Abstract
This study aimed to evaluate combinations of low-cost raw materials wheat bran, cottonseed cake, and potential inductors (olive oil, tween 80, triton X-100, and the oil extracted from the viscera of the Nile tilapia) for lipase production by Aspergillus niger IOC 4003 in solid-state fermentation (SSF). In addition, the lipase obtained was submitted to partial purification by precipitation with organic solvents, and its stability against pH and temperature was evaluated. The effects of SSF operating conditions (cultivation time, pH, inoculum, moisture), tilapia viscera oil, nutrients (glucose, urea, yeast extract, cottonseed cake/wheat bran ratio), and salts (KH2PO4 and MgSO4.7H2O) were screened for lipase production by Plackett–Burman Design followed by a Central Composite Rotatable Design (CCRD). The highest activity obtained using the CCRD was 0.091 U/g. The addition of olive oil, non-ionic surfactants, and tilapia viscera oil in different concentrations did not increase lipase production. Acetone and ethanol precipitation was able to increase the specific activities of lipase up to 16.00 and 4.91 times, respectively. The concentrated acetone fraction lipases showed high stability to pH and temperature stresses, maintaining enzymatic activity greater than 70% at pH 3 to 10 and not less than 80% at 30 to 60 °C after 6 h of exposure, which can be of great interest in various industrial processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzymes are important industrial inputs because they achieve results which cannot be obtained by chemical synthesis (Dayanandan et al. 2013). In addition, they have high substrate binding specificity (Adjonu et al. 2013). The global market of enzymes has reached USD 5 billion in 2016. It is projected to reach USD 8.7 billion by 2026 (Global markets for enzymes in industrial applications 2021). Thus, the enzyme market is advancing and lipases correspond to 10% of this market (Salihu et al. 2016).
Lipases are enzymes that catalyze the hydrolysis of the triacylglycerol ester bonds to diacylglycerols, monoacylglycerols, and free fatty acids. They also catalyze the synthesis of esters, transesterification, and interesterification of lipids (Geoffry and Achur 2018). Lipases have advantages such as the ability to act in wide pH and temperature ranges, stability against organic solvents, potential to replace conventional chemical catalysts, biodegradability, specificity, and selectivity (Thakur 2012; Torres et al. 2006). These enzymes are applied in catalysis in several productive sectors such as chemical (detergent formulations), environmental (biosurfactants, biofuels), food (dairy industry, food additives), and health (digestive enzymes and cosmetics) areas (Geoffry and Achur 2018; Nema et al. 2019). The catalytic role of lipases in transesterification reactions has been used in oils for biofuel production (Selvakumar and Sivashanmugam 2017). In turn, the cosmetic industry has employed them for synthesizing ingredients to improve the emollience and fragrance of products (Khan and Rathod 2020). However, the economic viability of industrial processes using lipases is associated with the costs of producing these enzymes (Geoffry and Achur 2018).
The main lipase production processes are submerged fermentation (SmF) and solid-state fermentation (SSF) (Geoffry and Achur 2018). The interest in SSF is attributed to the high yield and productivity of the product, less generation of effluents, low energy consumption and the use of low-cost raw materials simulating the natural habitat of microorganisms (Nema et al. 2019; Yazid et al. 2017).
Organic wastes can be good candidates for SSF subtracts due to providing carbon, nutrients, and moisture at low cost, with minimal or no prior treatment (Yazid et al. 2017). Among the agricultural residues studied, wheat bran had the highest productivity in obtaining lipase in the SSF when combined with olive oil as an inductor (Kumar and Ray 2014). Cottonseed cake was also investigated for lipase production in SSF with good results (Nema et al. 2019; Thirunavukarasu et al. 2016). In turn, no studies were found for other wastes such as tilapia viscera oil on its use for lipase production.
Cottonseed cake is the material that remains after extracting cotton oil and has high levels of crude protein and lipids (McCartney and Tingley 1998). The lipid constitution is mainly linoleic (56.9%), oleic (16.9%), and palmitic acids (23.3%) (Ferreira et al. 2019). These are also the main compounds of olive oil, which is the best lipase production inductor (Wang et al. 2008). In the same way, the oil extracted from Nile tilapia viscera is rich in unsaturated fatty acids, mainly oleic acid (López et al. 2010). This lipid profile similar to olive oil has the potential to induce the production of lipases.
Selectively recovering a target protein from a crude extract can represent 50–90% of total production costs (Sousa Junior et al. 2016). On the other hand, there are products whose application does not require a high degree of purity. In this case, reducing the number of purification steps is fundamental for economic viability (Trentini et al. 2015). Separating macromolecules by extract precipitation is the most traditional method for recovery and partial purification. It is a low-cost technique as it does not require complex equipment and consumes little energy (Trentini et al. 2015; Preczeski et al. 2018).
In this context, this study aimed to evaluate the potential of low-cost raw materials of wheat bran, cottonseed cake, and tilapia viscera oil and supplementation with surfactants and olive oil in different concentrations for lipase production by Aspergillus niger in solid-state fermentation. In addition, the lipase obtained was submitted to partial purification by precipitation with organic solvents, and its stability against pH and temperature was evaluated.
