tissue were degenerated, spermatogonia cells were scanty and scattered. It also had few abnormal spermatids and disruption of basal layer of germinal cells. Seminiferous tubules were degenerated (Fig. 1G2).
The photomicrograph of transverse section of the testis from group 3, showed a normal seminiferous tubular histoarchitecture and arrangement of the spermatogenic cells. Normal seminiferous tubules, presence of interstitial cells of Leydig and basal germinal cells were observed. Cells of seminiferous tubules demonstrated regenerations, but with few dividing spermatocytes and spermatids with migrating spermatozoa (Fig. 1G3).
The photomicrographs of transverse section of the testes from groups 4 and 5, demonstrated a normal histoarchitecture and arrangement in spermatogenic cells. Distinct presence of the interstitial cells of Leydig and basal germinal cells were observed. There were also numerous dividing spermatocytes and spermatids with migrating mature spermatozoa towards the central part of the seminiferous lumen (Fig. 1G4 and G5).
The photomicrograph of transverse section of the testis from group 6, presented a normalizing seminiferous histoarchitecture and arrangement of spermatogenic cells. Seminiferous tubules were distinct, but indicated reduced interstitial connective tissue cells. There were presence of interstitial cells of Leydig and basal germinal cells. Spermatids were fewer than normal, and there were migrating spermatozoa towards the central part of the seminiferous lumen (Fig. 1G6).
Discussion
This study was to determine the protective effect of C. papaya ripe fruits against ethanol induced toxicity to testicular tissue. Fertility impairment caused by alcohol consumption has been on a rise, and different measures have been employed to counter these effects. Ethanol and its derivatives have been reported to cause testicular injury [2]. The consequences of long term alcohol ingestion may include gynecomastia, impotence, testicular atrophy, and loss of libido [31]. Antioxidant capacity of juice (fruit blends) has been linked to in vivo protection from oxidative stress and the scavenging of free radicals [32]. The antioxidant activity may be explained by the phytochemicals and vitamins present [33].
The result of C. papaya fruit on acute lethal toxicity test showed there was no mortality at up to 5000 mg/kg body weight Wistar rats. This result verifies the fact that C. papaya is very safe for consumption and does not pose any health threat even at high concentration.
Phytochemical screening of ethanolic extract of C. papaya ripe fruit showed the moderate presence of alkaloids and anthraquinone, rich in saponins, flavonoids, cardiac glycosides, deoxy-sugar, phlobatanins, and carbohydrate. The screening also indicated the absence of tannins and phenol. The absence of phenol correlates with the findings that phenol increases in immature C. papaya and decreased at mature and ripened stages [34]. The various biological activities of C. papaya are believed to be influenced by their phytochemical constituents. One of the important phytochemical is the flavonoid. Bioflavonoids are a group of natural benzo-g-pyran derivatives, and are found to possess strong antioxidant activities [35, 36]. Bioflavonoids widely distributed in fruits and vegetables, are reported to exert multiple biological effects including free radical-scavenging activity [37]. Pharmacological activities of flavonoids have been reported for its anti-toxicity, anti-inflammatory and antitumor properties [38]. Saponins have cytotoxic and anti-tumor activities [39], and plants containing saponins have been used for medicinal purposes since ancient times [40]. Carbohydrate presence in C. papaya gives reasons for the high level energy availability from the plant. Other phytochemicals such as phlobatannins, alkaloids, terpenes and steroids have also been documented to possess cytoprotective properties.
Morphometric study of the testicular parameters showed no significant difference in the testicular weight, length and volume in both right and left testes. The significant decrease observed in the right testicular diameter of the rats administered C. papaya 500 mg/kg and C. papaya 1000 mg/kg + 40% ethanol may be influenced by the absence of any testicular inflammation as compared to the animals given 40% ethanol. Ethanol is experimentally reported to induce inflammation of tissues [2]. Hepatic and renal inflammations by ethanol have been documented, and testis is also prone to this effects. However, this finding shows that the extract may not have had any significant influence on the testicular weight, testicular length and testicular volume, and may not have significantly interfered with genetic factors influencing these parameters.
