Abstract
Membrane-based technology is a low-cost alternative for gas purification processes due to its easy implementation and low energy consumption. However, the participation of membrane systems in the CO2 separation technologies market for natural gas sweetening is only 10% due to the trade-off between permeability and selectivity and poor stability of the polymeric materials available. Therefore, to expand current operating conditions or meet adverse requirement in new applications, such as low pressure and high flue gas temperatures, new membrane materials need to be developed. Mixed matrix membranes (MMMs) formed by the incorporation of inorganic fillers in polymeric matrices can overcome the high cost of inorganic membranes and reduce polymeric membrane limitations. The main challenge is the development of MMMs with a defect-free interfacial morphology. In this sense, the type of load is one of the most important factors. Among the inorganic fillers, carbon nanotubes (CNTs) have great potential for chemical adaptation, as well as high mechanical and thermal properties, which can result in high performance MMMs. This review was prepared to summarize the advances achieved with polymeric matrices incorporated with CNTs (CNTs-MMMs) and contribute to their development by showing the possibilities of combining CNTs with different polymers and their respective properties. It has been observed that CNTs can increase the mechanical, thermal and transport properties of polymeric membranes. Furthermore, Robeson's upper bound revealed that some CNTs-MMMs were suitable for industrial use because of their excellent performances and with greatest application potential for CO2/N2 separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climatic warming caused by greenhouse gas emissions, with a greater contribution of carbon dioxide (CO2), can raise the Earth's temperature during this century by 1.0–3.7 °C (Anderson et al. 2016). Higher temperatures imply a greater occurrence of extreme weather events, such as storms, floods and sea level rise (Sreenivasulu et al. 2015). The use of fossil fuels as a primary energy source generates the greatest contribution to CO2 emissions (Friedlingstein et al. 2019). However, oil, coal and natural gas are primarily responsible for supplying energy to the world (British Petroleum 2020). Through approaches such as carbon capture and storage (CCS) and carbon capture and utilization (CCU), CO2 can be destined for advanced oil/gas recovery, stored in geological structures or be chemically or biologically transformed into high added value products and, consequently, reduce its emission to the atmosphere (Ramírez-Santos et al. 2018). Therefore, separating CO2 from other gases has become one of the most important research areas of this century.
Natural gas is considered to be the most appropriate fossil fuel to make the low-carbon energy transition. The CO2 emissions from natural gas are about 26% less than oil and coal, although producing the same amount of energy (Zhang et al. 2019). The composition of raw natural gas includes methane (CH4), water vapor, CO2, hydrogen sulfide (H2S), nitrogen (N2) and heavier gaseous hydrocarbons (Dai et al. 2016). However, high CO2 content is present in 40% of the world's natural gas reserves identified with potential for production. Among these acid reserves, almost 27% contain more than 10% CO2 (Hasegawa et al. 2017). CO2 decreases the calorific power of natural gas and forms acid solutions with water that cause corrosion in pipelines and equipment (Alcheikhhamdon and Hoorfar 2017). To avoid these problems, the maximum limit of CO2 content recommended for the transport of natural gas through pipelines can vary between 2 and 4% according to the specifications of each country (Grande et al. 2017). Thus, at the stage known as sweetening, CH4 needs to be separated from CO2. Natural gas and other fossil fuels can be used in industry to heat the ovens or generate energy. After combustion, the composition of flue gases is 78–80% of N2 and 10–12% of CO2 (Dai et al. 2016). Among the largest stationary sources of CO2, the heat and power generation, iron and steel production, petrochemical processes and cement production can be highlighted (Cormos et al. 2020). Power plants contribute more than a third of the world’s CO2 emissions (Mukhtar et al. 2020).
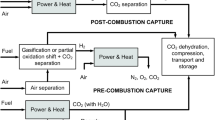
Through steam methane reforming (SMR), natural gas is also responsible for about half of the world’s production of hydrogen (H2) on industrial scale (Taghizadeh and Aghili 2019). SMR basically takes place in two stages: (i) CH4 reaction with water to generate syngas (carbon monoxide (CO) and H2) and; (ii) CO reaction with water generating H2 and CO2, to increase H2 production yield (Alqaheem et al. 2017). An effective CO2/H2 separation is required to meet the various purity requirements for different applications of H2 (Dai et al. 2016). The separation of gases using polymeric membranes is mainly affected by the kinetic diameter and solubility of gas molecules. Thus, the separation of CO2/CH4 and CO2/N2 is possible to be performed because CH4 and N2 are larger and less condensable than CO2. On the other hand, polymeric membranes do not have acceptable selectivity for CO2/H2 separation, because H2 has a smaller kinetic diameter, while CO2 has greater condensability (Harami et al. 2019a). This way, despite the importance of CO2/H2 separation, the focus of this work will be on separating CO2/N2 and CO2/CH4. For hydrogen separation dense metal membranes, porous membranes, and proton-conducting membranes generally are used (Gallucci et al. 2013, 2017; Taghizadeh and Aghili 2019). These membranes have the parameters (perm-selectivity, flux and temperature range) required for the SMR in membrane reactors, which is carried out at temperatures around 600 °C, far above the limit of polymeric membranes (< 100 °C) (Gallucci et al. 2013). Figure 1 highlights the gases of interest in this study and the strategies for a closed carbon loop cycle during the production and use of natural gas.
Conventional methods used for CO2 separation include absorption, adsorption and cryogenic distillation (Oko et al. 2017). Chemical absorption is the main technology used in the market for CO2 separation from gaseous mixtures (Sohaib et al. 2021). Amines are widely used absorbents due to their high reactivity with CO2, which results in small hydrocarbon losses and a high degree of CO2 removal (Yeo et al. 2012). However, absorption by amines is extremely energy intensive, particularly for natural gas with high CO2 loadings (Safari et al. 2009; Rezakazemi et al. 2017). Solvent loss and equipment corrosion must also be considered, since they can aggravate environmental degradation and increased costs (Murai et al. 2013; Borhani et al. 2015; Swati et al. 2021).