Material and methods
Agro-industrial by-products
Cottonseed cake (CTC) and wheat bran (WB) were obtained from the local market in Natal, RN, Brazil, and stored at room temperature. The following physicochemical characterizations were performed: moisture (McCartney and Tingley 1998); ash, fat, crude protein, crude fiber, and total carbohydrates (AOAC 2003) water activity (Sancho et al. 2015); and water absorption index (López et al. 2018).
Tilapia viscera oil (TVO) was provided by Piscis Industry and Commerce LTDA (Jaguaribara, CE, Brazil). The sample was stored at room temperature and protected from light. Acidity and peroxide values were evaluated according to the Association of Official Analytical Chemistry methodologies (AOAC 2003). The fatty acid profile of the oil was determined on a Shimadzu GCMS-QP2010 gas chromatograph mass spectrometer system (Shimadzu, Kyoto, Japan). In this case, methylation of fatty acids was performed to obtain the corresponding methyl esters (Hartman and Lago 1973) and the chromatographic method was carried out according to Alcântara et al. (2019). The percentage of fatty acids in the sample was determined by a calibration curve with methyl ester standards.
The use of these by-products was authorized by the National System for Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under registration number AD83CB1.
Microorganism
Aspergillus niger IOC 4003 was provided by the Collection of Culture of Filamentous Fungi of the Oswaldo Cruz Institute (Rio de Janeiro, Brazil). The growth of the microorganism occurred in Potato Dextrose Agar at 30 ± 2 °C for 7 days. Then, spore propagation was performed in corn cob medium, as previously described by Guilherme et al. (2008).
Lipase production
The Plackett–Burman (PB) experimental design was used to screen the conditions responsible for the increase in lipase production in SSF. Eleven factors were evaluated at 2 levels using 12 assays and three central points, totaling 15 experiments. The PB design matrix was obtained using the Statistica 7.0 software program. All the experiments were carried out in duplicate.
The independent variables evaluated were cottonseed cake/wheat bran ratio, tilapia viscera oil, cultivation time, pH, inoculum, moisture, glucose, urea, yeast extract, KH2PO4, and MgSO4.7H2O. The real and coded values are shown in Table 1. Fermentations were carried out with 5 g of waste or a combination of wastes in 250 mL Erlenmeyer flasks. The pH and moisture of culture media were adjusted by the addition of citric acid- sodium phosphate buffers (pH 5, 6, and 7). All nutrient solutions were autoclaved separately. A salt solution containing CaCl2 (0.31 g/L), FeSO4.7H2O (0.006 g/L), MnSO4.7H2O (0.019 g/L), and ZnSO4.7H2O (0.037 g) was added to all assays.
Cultivation was performed at 30 ± 2 °C in an incubator with humidified air injection. The flasks were shaken daily with autoclaved glass rods in a laminar flow cabinet.
After cultivation, the enzyme was extracted by adding 10 mL tris–HCl buffer (0.05 M, pH 8) per gram of agro-industrial by-product. Then, the mixture was incubated at 30 ± 2 °C and 150 rpm for 30 min. Next, the sample was filtered and subjected to centrifugation at 2833 xg for 15 min. The enzymatic extract was filtered by 0.22 μm membrane and stored at -20 °C for the lipolytic activity analysis (variable response of PB design).
The most important variables determined by the PB experimental design were then selected for further evaluation by a 23 Central Composite Rotatable Design (CCRD). CCRD matrix is formed by 19 runs, which include 6 axial points and 5 replicates at the central point. The independent variables (cultivation time, cottonseed cake/ wheat bran ratio and moisture) were evaluated according to Table 2.
In 250 mL Erlenmeyer flasks, 5 g of the cottonseed cake/wheat bran combination were weighed according to the CCRD matrix. Yeast extract (3%, w/v), salt solution, and an inoculum of 2 × 107 spores/g of residue were added. The pH of the culture medium and the moisture were adjusted with citric acid-phosphate buffer (pH 7). Cultivation was carried out at 30 ± 2 °C for the time provided in the matrix. After the incubation time, the enzyme extract was obtained with tris-HCI buffer (0.05 M, pH 8) as described above. Then, the lipolytic activity (dependent variable) was measured to verify the effects of the analyzed factors.
Supplementation with surfactants and olive oil
The effect of supplements on SSF was also investigated on the production of lipases. The assay with the highest lipolytic activity found by CCRD (run 2) was reproduced and considered the control experiment. Assays were performed under the same conditions (combination of 30% CSC and 70% WB, 240 h and 50% moisture) and added in the fermentation medium oil olive (1.0%, 2.0%, 4.0%, 6.0%, 8.0% and 10.0% w/w), tween 80 (0.5% and 1.0%, w/w) and triton X-100 (0.5% and 1.0%, w/w). The lipolytic activities were subsequently analyzed and compared with a control experiment (without supplementation).
Precipitation with organic solvents
Lipase precipitation tests were conducted using acetone and ethanol based on the methodology of Tacin et al. (2019) with modifications. First, 20% solvent (w/w) was slowly added to the crude extract (triplicate). The mixture was maintained by magnetic stirring for 20 min at 4 °C and then centrifuged at 3237 xg for 20 min. The precipitate was resuspended with 0.05 M tris–HCl buffer (pH 8) and the final volume of the supernatant was recorded. Then, 40% solvent (w/w) was added to the supernatant, and the above procedures were repeated. Proportions of 60% (w/w) and 80% (w/w) organic solvents were also used, with four fractions of each solvent being obtained. The fraction precipitates and the crude extract were submitted to lipolytic activity and total protein determinations.