The sperm activities investigated were observed to show significant increase in sperm count and motility in the groups administered with 500 mg/kg, 1000 mg/kg and 1500 mg/kg C. papaya + 40% ethanol when compared to the group 2 given 40% ethanol. This supports the fact that the extract promotes spermatogenic activities compared to the standard drug. The significant decrease observed in the group given 40% ethanol may be as a result of oxidative stress induced by ethanol on the spermatogenic cells [2].
Furthermore, spermatozoa are particularly susceptible to oxidative stress-induced damage due to their plasma membranes containing large quantities of polyunsaturated fatty acids (PUFAs) present in the plasma membrane and low concentrations of scavenging enzymes in the cytoplasm [41,42,43]. Mitochondria produce ATP required for the movement of the flagella of sperm cells. Hence, a reduced or impaired mitochondrial function will impede sperm motility [43] as observed in the ethanol treated group in this study. This explains the antifertility property of addictive consumption of ethanol, and the deposition of cytotoxic radicals which causes deleterious effect to the testicles and spermatogenic cells. The decrease in activities of spermatozoa by 50 mg Clomiphene citrate + 40% ethanol may explain a slower cyto-corrective potency of Clomiphene citrate to testicular tumor induced by ethanol as compared to C. papaya. C. papaya proves to be a good source of nutrient that boost these sperm activities and protect them from cytotoxic agents.
Testosterone is observed to be significantly increased by C. papaya, as evident in the group administered with 500 mg/kg C. papaya + 40% ethanol compared to the group given 40% ethanol alone. The mechanism in which C. papaya raises testosterone level is not known, but could be linked to the abundance of flavonoids, which is an effective oxidative radical inhibitor found in C. papaya. It is also known that the normal method of physiological response to reduced primary spermatogenesis is to relatively elevate blood gonadotropin level [28]. These findings also suggest that the release of follicle stimulating hormone into the blood were not influenced by any factor. The mechanisms responsible for the rapid increase in luteinizing and follicle stimulating hormone, upon administration of 40% ethanol alone as evident in group 2 were not clear. These finding correlates with the findings of the hormonal changes in Cisplatin induce testicular toxicity as reported by [28]. With constant administration of 40% ethanol alone for 28 days, the level of testosterone in the peripheral blood decreased while lutenizing hormone increased. It may be explained from the fact that Leydig cells and withdrawal of the controlling effects of the germinal epithelium on the LH-Leydig cells axis could bring about the changes in serum hormone levels as corroborated by other studies [28, 44].
Histopathological findings in this study (Fig. 1) confirmed ethanol toxicity in the animals as shown by extensive cellular necrosis, degeneration of seminiferous tubules and defoliation of spermatocytes, which correlates with findings in other studies [2, 31, 43]. The photomicrograph result correlates with the hepatoprotective and testiculo-protective properties of unripe fruit of C. papaya [45]. The ethanol administered tissue shows cellular swellings, depleted seminiferous epithelium, exfoliated germ cells, degenerated intertubular connective tissue and reductions in spermatogenic cells is similar to the degenerations and effects in testicular histology reported by [2] in a research study. These effects may be influenced by free radical activities induced by ethanol methabolism in vivo. Free radicals attack all major classes of biomolecules, mainly the polyunsaturated fatty acids of cell membranes. The oxidative damage of PUFA, known as lipid peroxidation is particularly destructive, because it proceeds as a self-perpetuating chain reaction [46]. It was also observed that there were indications of regenerating cells and normalizing germinal epithelium in the rats administered with Clomiphene citrate + 40% ethanol as compared to the control. It is evident that Clomiphene citrate also has a cytoprotective potency which may be due to hormonal mechanism, but this potency may be achieved faster with total withdrawal of ethanol intake. However, the protective ability of Clomiphene citrate to testicular cyto-structure is not as effective as that of C. papaya. It is, however, observed that the testicular effect by C. papaya 1500 mg/kg administration is similar to those effects in Clomiphene citrate treatment. This may suggest that higher dose of C. papaya may also affect the antioxidant property of the plant, or also deposit radical, due to the inability for the body to completely metabolize the C. papaya, hence also causing oxidative stress on the tissue, as against the dose of C. papaya 500 mg/kg and 1000 mg/kg.