In general, conventional methods use large equipment, with complexity of operation and high energy consumption (Alqaheem et al. 2017; Zhang et al. 2019). On the other hand, membranes can separate CO2 from other gases without changing the phase and without using dangerous chemicals. This makes membranes a simpler operating technology, favorable to the environment and with less energy consumption (Zhou et al. 2017). In addition, it becomes even more efficient and economical as the CO2 concentration in the feed increases (Baker et al. 2018). Membranes can be installed in modules that have no moving parts and do not require utilities such as water and instrument air (Mokhatab et al. 2019). These are important advantages for the gas separation process in remote places and, therefore, it is a preferred solution in offshore applications (Alcheikhhamdon and Hoorfar 2017). For example, the easily accessible oil and gas reservoirs are running out (Dalane et al. 2018). A new perspective for the production of gas and oil points to subsea treatment processes, which can maximize the oil and gas recovery, to increase the production from existing fields; to reduce capital expenditure and operating costs for new installations; to explore harsh environments (deep waters or arctic regions) and develop new fields that have been left undeveloped due to technical and/or economical limitations (Dalane et al. 2017). However, some challenges need to be overcome, including subsea design and installation limitations, operation at high pressure and limited accessibility for the manual control, operation and maintenance of the process (Dalane et al. 2017). Among CO2 separation technologies, membranes can meet the largest number of requirements, with emphasis on the size and weight (the lifting crane has a weight limitation), low maintenance and high modularity (Dalane et al. 2019).
Another important advantage of membrane technology is the flexibility with which it can be integrated with other separation technologies in the form of hybrid systems. The technical and economic limitations that apply to at least one of the units can be overcome by integrating processes (Rezakazemi et al. 2017). The membrane separation unit can be used, for example, in a first step to separate CO2 from other gases, while final purification can be performed via an absorption unit (Rezakazemi et al. 2017) or adsorption unit (Ohs et al. 2019). Another important hybrid system is the membrane contactor, with integration between membrane and absorption process being performed directly in the membrane module. In this system, the membrane plays an interface role between the liquid and gaseous phase to increase the mass transfer area between the gas and the absorber (Sohaib et al. 2021). The main advantages are a fast mass transfer rate, high operational flexibility, no liquid ubiquity and mist entrainment, simple equipment, low energy consumption, low operating costs and no secondary pollution (Zhang et al. 2020a).
The membranes can be prepared with polymeric or inorganic materials. Despite having good thermal, mechanical and chemical stability, the fragility and high preparation and operation cost of inorganic membranes hamper their industrial application (Vinoba et al. 2017). Thus, polymers dominate the market for membrane preparation due to manufacturing simplicity, low cost and easy operation (Sharif 2018). However, polymeric membranes need to overcome some challenges that limit their industrial viability. In addition to the well-known trade-off between productivity (permeability) and efficiency (selectivity), the thermal and mechanical properties also prevent the operational conditions to which these membranes can be subjected from being broader.
Generally, elastomeric polymers have high permeation and low selectivity. On the other hand, glassy polymers have high selectivity, but low permeation. As it is difficult to combine in a single polymer high permeability and high selectivity, membranes are not economically viable for treating large gas volumes. For the treatment of natural gas, it is recommended that feed flow rates be between 5 to 27 MMSCFD (million standard cubic feet per day) over the entire CO2 concentration range (Baker and Lokhandwala 2008; Rezakazemi et al. 2017). The membrane technology does not have a wider application range, as the loss of methane in feed with CO2 permeation is current by ± 4%, much higher than compared to the amine system, which is usually 1% (Galizia et al. 2017). More selective polymers can reduce hydrocarbon losses, although generally at a higher cost due to the need to increase the membrane area (Mokhatab et al. 2019). In some cases, the high surface-to-volume ratio offered by hollow fiber membranes overcomes this disadvantage (Younas et al. 2020b).
Due to their better thermal and mechanical stability, most of the polymers used for gas separation are glassy. However, they operate in a state of thermodynamic non-equilibrium and inherently undergo a spontaneous evolution towards balance, known as physical aging (Lock et al. 2018). This phenomenon causes a densification in the membrane that results in a gas permeation drop over time (Xia et al. 2012).
Current commercial membrane operations are limited to temperatures between 30 and 50 °C (Galizia et al. 2017). Potential applications of membrane technology for CO2 capture can involve extremely high temperatures (Younas et al. 2020a). For example, the pre-combustion process would benefit from a membrane system that could treat syngas at temperatures close to 300 °C (Rowe et al. 2010). In post-combustion, under typical operating conditions, the flue gas temperature can reach 80 °C (Zhao et al. 2008). Even with the development of polymers which exhibit enhanced thermal stability, the inability of polymeric membranes to withstand high temperatures with acceptable separation properties and performance characteristics still persists as one of the main challenges for membrane-based technology (Rezakazemi et al. 2018).
The stress of a membrane under pressure increases with ℓ−2/3, where ℓ is the thickness of the membrane (Cohen-Tanugi and Grossman 2014). Commercial membranes are necessarily prepared with a thin selective layer to obtain high gas productivity. Therefore, they are more susceptible to stress-related disruptions. This makes it difficult to apply these membranes under higher pressures, as in the processing of natural gas. In addition, the continuous reduction in membrane performance caused by compaction under high pressure and high temperatures conditions is a challenge for polymeric membranes (Zhao et al. 2014b).