Stability of lipases
The fraction containing pre-purified lipases (40% acetone fraction) was subjected to pH and temperature stresses according to the modified methodology by Tacin et al. (2019). The following buffers were used: 0.1 M sodium citrate (pH 3); 0.2 M sodium acetate (pH 4–5); 0.2 M sodium phosphate (pH 6–8); and 0.2 M glycine–NaOH (pH 9–10). The fractions were subsequently incubated at different temperatures (30, 40, 50, and 60 °C) to evaluate temperature stability. Lipolytic activity was determined after 6 h of exposure and compared with the enzyme activity of the fraction without incubation (0.05 M tris–HCl buffer pH 8). The results were expressed as a percentage of relative lipolytic activity.
Determination of lipolytic activity
Lipase activity was determined by the hydrolysis of the chromogenic substrate p-nitrophenyl palmitate (pNPP) according to the method described by Silva et al. (2005). One unit (U) of lipase activity was defined as the amount of enzyme that releases one micromol of p-nitrophenol per minute under standard assay conditions. The lipase activity result was expressed as U/g of the substrate. Each enzymatic extract was analyzed eight times.
Determination of protein concentration
The protein concentrations of the samples were obtained by the method of Lowry et al. (1951) adapted for microplates. The wavelength used was 660 nm and Bovine Serum Albumin was used as a standard. All measurements were made in triplicate.
Statistical analysis
The results were expressed as mean ± standard deviation. The Statistica 7.0 software program (StatSoft Inc., Tulsa, OK, USA) was used for Analysis of Variance (ANOVA) with Tukey HSD post-hoc test, considering 95% confidence level (p < 0.05) as a significant result.
All the experiments of the Plackett–Burman experimental design and CCRD were performed randomly, and the data were treated with the aid of the Statistica 7.0 software program. The statistical significance of the second-order model equation was determined by the F-test (ANOVA).
Results and discussion
Physico-chemical characterization of by-products
The physicochemical characterization of CSC and WB are shown in Table 3. Moisture, ash, and fat results were similar to the CSC evaluated by Pereira et al. (2016). However, the protein concentration in the present study was higher. The high crude protein and lipid levels are important factors for selecting low-cost raw materials to produce lipases by SSF because the microorganism can use it as a source of carbon and nitrogen. Moreover, the lipids of CSC can act as inductors for lipase production (Geoffry and Achur 2018; Salihu et al. 2012). The WB composition was similar to that found in other studies, except for the crude fiber and total carbohydrate contents (Han et al. 2019; Zhao and Dong 2016). According to Mahadik et al. (2002), one of the reasons for the success of WB in enzymatic production by SSF is that it has sufficient nutrients for microbial development.
The WB water activity is close to ideal for fungi, which is around 0.5 and 0.6 (Thomas et al. 2013). However, the water activity of the CSC was lower. Passamani et al. (2014) found that the optimal growth of A. niger in a semisynthetic grape culture medium needed water activity higher than 0.95. Therefore, both agro-industrial by-products would need to have water added to promote the development of A. niger. The water absorption index (WAI) of the WB was higher than the CSC. WAI indicates the amount of water that can be absorbed by the matrix. Thus, higher WAI values are preferred for SSF because the moisture can be modified during cultivation (Azevedo et al. 2020).
Analysis of the physicochemical characteristics of the oil is important for investigating oil properties and oil quality. The quality analysis of the TVO through acidity index (11.93 ± 0.64 mg of KOH/100 g of oil) revealed that it has some degree of degradation because the result verified was higher than what is accepted by the Codex Alimentarius (maximum 3 mg KOH/100 g fish oil) (Codex Alimentarium Comission 2017). Enzymatic activity continues in the viscera and muscles after the fish’s death, resulting in hydrolysis of triacylglycerides, leading to the formation of free fatty acids. Therefore, it is inferred that the tilapia viscera were stored for long periods before extraction.
Peroxides are intermediate products of oxidation reactions. Thus, the peroxide index is regularly used to evaluate the primary oxidation of oils (Ribeiro et al. 2021). The peroxide index was 2.99 ± 0.29 mEq of reactive oxygen/Kg of oil. This result is in accordance with that recommended by Codex Alimentarium Comission (2017), which allows up to 5 mEq of reactive oxygen/Kg of oil.
The fatty acid profile of the TVO (Fig. 1) revealed the predominance of oleic acid (37.3%), palmitic acid (27.0%), and linoleic acid (12.7%). These constituents presented are the same as olive oil (Ferreira et al. 2019), corroborating the potential inductor of lipases of TVO.
Screening of SSF conditions by PB experimental design
Table 4 shows the PB matrix and the results of lipolytic activity of crude extract from SSF of A. niger IOC 4003.
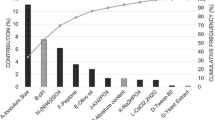
The Pareto chart (Fig. 2) shows the effects that the variables had on lipolytic activity. The factors which significantly influence the production of lipases (p < 0.05) were: cultivation time, cottonseed cake/wheat bran ratio, glucose, yeast extract, and pH. The positive sign of the effects showed that an increase in these variables (level -1 to level + 1) increased lipase production. Only the glucose concentration variable had a negative effect on lipolytic activity.