Conclusions
The supplementation of ripe C. papaya fruit exerts strong testicular protective potential against ethanol-induced testicular toxicity in Wistar rats in a dose dependent manner, which may be attributed in part to the bioavailability and bioactivity of antioxidant like flavonoids in vivo.
References
World Health Organisation (2012) Global Health adds life to years. Global brief for World Health Day 2012. Geneva, Switzerland. Ref. No.: WHO/DCO/WHD/2012.2. Accessed 29 April 2017
Dosumu OO, Osinubi AA, Duru FI (2014) Alcohol induced testicular damage, can abstinence equal recovery? Middle East Fertil Soc J 19:221–228. https://doi.org/10.1016/j.mefs.2014.01003
Muthusami KR, Chinnaswamy P (2005) The effect of chronic alcoholism on male fertility. Fertil Steril 84:919–924. http://www.ncbi.nlm.nih.gov/m/pubmed/16213844. Accessed 9 May 2018
Zegers-Hochschild F, Adamson GD, De Mouzon J, Ishihara O, Mansour R (2009) The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology. Hum Reprod 24:2683–2687. https://doi.org/10.1093/humrep/dep343
Jungwirth A, Giwercman A, Tounaye H, Diemer T, Kopa Z, Dohle G, Krausz C (2015) Guidelines on male infertility. Eur Urol 17:1–42. http://www.ncbi.nlm.nih.gov/m/pubmed/22591628. Accessed 7 July 2018
Alina B (2017) Testicles: facts, function and diseases. live science. https://www.livescience.com/58838-testicle-facts.html. Accessed 10 Sept 2017
Snell RS (2000) Structures of the Anterior Abdominal wall: scrotum, testis, and epididymides. Clinical anatomy for medical students, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 214–230
Moore K, Daley A (2006) Inguinal region. Clinical oriented anatomy, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 214–230
Guyton A, Hall J (2006) Textbook of medical physiology. Churchill LivingStone Elsevier, Philadelphia, p 452
Gabriela F, Jorge MS (2014) Papaya (Carica papaya L): origin, domestication, and production. In: Genetics and genomics of papaya, crop and models, vol 10, https://doi.org/10.1007/978-1-4614-8087-7_1
Mahmood AA, Sidik K, Salmah I (2005) Wound healing activity of Carica papaya L. aqueous leaf extract in rats. Int J Mol Med 1:398–401 (ijmmas.2005.398.401)
Runnie I, Salleh MN, Mohamed S, Head RJ, Abeywarden MY (2004) Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J Ethnopharmacol 92:311–316. https://doi.org/10.1016/j.jep.2004.03.019
Owoyele B, Adebukola O, Funmilayo A, Soladoye A (2008) Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology 16:168–173. https://doi.org/10.1007/s10787-008-7008-0
Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C (2010) Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol 127:760–767. https://doi.org/10.1016/j.jep.2009.11.024
Seigler DS, Pauli GF, Nahrstedt A, Leen R (2002) Cyanogemic allosidies and glucosides from Passiflora edulis and Carica papaya. Phytochemistry 60:873–882. https://www.ncbi.nlm.nih.gov/m/pubmed/12150815/. Accessed 12 Mar 2018
Yushau M, Onuorah FC, Murtala Y (2009) In-vitro Sensitivity pattern of some urinary tract isolate to Carica papaya extract. Bajopas 2:75–78. https://doi.org/10.4314/bajopas.v2i2.63786
Rodrigues ALS, Oliveira BGRB, Futuro DO et al (2015) Effectivesness of papain gel in venous ulcer treatment: randomized clinical trial. Rev Latino-Am Enfermagem 23:458–465. https://doi.org/10.1590/0104-1169.0381.2576