The dissolution of CO2 in the polymer can alter the segmental mobility of the polymer chains through the phenomenon known as plasticization (Sanders et al. 2013). This causes an increase in the diffusion coefficient of all penetrants, which results in a drastic drop in membrane selectivity. In the processing of natural gas, plasticization affects the reliability of the membrane separation process, because as plasticization progresses, the loss of methane increases (Adewole et al. 2013).
Therefore, although natural gas sweetening is already an established membrane application, the limitations discussed above prevent their greater participation in the gas separation market, which currently corresponds to only about 10% (Wang et al. 2020). On the other hand, membranes for flue gas treatment are not an economical option because their streams are typically at atmospheric pressure. This fact, associated with the low concentration of CO2, results in the low partial pressure of CO2 and requires compression or vacuum pumping to generate driving force for the gas separation by membranes, making the process more expensive (Khalilpour et al. 2015). In addition, the flow rate and temperature of flue gas are higher than commonly found in natural gas processing. Therefore, the development of CO2 capture technologies at the industrial level is a major step towards a sustainable environment (Younas et al. 2020a). Table 1 shows the typical operating conditions found in natural gas sweetening and flue gas treatment.
Therefore, to increase the participation of membranes among CO2 separation technologies from natural gas, it will be necessary to develop materials with better mechanical, thermal and transport properties, with a superior balance between selectivity and permeability. In addition, these materials could also expand the application of membranes for subsea treatment and CO2 capture.
The incorporation of inorganic fillers in the polymer is an attractive strategy to improve the properties of polymeric membranes, resulting in a class of membranes called mixed matrix membranes (MMMs). MMMs can combine in a single material the properties of inorganic materials, such as high selectivity and high thermal and chemical stability, with the low cost and easy processability of polymers (Wang et al. 2016). Loads such as zeolites, carbon nanotubes (CNTs), carbon molecular sieves (CMS), graphene, metal organic framework structures (MOFs), zeolitic imidazolate frameworks (ZIFs) and materials Institute Lavoisier (MIL) have been combined with various polymeric materials, showing promising results for CO2 separation (Vinoba et al. 2017). Among these materials, CNTs can also improve the mechanical (Dai and Sun 2016) and thermal (Cangialosi et al. 2013) properties of composite materials, which indicates the great potential of CNTs for MMMs preparation with high performance. Thus, this article discusses the thermal, mechanical and transport properties of MMMs preparated with CNTs (CNTs-MMMs). In addition, it performs a comprehensive analysis of the separation performance of CO2/CH4 and CO2/N2 with CNTs-MMMs and the feasibility for their practical application.
Fundamentals of gas separation
The development of membranes with enhanced properties depends on an understanding of the basics of transporting gas across the membrane.
Gas permeability
Most polymeric membranes, including commercial ones, separate gases by the sorption–diffusion mechanism. In this mechanism, it is the relationships between the properties of the membrane material (physical, chemical, etc.) and the nature of the gas (size, polarity, shape, condensability, etc.) that control separation (Xie et al. 2019). The sorption of gas molecules in the membrane depends on the affinity of the gas molecule for the polymeric matrix and the condensability of the gas (Wang et al. 2016). This relationship is characterized by the solubility coefficient, S. The diffusion of gas molecules occurs through the empty spaces between the polymer chains, called free volume. The speed of diffusion depends on the amount of free volume, characteristic of each polymer, and the size of the gas molecule (Wang et al. 2016). This relationship is characterized by the diffusion coefficient, D. Therefore, the sorption–diffusion mechanism proposes that the permeability of a gas i, Pi, can be defined by Eq. (1).
In a more practical way, permeability represents the productivity of the membrane. Although not a unit of the International System (SI), a unit most used to scale it is the Barrer (1 Barrer = 1 × 10–10 cm3 (STP) cm−1 s−1 cmHg−1, STP stands for standard temperature and pressure), in honor of Professor Richard Maling Barrer's important contributions (Dai et al. 2016). The diffusion coefficient is commonly expressed in cm2 s−1 and the solubility coefficient is often expressed in cm3 (STP) cm−3 cmHg−1.
As it is often difficult to accurately define the thickness of the dense layer, ℓ, the permeance, Qi, represented by Eq. (2), is commonly used to evaluate the productivity of membranes with asymmetric structure (Dai et al. 2016).
The unit most commonly used for permeance is the GPU (Gas Permeation Unit), with 1 GPU = 1 × 10–6 cm3 (STP) cm−2 s−1 cmHg−1 or 3.35 × 10–10 mol m−2 s−1 Pa−1 (Dai et al. 2016).
Gas selectivity
The most used parameter to determine the efficiency of the membrane in separating two components is the ideal selectivity, α, which can be defined as the ratio between the permeability of the most permeable gas, i, and the least permeable, j, resulting from a single gas test, according to Eq. (3).
Thus, the most permeable gas can be separated on the permeate side. Considering the same polymer and the same operational conditions, it is only the nature of the gas that distinguishes the permeability between the different gases that make up the feed. The condensability of the gas can be characterized by its critical temperature. The higher the critical temperature, the more condensable the gas becomes and the easier it is to solubilize it in the polymer. The size of the gas molecule can be characterized by the kinetic diameter. The smaller the gas, the faster it diffuses through the membrane (Sanders et al. 2013). As shown in Table 2, the smaller size and greater condensability gives CO2 greater permeability in relation to CH4 and N2. Therefore, it is possible to separate it from other gases using different polymers.