The highest results were obtained in fermentations with a pH equal to or greater than 7. Unlike previous studies showing the effect of supplementation with MgSO4.7H2O and KH2PO4 on the production of lipase (Falony et al. 2006; Pokorny et al. 1994), the results of this study showed that MgSO4.7H2O and KH2PO4 did not influence lipase production.
Yeast extract was a significant variable for nitrogen sources; however, urea had did not influence lipase production. Similar results were found in other studies (Cihangir and Sarikaya 2004; Contesini et al. 2009; Kamini et al. 1998). Fungus requires nitrogen for its maintenance and growth to synthesize amino acids, vitamins, nucleic acids, and chitin (Pastore et al. 2011). Because the by-products evaluated possess considerable crude protein levels (CSC: 47.88 ± 1.01 g/100 g; WB: 21.97 ± 0.28 g/100 g), it is possible to infer that the nitrogen supply in the studied conditions was from the yeast extract and the protein content of the by-products used.
Glucose was the only statistically significant factor in that supplementation reflected lower lipase activity. Other studies have also reported repression of lipase production by SSF using medium supplemented with carbohydrates (Cihangir and Sarikaya 2004; Salihu et al. 2016). In addition, the carbon source that strongly influences the production of lipases is particularly lipid (Geoffry and Achur 2018). Thus, the lipids present in the low-cost raw materials may have also been the carbon source used. TVO did not influence lipase production. A hypothesis for this is related to the high degree of oil deterioration verified by the acidity index. Further studies with other samples of TVO are recommended. On the other hand, it could be inferred that CSC contributed as an inductor of lipase production due to the considerable lipid content. However, the amount of lipids used cannot be excessive. Aliyah et al. (2016) reported that excessive levels can form a two-phase system to inhibit the transfer of oxygen and nutrient-absorbing fungi.
The three variables with the highest statistical significance on lipolytic activity (cultivation time, cotton cake/wheat bran ratio, and moisture) were selected and studied through a Central Composite Rotatable Design (CCRD) 23.
Central rotational composite design
The effects of the independent variables cultivation time, CSC/WB ratio, and moisture were evaluated on lipase production using a CCRD 23. According to the experimental conditions (Table 5), the lipolytic activity values reached between 0.001 U/g (run 14) and 0.091 U/g (run 2).
The Pareto chart (Fig. 3) showed that the quadratic effects of variables CSC/WB ratio and moisture were significant with a confidence level of 95%. Furthermore, the linear effect of the variable CSC/WB ratio and the interaction between the cultivation time and moisture were also statistically significant (p < 0.05) on enzyme activity, with a negative effect.
The ana
The analysis of variance (ANOVA) for the CCRD was performed. Based on the F-test results (Supplementary file 1), ANOVA do not show statistical significance for the regression model at 90% of confidence level (p < 0.1), since the calculated F-value (2.12) was lower than the listed F (2.44). Therefore, it was not possible to obtain a statistically significant mathematical model. This can be attributed to the difficulty in reproducibility in experiments using SSF and agro-industrial by-products as well as to the complexity of factors related to the lipolytic activity. Thus, in the next steps in this study, run 2 was selected, which showed the highest lipolytic activity value (0.091 U/g). The experimental conditions for this run were a combination of 30% CSC and 70% WB, 240 h of cultivation, and 50% moisture.
Some studies have also shown higher lipase production by Aspergillus sp. by combining agro-industrial by-products: WB and sugarcane bagasse, WB and soybean (Fleuri et al. 2014), WB and gingelly oil cake (Mala et al. 2007), and WB and cacay butter (Azevedo et al. 2020). Since some nutrients may be available at less than optimal concentrations or absent in the low-cost raw materials, exogenous supplementation or a combination of low-cost raw materials is needed (Costa et al. 2017; Passamani et al. 2014; Zhao and Dong 2016). Although WB has interesting properties for lipase production, the combined use with by-products of oil extraction from seeds provides additional properties. Cottonseed cake oil is constituted by linoleic (56.9%), oleic (16.9%), and palmitic acids (23.3%), which are also the main compounds of olive oil (Ferreira et al. 2019). This lipid profile similar to olive oil has the potential to induce lipase production.
Moisture influenced lipolytic activity at extreme values. Low moisture values imply low fungal development due to reduced nutrient diffusion and less swelling of the low-cost raw material. On the other hand, moisture greater than 80% can decrease fungal growth because reducing the porosity of the low-cost raw material and increase the viscosity of the medium (Baysal et al. 2003; Mahadik et al. 2002).
In a similar study, the best combination for lipase production of Aspergillus sp. was 7.5 g of wheat bran and 2.5 g of sugarcane bagasse with 40% moisture. The production was optimized by SSF using lipases in wheat bran varying the initial water content of 20 to 84%. The best result was observed with 20% moisture (Contesini et al. 2009). Nema et al. (2019) achieved the highest lipolytic activity for A. niger in SSF with 75% moisture using a mixture of rice husk, cottonseed cake, and red grass husk (2:1:1). Azevedo et al. (2020) evaluated the water absorption index of different low-cost raw materials before cultivating A. terreus NRRL-255 in SSF for producing lipases and found that some of them absorbed more water than others. Therefore, moisture adopted in the fermentation process is related and should consider the characteristics of the selected low-cost raw material.