Lohiya NK, Manivannan B, Mishra PK, Pathak N, Sriram S, Bhande SS, Panneerdoss S (2002) Chloroform extract of Carica papaya seeds induces long-term reversible azoospermia in langur monkey. Asian J Androl 4:17–26. http://www.ncbi.nlm.nih.gov/m/pubmed/11907624/. Accessed 12 Mar 2018
Tan RS, Vasudevan D (2003) Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil Steril 79:203–205. http://www.ncbi.nlm.nih.gov/pubmed/12524089. Accessed 15 July 2018
Richard S (2016) Clomid and metformin for PCOS: glucophage plus clomiphene for fertility treatment and pregnancy with polycystic ovarian syndrome. Adv Fertil Cent Chic 8:662–1818. https://doi.org/10.3978/j.issn.2305-5839.2014.04.15
Ahmad A, Kikuchi H, Takami Y, Nakayama T (2005) Different roles of N-terminal and C-terminal halves of HIRA in transcription regulation of cell cycle—related genes that contribute to control of vertebrate cell growth. J Biol Chem 280:32090–320100. http://www.ncbi.nlm.nih.gov/m/pubmed/1602492. Accessed 11 July 2018
Bhardwaj RL, Pandey S (2011) Juice blends—a way of utilization of under utilized fruits, vegetables, and spices: a review. Crit Rev Food Sci Nutri 51:563–570. https://doi.org/10.1080/10408391003710654
Sofowora A (2008) Medicinal plants and traditional medicine in African. 3rd edn. Spectrum Books Limited, Ibadan, pp 199–204. https://doi.org/10.12691/ajbr-5-2-1
Trease GE, Evans WC (1989) Pharmacognosy, 11th edn. Macmillan Publishers, London, pp 1–7
Lorke D (1983) A new approach to practical acute toxicity testing. Toxicology 54:275–287. http://www.ncbi.nlm.nih.gov/m/pubmed/6667118/. Accessed 9 May 2018
Okokon JE, Oguamanam WN, Umoh EE (2017) Hepartoproterctive and neuroprotective activities of solenostemon monostachyus P. beauv (Lamiaceae) leaf extract. Afr J Pharmacol Ther 6:123–133. http://journals.uonbi.ac.ke/ajpt/article/view/1602. Accessed 9 May 2018
National Institute of Health (2011) Guide for the care and use of laboratory animals. 8th edn, Natsional Academies Press, Washington (DC), pp 1–217. https://doi.org/10.17226/12910
Azu OO, Duru FI, Osinubi AA, Oremusu AA, Norohna CC, Okanlawon AO, Elesha SO (2011) Long-term treatment with Kigelia Africana fruit extract ameliorates the testicular toxicity following cisplatin administration in male sprague-dawley rats. J Med Plants Res 5:388–397
Osinubi AA, Daramola AO, Noronha CC, Okanlawon AO, Ashiru AO (2007) Effect of quinine and ascorbic acid on rat testes. West Afr J Med 26:217–221. http://www.ncbi.nlm.nih.gov/m/pubmed/18399338/. Accessed 7 July 2018
Dhurba G (2015) Hematoxylin and eosin staining: principle, procedure and interpretation. Histopathology 4:125–130. http://laboratoryinfo.com/hematoxylin-and-eosin-staining/. Accessed 9 May 2018
Adaramoye OA, Arisekola M (2013) Kolaviron, a biflavonoid complex from garnicia kola seeds, ameliorate ethanol induced reproductive toxicity in male wistar rats. Niger J Physiol Sci 28:9–15. http://www.ajol.info/index.php/njps/articl/download/95095/84442. Accessed 9 May 2018
Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG (2008) In vitro and in vivo antioxidant and anti-inflammatory capacity of an antioxidant-rich fruit and berry juice blend. result of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem 56:8326–8333. https://doi.org/10.1021/jf8016157