The diffusivity selectivity, αD, calculated as the ratio of the diffusion coefficients of two gases, indicates to what extent the size difference between the two molecules can contribute to separation. On the other hand, solubility selectivity, αS, calculated as the ratio of the sorption coefficients, indicates how gases can be separated by the respective solubilities in the membrane material (Ansaloni and Deng 2017). Equation (4) represents the relationship between these parameters.
The separation factor, α*, is commonly used instead of ideal selectivity when the composition of the feed is formed by a mixture of gases (Dai et al. 2016). The separation factor is calculated from the ratio between the composition of the feed gas and the permeate gas, according to Eq. (5).
where yi and yj are the molar fractions of gases i and j on the permeate side, while xi and xj are the molar fractions of the gaseous species i and j on the feed side.
Equation (6) shows that, considering the partial feed pressure much higher than the partial pressure of the permeate, the separation factor can be approximated to the ideal selectivity (Dai et al. 2016).
However, the separation factor is generally smaller than the ideal selectivity due to the competitive sorption between gases and the plasticization effect (Wang et al. 2016). Thus, the separation factor is more sensitive to operating conditions, for example, feed and permeate pressures and composition of the feed gas (Dai et al. 2016).
Effect of upstream pressure
Solubility of sparingly soluble gases in rubbery polymers is characterized by a linear isotherm in which the gas concentration inside the polymer, C, is proportional to the applied pressure, p, described by Henry’s law, given by Eq. (7) (Mukaddam et al. 2016).
where, in an linear isotherm, S corresponds to the equilibrium Henry’s law coefficient, kD.
However, deviations from Henry’s model in rubbery polymers are observed when there is a mutual miscibility between the polymer and the gas. In this case, sorption and diffusion coefficients cannot be considered constant, changing with the concentration of the permeating gas (Crespo and Brazinha 2015). For example, under medium–high pressure, condensable gases such as CO2 and hydrocarbons can be physically dissolved in the polymeric phase during permeation, causing an increase in the concentration of these gases in the polymer. As a consequence, the permeation of these highly soluble penetrants induces a significant swelling of the polymer matrix (Minelli et al. 2019). The change in the membrane structure is quantified by an increase in the diffusion coefficient due to the creation of extra free volume and by an increase in the solubility coefficient that can be described by Eq. (8), according to the Flory–Huggins model (Crespo and Brazinha 2015). The Flory–Huggins parameter, χ, allows for quantifying the affinity between polymer and gas (Langer et al. 2020).
where a is the penetrant activity and ϕ is the volume fraction occupied by the sorbed penetrant molecule. The penetrant activity is expressed as p/psat, where psat is the penetrant saturation vapor pressure at the temperature of the sorption experiment (Mukaddam et al. 2016).
Gas solubility in glassy polymers using the dual mode sorption model correlates well with the experimental sorption behavior of these polymers. This model divides the total sorbed gas into two mechanisms: based on Henry’s law, some molecules dissolve into the dense portion of the polymer, while those saturating the microvoids of the excess free volume that characterizes the glassy state are described by a Langmuir type isotherm (Ricci and De Angelis 2019). Thus, the cumulative sorption occurring in the Langmuir holes and via Henry’s mode results in Eq. (9).
where C′H is the Langmuir saturation capacity parameter that describes the non-equilibrium excess free volume features of the glassy state, and b is the affinity constant between the penetrant and the Langmuir sites.
Deviations from the ideal permeability behavior are caused by the effect of pressure on the gas solubility and/or the diffusion coefficient. Therefore, the dependence of the upstream pressure with the gas permeability in the polymers can be summarized in four distinct cases: (i) constant permeability value, commonly associated with the permeability of light and sparingly soluble gases in the polymer matrix; (ii) an increasing dependence, associated with the permeation of highly soluble penetrants that induce a significant swelling of the polymeric matrix; (iii) a decreasing dependence due to the compactness of the polymeric segments; (iv) a combination of cases ii and iii, in which, under lower pressures, the permeability is reduced until reaching a minimum value, after which the permeability increases under higher pressures, resulting in the phenomenon known as plasticization (Minelli and Sarti 2013). Generally, i and ii are seen in elastomeric polymers and iii and iv in glassy polymers.
Effect of operation temperature
The operation temperature can also cause major changes in the gas transport properties of membranes. The dependence of solubility on temperature is typically written in terms of a van't Hoff relationship through Eq. (10) (Rezakazemi et al. 2018).
where S0 is the pre-exponential factor, ΔHs is the partial molar enthalpy of sorption, R is the universal gas constant and T is the absolute temperature.
For less condensable gases, the interaction between the gas molecule and the polymer is weak. As the temperature increases, the change in the mixing enthalpy is positive (ΔHs > 0) and the solubility of the gas increases. For condensable gases, the increase in temperature results in a negative variation of the enthalpy (ΔHs < 0) and the solubility decreases. In this way, the selectivity of solubility between CO2 and less condensable gases is reduced with increasing temperature (Rezakazemi et al. 2018).
The diffusion of gas molecules in a dense membrane is usually expressed by the Arrhenius relationship, shown in the Eq. (11) (Rezakazemi et al. 2018).
where D0 is the pre-exponential fator and ED is the activation energy of diffusion.
The increase in the operating temperature results in greater gas diffusion due to the increase in the gas kinetic energy and the relaxation of the polymer chains (Xue et al. 2017). However, the reduction in diffusivity selectivity occurs because the mobility of molecules with a larger kinematic diameter increases more dramatically than those with a smaller diameter. Thus, when a component is smaller and at the same time more soluble, such as CO2 in relation to N2 and CH4, the selectivity of diffusivity also decreases as the temperature increases (Rezakazemi et al. 2018).