Considering that the effects of this variable are very close to the limit of the 95% confidence level and that by adopting a 90% confidence level (data not shown) the variable would have significance, it is possible to infer that the cultivation time has some positive influence on lipase production. This fact confirms the test of the highest lipolytic activity (run 2), in which the cultivation time was 240 h (level + 1). Other lipase production studies by A. niger in SSF had higher lipase activity with 14 days of cultivation using waste wineries and oil industries (Salgado et al. 2014) and after 21 days of culture using olive pomace and wheat bran as substrates (Oliveira et al. 2016).
In contrast, Contesini et al. (2009) evaluated enantioselective lipases produced by A. niger AC-54 in SSF for 72, 96, and 120 h. Maximum activity was achieved after 96 h for all assays and then decreased activity. In turn, Fleuri et al. (2014) evaluated the lipolytic activity in SSF using Aspergillus sp. and the agro-industrial by-products wheat bran, soybean meal, and sugarcane bagasse combined with soybean meal and observed maximum activity (11.25 U/mL) at 96 h of cultivation.
Based on the obtained results, the condition with the highest lipolytic activity (run 2) was selected to continue studies.
Supplementation with surfactants and olive oil
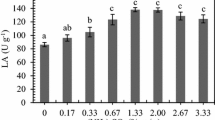
The best cultivation conditions (extract obtained from run 2 of the CCRD) were reproduced with the addition of potential inductors in different concentrations to increase lipase production. However, the results (Fig. 4) showed that none of the supplements significantly increased lipase production.
Olive oil has been used in many studies with good results, which is attributed to its high constitution of unsaturated fatty acids, such as oleic acid (Wang et al. 2008). However, Edwinoliver et al. (2010) found that supplementation of 5% olive oil showed no effect on lipase production, thus choosing not to supplement the medium, reducing costs and risks of contamination. Sharma et al. (2001) also reported that although lipid carbon sources seem essential for a high lipase yield, some authors have produced good yields in the absence of fats and oils.
In turn, surfactants can increase cell permeability as they are amphiphilic molecules, facilitating the excretion of molecules and also facilitating contact between the enzyme and the substrate (Geoffry and Achur 2018; Kumar and Ray 2014). Similar to the present work, Kumari and Rani (2012) also showed that there was no increase in the lipolytic activity of the fungus Trichosporon asahii with the supplementation of tween 80.
In summary, the lipolytic activity of the tested extract did not increase with the presence of olive oil, tween 80, or triton X-100 at different concentrations. One hypothesis may have been the biocidal action of surfactants on some microorganisms in which the concentration may be enough to reduce cell viability and therefore do not increase the lipase activity (Mulligan et al. 2014).
This experiment shows that even supplementation with substances already well described in the literature does not always indicate an increase in enzymatic activity.
Precipitation with organic solvents
The results of precipitation carried out with organic solvents are shown in Table 6. The purification factors with both solvents were higher in the first steps (20% acetone, 40% acetone, and 20% ethanol). The highest yields were obtained in the 40% acetone and 20% ethanol fractions.
Tacin et al. (2019) precipitated lipases from Aspergillus sp. using the same solvents in concentrations above 50%. The purification factor using ethanol was 10.72 ± 0.29 after three cycles of precipitation. The purification factor with acetone reached 16.76 ± 0.43 and an enzymatic activity of 70.50 U/mL employed four cycles of precipitation (Tacin et al. 2019). Two hypotheses are raised that there was a loss of enzymatic activity in the current study due to the accelerated dripping of solvents and the precipitation was not as significant due to the shorter contact time between the solvents and the extract.
Precipitation is a common and low-cost method to concentrate macromolecules. However, the use of organic solvents to precipitate enzymes can cause denaturation in some cases, and consequent loss of activity (Soares et al. 2012).
The precipitation in this study was an effective choice for the concentration of A. niger lipases because it was able to increase the specific activity by 16 times, while the increase in other studies using precipitation was 4.29 times (Mhetras et al. 2009) and 2.01 times (Sethi et al. 2016). Moreover, this process is aligned with the Green Chemistry since the organic solvents can be recovered by distillation, reducing environmental impacts.
Stability of lipases
The stability of pre-purified lipases of the 40% acetone fraction was evaluated after 6 h of exposure to pHs in the range 3 to 10, and the results are shown in Fig. 5. It was observed that the lipases were less stable at pH 3, showing the residual lipolytic activity of 74.62%. It was also observed that increasing stability was associated with the increase in pH, with the best results obtained at pH values of 8 (88.61%), 9 (97.07%), and 10 (108.35%).
A similar profile was verified by Mhetras et al. (2009), in which the lipase produced by A. niger had greater stability at alkaline pHs (pH 8–11) retaining 100% of its original activity after incubation for 24 h. Colla et al. (2015) also found greater stability after 24 h at a pH greater than 7 with residual activities greater than 60% and approximately 50% with a pH between 4 and 6. In turn, Tacin et al. (2019) evaluated the lipase from A. niger stability after 6 h of exposure. Maximum stability was 90% activity at pHs 7 and 7.5, while less than 50% of the enzymatic activity was observed for pHs 8, 9, and 10.
The results of the thermal stability of pre-purified lipases of the 40% acetone fraction after 6 h of exposure are shown in Fig. 6. It can be seen that the enzymatic activity decreases with increasing temperature, but the most important loss of activity was 15.10%, which only occurred at 60 °C.