Szeto YT, Tomlinson B, Benzie IF (2002) Total antioxidant and ascorbic acid content of fresh fruits and vegetables; implications of dietary planning and food preservation. Braz J Nutr 87:55–59. http://www.ncbi.nlm.nih.gov/m/pubmed/11898770/. Accessed 7 July 2018
Addai ZR, Abdullah A, Mutalib SA, Musa KH, Douqan EM (2013) Antioxidant activity and physiochemical properties of mature papaya fruit (Carica papaya L. cv. Eksotika). Adv J Food Sci Technol 5:859–865. https://ukm.pure.elsevier.com/en/publications/antioxidant-activity-and-physiochemical-properties-of-mature-pap. Accessed 11 July 2018
Pietta P (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. http://www.ncbi.nlm.nih.gov/m/pubmed/10924197. Accessed 9 May 2018
Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA (2001) Flavonoids: a review of probable mechanism of action and potential applications. Am J Clin Nutr 74:418–425. http://www.ncbi.nlm.nih.gov/m/pubmed/11566638/. Accessed 9 May 2018
Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv 5:27986. https://doi.org/10.1039/C4RA13315C
Mendel F (2007) Overview of antibacterial, antitoxin, antiviral and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res 51:116–134. http://www.ncbi.nlm.nih.gov/m/pubmed/17195249. Accessed 7 July 2018
Irma P, Agnieszka G, Danuta S (2010) Saponins are cytotoxic agent: a review. Phytochem Rev 9:425–474. http://www.ncbi.nlm.nih.gov/m/pubmed/20835386. Accessed 11 July 2018
Flore C, Elsenhut M, Ragazzi E (2005) A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol 99:317–324. http://www.ncbi.nlm.nih.gov/m/pubmed/15978760. Accessed 9 May 2018
Alvarez JG, Storey BT (1995) Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev 42:334–346. http://www.ncbi.nlm.nih.gov/m/pubmed/8579848. Accessed 9 May 2018
Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility. Urology 48:835–850. http://www.ncbi.nlm.nih.gov/m/pubmed/8973665. Accessed 9 May 2018
Oremusu AA, Akang EN (2015) Impact of alcohol on male reproductive hormones, oxidative stress and semen parameters in Sprague-Dawley rats. Middle East Fertil Soc J 20:114–118. https://doi.org/10.1016/j.mefs.2014.07.001
Aydiner A, Aytekin Y, Topuz E (1997) Effects of cisplatin on testicular tissue and the leydig cell-pituitary axis. Oncology 54:74–78. http://www.ncbi.nlm.nih.gov/m/pubmed/8978597. Accessed 9 May 2018
Erukainure OL, Okafor OY, Obode OC, Ajayi A, Oluwole OB (2012) Blend of roselle calyx and selected fruit modulates testicular redox status and sperm quality of diabetic rats. Diabetes Metab 3:214. https://doi.org/10.4172/2155-6156.1000214
Jenkinson AM, Collins AR, Duthie SJ, Wahle KW, Duthie GG (1999) The Effect of increased intakes of polyunsaturated fatty acids and vitamin E on DNA damage in human lymphocytes. J Leukoc Biol 13:2138–2142. http://www.ncbi.nlm.nih.gov/m/pubmed/10593860. Accessed 7 July 2018
Funding
Self funded research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asuquo, I.E., Edagha, I.A., Ekandem, G.J. et al. Carica papaya attenuates testicular histomorphological and hormonal alterations following alcohol-induced gonado toxicity in male rats. Toxicol Res. 36, 149–157 (2020). https://doi.org/10.1007/s43188-019-00017-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-019-00017-1