As with diffusion, dependence between temperature and permeabilities of gases in the membranes can be expressed by the Arrhenius relationship, shown in the Eq. (12) (Dixon-Garrett et al. 2000).
where P0 is the pre-exponential fator. Permeability is the product of solubility and diffusivity, so the activation energy of permeation, EP, is the sum of the contributions of the enthalpy of sorption and the the activation energy of diffusion (ΔHs + ED).
ED is always positive, however EP depends on the magnitude of the difference between ED and ΔHs (Dixon-Garrett et al. 2000). For gases, the EP is generally positive and the increase in the operating temperature results in greater gas permeation through the membrane. On the other hand, gases that are less condensable than CO2 have a higher EP, which results in a greater increase in the permeation of these gases with an increase in temperature (Zhao et al. 2014b). In this way, the selectivity between CO2 and less condensable gases is reduced with increasing temperature.
Therefore, the permeability of gases through nonporous polymeric membranes is governed by a number of factors, which include driving forces (temperature and pressure), features of the permeating gas (molecular size and chemical nature), the composition of the feed mixture, and the properties of polymer, mainly the free volume.
Free volume
Free volume can be defined as the sum of the microvoids not occupied by polymer chains by which the permeation of molecules in non-porous polymeric membranes occurs (Tofighy and Mohammadi 2020). The diffusion of a molecule from one void to another occurs based on the size of the void being sufficient for the molecule to occupy it (Swapna et al. 2020). Thus, size and distribution of the free volume, as well as the mobility of the polymer chains and the nature of the penetrant are fundamental for defining the transport properties of gases in polymers. Therefore, changes in free volume directly impact transport properties. While the increase in free volume caused by plasticization increases permeation and reduces selectivity, the process of reduction of excess free volume in the membrane, referred to as physical aging, increases selectivity and reduces permeability.
The free volume of a polymer can be determined by the difference between the specific experimental volume, V, and the theoretical volume occupied by the polymer chains, V0 (Sanders et al. 2013). However, when it is desired to compare this property between several polymers, the free volume fraction parameter, FVV, is more convenient. As Eq. (13) shows, the FVV parameter is normalized in relation to the specific volume of the polymer.
where V0 = 1.3VW, ρ represents the experimental density of the membrane and VW is the van der Waal's volume of the polymer repeat unit, which can be obtained using the Bondi group contribution method (Sanders et al. 2013). Density can be determined by experimental methods such as flotation (Murali et al. 2010) or hydrostatic weighing (Matteucci et al. 2008).
Changes in free volume can also be assessed by measuring the gas diffusion coefficient (Kim et al. 2006; Cong et al. 2007; Yu et al. 2013). This relationship presented in Eq. (14) indicates that the increase in free volume positively affects the diffusion coefficient (Ansaloni and Deng 2017). In this way, as the gas solubility normally depends weakly on free volume, the gas permeability is also directly related to FVV (Sanders et al. 2013).
where Ai is a constant related to the characteristics of the gas molecule and Bi is a parameter obtained from relationships between the penetrant and polymer.
Positron annihilation lifetime spectroscopy (PALS) is the most accepted method for experimental investigation of the free volume in polymers (Tanh Jeazet et al. 2013). The reliability of this method comes from the fact that the positronium (Ps) is preferentially located in regions with low electron density, such as free volume, interfacial holes and pores, which makes it possible to estabilish a direct relationship between the lifetime of the ortho-positronium (o-Ps) and free volume units (Awad et al. 2012).
Separation efficiency for MMMs
The gas separation performance of polymeric membranes was investigated by Robeson in two papers, one in 1991 (Robeson 1991), and another in 2008, with the update of new materials (Robeson 2008). He gathered the permeability and selectivity data of membranes prepared with different types of polymers and was able to clearly demonstrate the inverse relationship between the permeability and selectivity of polymeric membranes. The relationships obtained for different gas pairs are widely used to obtain the maximum permeability value for a given selectivity value that current polymeric membranes cannot overcome. Therefore, as these limits represent the separation performance that can be expected for the best membrane materials, it has become a very useful tool to qualify the newly developed membranes. However, the transport properties of new materials can be significantly changed, even if they do not exceed the Robeson limit. In addition, asymmetric membrane data commonly provided in GPU cannot be included. Thus, another way to assess the performance of MMMs is through the permeability factor, PF, and the selectivity factor, FS, calculated from the ratio between the transport properties of MMMs and individual polymeric membranes, using Eq. (15) and Eq. (16), respectively.
According to performance, the MMMs can be categorized in different regions, as shown in Table 3.
Mixed matriz membranes (MMMs) and their challenges
MMMs are hybrid membranes formed by a continuous phase (polymer) that incorporates a second dispersed phase (inorganic filler). In these membranes, gases can permeate through the polymeric phase and the inorganic phase, depending on the interfacial morphology that the MMM assumes (Fig. 2). With the incorporation of fillers, the permeation of gases through the polymeric phase (sorption–diffusion) can be improved by adjusting the size of the elements of free volume (Wang et al. 2016). In an ideal interfacial morphology (Fig. 2a), the polymeric chains are continuously connected to the external surface of the filler in such a way that, upon reaching the dispersed phase, the gases are transported by the mechanism that characterizes the inorganic phase, for example, molecular sieving, surface adsorption, Knudsen or bulk diffusion (Ansaloni and Deng 2017). In this way, it becomes possible to simultaneously increase the selectivity and permeability of MMMs in comparison to the polymeric matrix.