Tacin et al. (2019) showed similar results, in which lipolytic activities were maintained at around 100% after 6 h of exposure to temperatures of 30, 40, and 50 °C, and enzymes subjected to 60 °C lost 30% of activity. Silveira et al. (2016) had lower results in observing residual activity between 80 and 90% under 40, 45, and 50 °C and residual activity of 70% under 60 °C.
Thus, the lipases obtained in this study showed interesting results regarding the term stability and pH tolerance, considering that the enzymatic activity was greater than 70% in 6 h at the pHs 3 to 10, and not less than 80% between 30 to 60 °C.
Knowledge of the pHs at which an enzyme is stable is an essential aspect for choosing the most suitable buffer solutions for the enzymatic process. In addition, it is necessary to know a temperature which enables supplying a reasonably high reaction rate in parallel to a reasonably low denaturation rate (Glogauer et al. 2011).
The stability characteristics found are of considerable interest in industrial applications because production processes commonly require maintenance of their component properties despite the use of high temperatures and different pHs. For example, thermostable lipases can be employed in biodiesel synthesis, biopolymers, pharmaceuticals, agrochemicals, and cosmetics (Colla et al. 2015).
Conclusions
It was possible to obtain lipases from Aspergillus niger IOC 4003 in SSF by experimental design using a combination of low-cost raw materials of cottonseed cake and wheat bran. Under the study conditions, supplementation with tilapia viscera oil, olive oil, tween 80, and triton X-100 did not affect the increase in lipolytic activity. Thus, they were not inductors of lipase production.
Concentrated lipase fractions were obtained by precipitation, which is an inexpensive method and aligned with Green Chemistry. The specific activity of lipase was increased up to 16.00 and 4.91 times with acetone and ethanol, respectively. Pre-purified lipases showed excellent stability at different pHs and temperatures.
Thus, the results offer a more economically viable and sustainable source for producing and concentrating thermostable and pH-tolerant lipases applicable in various industrial processes.
Availability of data and material
Not applicable.
References
Adjonu R, Doran G, Torley P, Agboola S (2013) Screening of whey protein isolate hydrolysates for their dual functionality: influence of heat pre-treatment and enzyme specificity. Food Chem 136:1435–1443
Alcântara MA, Lima AEA, Braga ALM, Tonon RV, Galdeano MC, Mattos MC, Brígida AIS, Rosenhaim R, Santos NA, Cordeiro AMTM (2019) Influence of the emulsion homogenization method on the stability of chia oil microencapsulated by spray drying. Powder Technol 354:877–885
Aliyah AN, Edelweiiss ED, Sahlan M, Wijanarko A (2016) Solid state fermentation using agroindustrial wastes to produce Aspergillus niger lipase as a biocatalyst immobilized by an adsorption-crosslinking method for biodiesel synthesis. Int J Technol 8:1393–1404
Association of Official Analytical Chemistry - AOAC (2003) Official methods and recommended practices of the American oil chemists’ society. AOCS Press, Champaign
Azevedo WM, Oliveira LFR, Alcântara MA, Cordeiro AMTM, Damasceno KSFSC, Assis CF, Sousa Junior FC (2020) Turning cacay butter and wheat bran into substrate for lipase production by Aspergillus terreus NRRL-255. Prep Biochem Biotechnol 50(7):689–696
Baysal Z, Uyar F, Aytekin Ç (2003) Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem 38(12):1665–1668
Cihangir N, Sarikaya E (2004) Investigation of lipase production by a new isolate of Aspergillus sp. World J Microbiol Biotechnol 20(2):193–197
Codex Alimentarium Comission (2017) Standard for fish oils, Rome. http://www.fao.org/fao-who-codexalimentarius. Accessed 10 Dec 2020
Colla LM, Ficanha AMM, Rizzardi J, Bertolin TE, Reinehr CO, Costa JAV (2015) Production and characterization of lipases by two new isolates of Aspergillus through solid-state and submerged fermentation. Biomed Res Int 2015:725959
Contesini FJ, da Silva VCF, Maciel RF, de Lima RJ, Barros FFC, de Carvalho PO (2009) Response surface analysis for the production of an enantioselective lipase from Aspergillus niger by solid-state fermentation. J Microbiol 47(5):563–571
Costa TM, Hermann KL, Garcia-Roman M, De Valle RCSC, Tavares LBB (2017) Lipase production by Aspergillus niger grown in different agro-industrial wastes by solid-state fermentation. Braz J Chem Eng 34(2):419–427
Dayanandan A, Rani SHV, Shanmugavel M, Gnanamani A, Rajakumar GS (2013) Enhanced production of Aspergillus tamarii lipase for recovery of fat from tannery fleshings. Braz J Microbiol 44(4):1089–1095
Edwinoliver NG, Thirunavukarasu K, Naidu RB, Gowthaman MK, Kambe TN, Kamini NR (2010) Scale up of a novel tri-substrate fermentation for enhanced production of Aspergillus niger lipase for tallow hydrolysis. Bioresour Technol 101(17):6791–6796
Falony G, Armas JC, Mendoza JCD, Hernández JLM (2006) Production of extracellular lipase from Aspergillus niger by solid-state fermentation. Food Technol Biotechnol 44(2):235–240
Ferreira MM, de Oliveira GF, Basso RC, Mendes AA, Hirata DB (2019) Optimization of free fatty acid production by enzymatic hydrolysis of vegetable oils using a non-commercial lipase from Geotrichum candidum. Bioprocess Biosyst Eng 42(10):1647–1659
Fleuri LF, de Oliveira MC, Arcuri MLC, Capoville BL, Pereira MS, Delgado CHO, Novelli PK (2014) Production of fungal lipases using wheat bran and soybean bran and incorporation of sugarcane bagasse as a co-substrate in solid-state fermentation. Food Sci Biotechnol 23(4):1199–1205
Geoffry K, Achur RN (2018) Screening and production of lipase from fungal organisms. Biocatal Agric Biotechnol 14:241–253
Global markets for enzymes in industrial applications (2021) BCC Research LLC, Wellesley. https://www.bccresearch.com. Accessed 07 Jan 2021
Glogauer A, Martini VP, Faoro H, Couto GH, Müller-Santos M, Monteiro RA, Mitchell DA, de Souza EM, Pedrosa FO, Krieger N (2011) Identification and characterization of a new true lipase isolated through metagenomic approach. Microb Cell Fact 10:54
Guilherme AA, Pinto AS, Rodrigues S (2008) Optimization of trace metals concentration on citric acid production by Aspergillus niger NRRL 2001. Food Bioproc Technol 1(3):246–253
Han W, Ma S, Li L, Zheng X, Wang X (2019) Impact of wheat bran dietary fiber on gluten and gluten-starch microstructure formation in dough. Food Hydrocoll 95:292–297
Hartman L, Lago RCA (1973) Rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22(6):475–476
Kamini NR, Mala JGS, Puvanakrishnan R (1998) Lipase production from Aspergillus niger by solid-state fermentation using gingelly oil cake. Process Biochem 33(5):505–511
Khan NR, Rathod VK (2020) Biocatalysis and agricultural biotechnology microwave mediated lipase-catalyzed synthesis of n -butyl palmitate and thermodynamic studies. Biocatal Agric Biotechnol 29:101741
Kumar DS, Ray S (2014) Fungal lipase production by solid state fermentation-an overview. J Anal BioanalTech 6(1):1000230
Kumari A, Rani G (2012) Purification and biochemical characterization of a novel magnesium dependent lipase from Trichosporon asahii MSR 54 and its application in biodiesel production. Asian J Biotechnol 4(2):70–82
López E, Deive FJ, Longo MA, Sanromán MA (2010) Strategies for utilisation of food-processing wastes to produce lipases in solid-state cultures of Rhizopus oryzae. Bioprocess Biosyst Eng 33(8):929–935
López DN, Galante M, Ruggieri G, Piaruchi J, Dib ME, Duran NM, Lombardi J, Sanctis M, Boeris V, Risso PH, Spelzini D (2018) Peptidase from Aspergillus niger NRRL 3: optimization of its production by solid-state fermentation, purification and characterization. LWT 98:85–491
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mahadik ND, Puntambekar US, Bastawde KB, Khire JM, Gokhale DV (2002) Production of acidic lipase by Aspergillus niger in solid state fermentation. Process Biochem 38(5):715–721
Mala JGS, Edwinoliver NG, Kamini NR, Puvanakrishnan R (2007) Mixed substrate solid state fermentation for production and extraction of lipase from Aspergillus niger MTCC 2594. J Gen Appl Microbiol 53(4):247–253
McCartney D, Tingley J (1998) Development of a rapid moisture content method for compost materials. Compost Sci Util 6(3):14–25
Mhetras NC, Bastawde KB, Gokhale DV (2009) Purification and characterization of acidic lipase from Aspergillus niger NCIM 1207. Bioresour Technol 100(3):1486–1490
Mulligan CN, Sharma SK, Ackmez M (2014) Biosurfactants research trends and applications, 1st edn. CRC Press, Boca Raton
Nema A, Patnala SH, Mandari V, Kota S, Devarai SK (2019) Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull Natl Res Cent 43:82
Oliveira F, Moreira C, Salgado JM, Abrunhosa L, Venâncio A, Belo I (2016) Olive pomace valorization by Aspergillus species: lipase production using solid-state fermentation. J Sci Food Agric 96(10):3583–3589
Passamani FRF, Hernandes T, Lopes NA, Bastos SC, Santiago WD, Cardosó MDG, Batista LR (2014) Effect of temperature, water activity, and pH on growth and production of ochratoxin a by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J Food Prot 77(11):1947–1952
Pastore NS, Hasan SM, Zempulski DA (2011) Produção de ácido cítrico por Aspergillus niger: avaliação de diferentes fontes de nitrogênio e de concentração de sacarose. Engevista 13(3):149–159
Pereira L, Bezerra LS, Barbosa AM, Carvalho GGP, Simionato JI, Freitas JE, Araújo MLGML, Silva RR, Lacerda ECQ, Carvalho BMA (2016) Nutritional characteristics of lambs meat fedd diets with cotton cake. J Food Qual 39:140–149
Pokorny D, Friedrich J, Cimerman A (1994) Effect of nutritional factors on lipase biosynthesis by Aspergillus niger. Biotechnol Lett 16(4):363–366