However, due to the nature of the fillers (inorganic) and polymers (organic), incompatibility and weak interaction between the filler and the polymeric chains can occur, resulting in discontinuity in the polymeric chain packaging and formation of interfacial voids between the two phases (Xiao et al. 2009). As the gases find less resistance to diffuse through the voids, the tendency is for the permeability to increase. In the morphology known as “sieve-in-a-cage” (Fig. 2b), the void formed is on the molecular scale of the gas; therefore, it can contribute to increased permeability and selectivity. However, the extent of the interfacial voids can increase considerably and form a morphology known as “leaky interface” (Fig. 2c) (Rezakazemi et al. 2014). In this case, selectivity is drastically reduced, as the interfacial void becomes larger than the gas molecules that permeate the membrane.
Other problems found in the interfacial morphology of MMMs can be caused by a high affinity of the filler with the polymer. In the “ matrix rigidification” (Fig. 2d), a strong connection between the phases reduces the mobility of the polymer chains that are in contact with the filler, resulting in formation of a rigid polymeric region that surrounds the load (Rezakazemi et al. 2014). As larger gases are more affected by rigidification, the permeation of these gases is reduced to a greater extent and generally selectivity increases. On the other hand, in “plugged sieves” (Fig. 2e), the pores of the fillers can be totally blocked by the penetration of polymer chains and form regions of reduced permeability without great effects on selectivity (Ansaloni and Deng 2017).
Among the problems found in MMMs morphology, interfacial voids are the most serious, because in addition to reducing the transport properties, they can also impair the mechanical and thermal properties. The formation of voids makes tension transfer between the load and the polymer difficult, causing the material to weaken (Yetgin 2019). Meanwhile, the membrane thermal stability is gradually reduced with the increase of polymer-filler interaction distance (Ismail et al. 2011).
Therefore, the most important challenge in the development of MMMs is the adjustment of the chemical nature between its components. The fillers need to have the potential for chemical adaptation, as well as good mechanical and thermal properties that can be transferred to the polymeric matrix. Various materials have been investigated and identified as potential fillers for MMMs, including CNTs, zeolites and MOFs (Norahim et al. 2018).
Selection of inorganic fillers
Inorganic fillers can be porous or nonporous and, when incorporated into the polymeric matrix, they offer different effects on the transport properties of MMMs. Although nonporous fillers can adjust the free volume of the polymer available for gas transportation, generally the increase in permeability causes a loss in selectivity (Casado-Coterillo 2019). This problem may be associated with the formation of non-selective voids. In another situation, the use of nonporous fillers enhances the selectivity of MMMs, but reduces the permeability (Sodeifian et al. 2019). In this case, an increase in tortuosity prolongs the molecule permeation paths across the membrane (Yang et al. 2020). On the other hand, the pore size distribution and surface chemistry of a porous filler give better compatibility with polymers than a nonporous filler (Vinoba et al. 2017), and gas separation with a porous filler can occur by molecular sieving, resulting in a greater possibility of simultaneous increase in permeability and selectivity for MMMs (Setiawan and Chiang 2019). Although the pore size is significantly larger than the size of the molecules, adsorption and selective surface flow mechanisms must be considered (Bastani et al. 2013). Therefore, porous fillers are preferred for the development of MMMs for gas separation.
The choice of a porous filler may depend on factors such as ease of synthesis, cost, stability, toxicity, pore size and efficiency for transporting and separating gases. Silica is a cost-effective nanomaterial with low toxicity (Yang et al. 2020), high surface area, high pore volume, tunable pore size and excellent mechanical and thermal stability (Zhou et al. 2017). However, mesoporous silica (2–50 nm) has pores that can be easily blocked by the penetration of polymeric chains, leaving the internal pores inaccessible. Even if unblocked, they are not very selective pores, since Knudsen's diffusion is predominant in these materials (Bastani et al. 2013). Greater selectivity can be achieved with microporous silica (< 2 nm). However, hydrogen bonding between hydroxyl groups in the silica filler and major polymer functional groups can form a strong interfacial network and create a rigid region around the filler (Setiawan and Chiang 2019). Other microporous materials, including CNTs, MOFs and zeolites demonstrate exceptionally narrow pore size distributions and can exhibit extreme mechanical and thermal stability.
Zeolites have excellent characteristics that can make the MMM separation performance superior in polymeric membranes (Harami et al. 2019b). For example, various rates of adsorption depending on molecular size, the effect of molecular sieving, the right solubility and diffusivity, excellent thermal stability in high-temperature operations, slight swelling and polymorphism (Harami et al. 2019a). Zeolites such as siliconaluminophosphate crystals (SAPO-34) perform better in CO2 separation from CH4 due to their specific structure with pores comparable to the size of the CH4 molecule (0.38 nm) and the relatively high ratio of silica to aluminum compared to other materials (Ahmad et al. 2016; Sodeifian et al. 2019). However, the weak point of zeolites is typically the water sensitivity (Zhou et al. 2017), since water is one of the contaminants of many processes, including raw natural gas.
MOFs are among the classes of microporous solids widely investigated for the development of MMMs because of their large surface area, well-defined and extended porosity and high pore volumes (Anastasiou et al. 2018). Unlike other fillers, which have an inorganic chemical composition, MOFs are organic–inorganic hybrids containing metal ions or clusters coordinated with organic ligands, resulting in a better compatibility with the polymer (Yang et al. 2020). Other advantages of MOFs compared to conventional porous fillers (carbons, zeolites and silicas) are the flexibility to change their composition through a straightforward change of the metal and/or the organic linker (Zhou et al. 2017), and the possibility of adjusting the pore diameter to a molecular sieving level (Casado-Coterillo 2019). ZIFs are one of the most studied MOFs for gas separation and are a good example of how flexible these materials are. In an isoreticular series of eight ZIFs, the pores were precisely controlled with diameters between 15.9 to 7.1 Å and functionalized with the desirable topology. Thus, the selectivity of CO2/N2, which was about 20, increased to 50 (Younas et al. 2020a). Furthermore, they are chemically stable in the presence of water, benzene and other aromatic hydrocarbons, which are typical impurities in natural gas (Anastasiou et al. 2018). However, some important disadvantages must be mentioned. The organic ligands that are involved in their synthesis are expensive (Ahmad et al. 2016). The performance of MOF based membranes could be changed by operational conditions such as pressure and temperature (Mirqasemi et al. 2020). For example, upon mechanical loading, problems such as partial pore collapse or even a change of phase in MOFs can occur (Younas et al. 2020a).
Carbon nanotubes (CNTs)
CNTs are basically graphene sheets (hexagonal structures) rolled up into cylindrical form and their morphology depends on the orientation and magnitude of the chiral vector on the graphene sheet (Mittal et al. 2015). CNTs can be synthesized having two structures: single-walled carbon nanotubes (SWNT) or multi-walled carbon nanotubes (MWNT). SWNT consist of a single layer of graphene packed in the form of a seamless hollow cylinder with a diameter of about 0.4–3.0 nm and lengths between 20 and 1000 nm. MWNT are formed by two or more SWNT structures with different radii, with separation between adjacent layers around 0.34 nm, diameter of 2–100 nm and lengths of 1–50 µm (Karimi et al. 2015). Table 4 shows that the properties of CNTs are highly dependent on morphology.
CNTs have attractive properties to be used as fillers in MMMs, such as: high mechanical strength, high strength-to-weight ratio, high aspect ratio, high thermal stability, very smooth internal surface and precise diameter (Aroon et al. 2010a), and highly selective sites, resulting in high gas permeability and selectivity (Dilshad et al. 2020). The interpenetrated and densely packed structure enables the formation of nanopores (Yang et al. 2020). This way, they can be used for selective separation based on size differences of both gases and molecules or ions in the liquid state (Mangukiya et al. 2016). Moreover, as their internal walls are smooth, the gas is transported in CNTs with rates several orders of magnitude faster than in zeolites or other microporous fillers (Li et al. 2015). Therefore, the effect of CNTs on the transport properties of CNTs-MMMs has the potential to overcome Robeson's upper limit (Vinoba et al. 2017). However, a variety of parameters should be considered for the preparation of CNTs-MMMs, including the synthetic process used to produce nanotubes; the nanotube’s purification process; the amount and type of impurities in the nanotubes and their diameter, length and aspect ratio (Bastani et al. 2013). CNTs are not perfect structures, and the defects formed during synthesis can change their properties (Karimi et al. 2015). It is also important to mention that CNTs can have harmful effects on the human body. For example, they can cause lung tumors (Kobayashi et al. 2017).
Pristine CNTs are usually severely contaminated with metallic catalysts and amorphous carbons (Madani et al. 2012). In addition, they have a chemically inert structure that makes it difficult to disperse them in typical organic solvents (Ahmad et al. 2014). Their tendency is to agglomerate when incorporated into the polymeric matrix, resulting in deteriorated properties of the individual components (Roy and Singha 2017). Modification of CNTs by functionalization has been the main method used to reduce these problems. Through functionalization, it is possible to fix chemical structures on the side walls or at the ends of CNTs. The methods can be grouped into chemical (covalent) and physical (non-covalent) functionalization, depending on the links between the added structures and the CNTs (Jeon et al. 2011).
The definition of the most appropriate type of functionalization depends on the type of application for which the CNTs are intended. For CNTs-MMMs, covalent functionalization through treatment with strong acids is widely explored due to its versatility. In this method, oxygenated functional groups are attached to the external structure of CNTs, especially carboxyls and hydroxyls (Karimi et al. 2015). The polar character of functional groups significantly alters the hydrophobic nature of CNTs. As a result, solubility in water and organic solvents can improve substantially (Dai and Sun 2016). In addition, acid treatment removes impurities from CNTs that can impair the gas flow and simultaneously reduce CNTs length, which favors dispersion in polymers (Kim et al. 2006). It can also be highlighted that, from the incorporation of oxygenated groups, CNTs can be further functionalized with amines, polymers and even some biomolecules (Jeon et al. 2011). Table 5 shows the changes observed in the SWNTs physical properties after acid functionalization with a mixture of HNO3:H2SO4 (1:3 v/v).
Covalent functionalization can damage the structure of CNTs. However, the disruption of the surface conjugated π network impacts with greater intensity only the electrical properties of CNTs, while their thermal and mechanical properties are maintained (Mallakpour and Soltanian 2016). Thus, for electrical applications, non-covalent functionalization may be a better option, as it preserves the structure of CNTs. On the other hand, for MMMs, the chemical structures that are added by non-covalent functionalization to the surface of CNTs, such as surfactants and polymers, can impair the interaction of CNTs with the polymeric matrix and, due to the weak forces that may be involved in the connection between these molecules and CNTs, a low charge transfer may occur (Mallakpour and Soltanian 2016).
Effect of CNTs on morphology
The filler dispersion in the polymeric matrix, the adhesion between components and the changes in the structure of membranes caused by incorporation of fillers in MMMs can be observed through morphological analysis, mainly, by scanning electron microscopy (SEM).
The main problems encountered in the preparation of CNTs-MMMs are the agglomeration of CNTs and the weak interfacial interactions between CNTs and the polymeric matrix (Dai and Sun 2016). Thus, the main strategy used to obtain MMMs with interfacial morphology as close as possible to ideal has been the superficial modification of CNTs. With functionalization, the van der Walls forces that keep CNTs aggregated can be overcome by electrostatic repulsion between functional groups inserted in the structure (Castro et al. 2017), facilitating their dispersion in the polymer. On the other hand, when CNTs are not modified, generally their tendency is to form agglomerates and present a low adhesion in the polymeric matrix, as observed in SEM images from different MMMs (Aroon et al. 2010b; Khan et al. 2013; Rajabi et al. 2013; Moghadassi et al. 2014; Sun et al. 2017).