Preczeski KP, Kamanski AB, Scapini T, Camargo AF, Modkoski TA, Rossetto V, Venturin B, Mulinari J, Golunski SM, Mossi AJ, Treichel H (2018) Efficient and low-cost alternative of lipase concentration aiming at the application in the treatment of waste cooking oils. Bioprocess Biosyst Eng 41:851–857
Ribeiro PPC, Damasceno KSFDSC, de Veras BO, de Oliveira JRS, Lima VLM, de Assis CRD, da Silva MV, de Sousa Júnior FC, de Assis CF, Padilha CEA, Stamford TCM (2021) Chemical and biological activities of faveleira (Cnidoscolus quercifolius Pohl) seed oil for potential health applications. Food Chem 337:127771
Salgado JM, Abrunhosa L, Venâncio A, Domínguez JM, Belo I (2014) Integrated use of residues from olive mill and winery for lipase production by solid state fermentation with Aspergillus sp. Appl Biochem Biotechnol 172(4):1832–1845
Salihu A, Alam MZ, AbdulKarim MI, Salleh HM (2012) Lipase production: an insight in the utilization of renewable agricultural residues. Resour Conserv Recy 58:36–44
Salihu A, Bala M, Alam MZ (2016) Lipase production by Aspergillus niger using sheanut cake: an optimization study. J Taibah Univ Sci 10(6):850–859
Sancho SDO, Raquel A, Nilson A, Dantas DS, Magalhães TA, Lopes GS, Rodrigues S, Maria J, André F, Fernandes N, Goretti M, Silva DV (2015) Characterization of the industrial residues of seven fruits and prospection of their potential application as food supplements. J Chem 2015:264284
Selvakumar P, Sivashanmugam P (2017) Optimization of lipase production from organic solid waste by anaerobic digestion and its application in biodiesel production. Fuel Process Technol 165:1–8
Sethi BK, Nanda PK, Sahoo S (2016) Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus terreus NCFT 4269.10. Braz J Microbiol 47(1):143–149
Sharma R, Chisti Y, Chand U (2001) Production, purification, characterization, and applications of lipases. Biotechnol Adv 19:627–662
Silva WOB, Mitidieri S, Schrank A, Vainstein MH (2005) Production and extraction of an extracelular lipase from the entomopathogenic fungus Metarhizium anisopliae. Process Biochem 40:321–326
Silveira EA, Tardioli PW, Farinas CS (2016) Valorization of palm oil industrial waste as feedstock for lipase production. Appl Biochem Biotechnol 179(4):558–571
Soares PAG, Vaz AFM, Correia MTS, Pessoa A, Cunha MGC (2012) Purification of bromelain from pineapple wastes by ethanol precipitation. Sep Purif Technol 98:389–395
Sousa Junior FC, Padilha CEA, Chibério AS, Ribeiro VT, Martins DRA, Oliveira JA, Macedo GR, Santos ES (2016) Modeling and simulation of breakthrough curves of recombinant 503 antigen using immobilized metal affinity expanded bed adsorption chromatography. Sep Purif Technol 164:34–40
Tacin MV, Massi FP, Fungaro MHP, Teixeira MFS, de Paula AV, Santos-Ebinuma VC (2019) Biotechnological valorization of oils from agro-industrial wastes to produce lipase using Aspergillus sp. from Amazon. Biocatal Agric Biotechnol 17:369–378
Thakur S (2012) Lipases, its sources, properties and applications: a review. Int J Sci Eng Res 3(7):1–29
Thirunavukarasu K, Purushothaman S, Sridevi J, Aarthy M, Gowthaman MK, Nakajima-Kambe T, Kamini NR (2016) Degradation of poly(butylene succinate) and poly(butylene succinate-co-butylene adipate) by a lipase from yeast Cryptococcus sp. grown on agro-industrial residues. Int Biodeterior Biodegrad 110:99–107
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 81:146–161
Torres R, Ortiz C, Pessela BCC, Palomo JM, Mateo C, Guisán JM, Fernández-Lafuente R (2006) Improvement of the enantioselectivity of lipase (fraction B) from Candida antarctica via adsorpiton on polyethylenimine-agarose under different experimental conditions. Enzyme Microb Technol 39(2):167–171
Trentini MMS, Toniazzo G, Zeni J, Pili J, Di Luccio M, Valduga E (2015) Purification of pectinases from Aspergillus niger ATCC 9642 by ethanol precipitation. Biocatal Agric Biotechnol 4(3):315–320
Wang D, Xu Y, Shan T (2008) Effects of oils and oil-related substrates on the synthetic activity of membrane-bound lipase from Rhizopus chinensis and optimization of the lipase fermentation media. Biochem Eng J 41(1):30–37
Yazid NA, Barrena R, Komilis D, Sánchez A (2017) Solid-state fermentation as a novel paradigm for organic waste valorization: a review. Sustainability 9(2):1–28
Zhao X, Dong C (2016) Extracting xylooligosaccharides in wheat bran by screening and cellulase assisted enzymatic hydrolysis. Int J Biol Macromol 92:748–752
Acknowledgements
The authors thank the Laboratory of Fuels and Materials of the Federal University of Paraíba for collaboration in the chromatographic analysis of tilapia viscera oil. The authors also acknowledge Ms. Jessica dos Santos by the determination of crude protein of the low-cost raw materials.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Medeiros, W.R.D.B., de Paiva, W.K.V., Diniz, D.S. et al. Low-cost approaches to producing and concentrating stable lipases and the evaluation of inductors. Braz. J. Chem. Eng. 40, 81–92 (2023). https://doi.org/10.1007/s43153-022-00223-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-022-00223-9