The agglomeration of CNTs occurs more frequently in glassy polymers due to the lower flexibility of polymer chains. Kim et al. (2007) observed that raw SWNT (rSWNT) formed agglomerates in a polysulfone (PSF) polymer matrix. However, for the same filler content, SWNT functionalized with amines (SWNT-NH2) were distributed homogeneously. Similar results were obtained by Ahmad et al. (2014) for MMMs prepared with cellulose acetate (CA) incorporated with raw MWNT (rMWNT) and MWNT functionalized with beta-cyclodextrin (MWNT-β-CD). They also observed that the morphology of MMMs was a result of the way CNTs were distributed in the polymeric matrix. CNTs modified the phase inversion kinetics, which resulted in MMMs with a more porous structure compared to the pure CA membrane. The interfacial layer of the CA/rMWNT membrane showed agglomerations and defects, while the CA/MWNT-β-CD membrane was formed with a smooth and defect-free interfacial layer. Thus, the homogeneous dispersion between the MWNT-β-CD and the CA matrix produced MMMs with the best performance in terms of permeance of CO2 and selectivity towards the separation of CO2/N2 due to better adjustment of the polymer/filler interface, as confirmed by X-ray diffraction (XRD) results. In addition, the membrane morphology also influenced the mechanical properties. The wide distribution of MWNT-β-CD produced an effective reinforcement, resulting in a strong network of the filler. As expected, the increase in pressure did not exert a significant change in selectivity and the additional mechanical strength of the MWNT-β-CD was transferred to the MMMs.
In fact, functionalized CNTs show good compatibility with polymers. In some cases, adhesion can be so high that a thin layer of polymer can settle on the surface of the CNTs. For example, in Fig. 3, the polymeric matrix formed by a blend of PSF and polyimide (PI) Kapton covers the MWNT functionalized with oxygenated groups (MWNT-O).

Reproduced from Soleymanipour et al. (2016) Copyright (2016), with permission from John Wiley and Sons
SEM shows the high adhesion between Kapton–PSF and MWNT-O
Effect of CNTs on thermal properties
Different techniques can be used to measure the change in a physical property of the material when it is heated, cooled or maintained isothermally to establish a connection between temperature and specific physical properties of materials (Rezakazemi et al. 2018). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are the main techniques used to investigate the thermal properties of polymers. TGA is used to assess the membrane's resistance to decomposition and degradation by measuring mass loss with increasing temperature. On the other hand, DSC can determine the glass transition temperature (Tg), which is used to identify changes in the mobility of the polymer chain.
The thermal stability of CNTs/MMMs clearly depends on the type of fillers and their loading rate. Ismail et al. (2011) investigated the thermal behavior of polyethersulfone (PES) incorporated with CNTs. The rMWNT were purified with acid mixtures (HNO3/H2SO4; v/v = 1:3) to remove carbonaceous impurities, and the purified MWNT (pMWNT) were submitted to surface functionalization with 3-aminopropyltriethoxylsilane (APTES) (pMWNT-APTES). The initial and final decomposition temperatures (Td) of PES increased with the incorporation of 0.5 wt% of pMWNT-APTES, which resulted in a reduction of mass loss. On the other hand, with the increase of its concentration to 1.0, 2.0 and 3.0 wt%, the thermal stability decreased, indicating a low adhesion between the phases for high concentrations. Probably, with the concentration increase of pMWNT-APTES, the polymer–filler interactions were gradually reduced due to the formation of clusters in greater proportions. The reduction in mobility of the polymer chains, as well as the formation of more rigid regions involving pMWNT-APTES with the incorporation of 0.5 wt%, may have resulted in a MMM with a Tg value slightly higher than that of the pure PES membrane, confirming the good interaction between phases at this concentration, while for the other MMMs, Tg was reduced.
As noted by Aroon et al. (2010b), the increase in the Tg in MMMs is more prominent using functionalized CNTs. Compared to the pure PI membrane, MMMs with MWNT functionalized by adding chitosan (MWNT-CS) had an increase in Tg of 14.3 °C, while MMMs with rMWNT had an increase of 12.8 °C. MWNT funcionalized with low molecular weight chitosan (LMWC) (MWNT-LMWC) (Aroon et al. 2018) and MWNT-β-CD (Aroon et al. 2013) have also been shown to be effective in increasing the thermal resistance of PI and PES, respectively.
Copolymer synthesis is a technique widely used in the preparation of membranes. For gas separation, in general, the objective is to combine the diffusivity selectivity of the glassy polymer with the solubility selectivity of the elastomeric polymer (Rezakazemi et al. 2018). For example, poly (ether-block-amide) (PEBA, commercial name of Pebax) is a copolymer consisting of soft polyether (PE) and hard polyamide (PA) segments. Although it has good transport properties, PEBA is expensive, which makes it is difficult to scale it up. Alternatively, Amirkhani et al. (2020) prepared MMMs by adding MWNT with different functional groups to PEBA. MWNT-NCO (MWNT functionalized with isocyanate groups (NCO)), MWNT-O and MWNT-NH2 were used as fillers. Gas permeation measurements showed that the higher CO2 permeability was observed with the optimum loading of 0.3 wt% for MWNT-NCO, 0.5 wt% for MWNT-NH2 and 0.75 wt% for MWNT-O. The MMMs with optimum MWNT loading showed better performance in terms of permeability and selectivity than pure PEBA. Another important observation was that MWNT-NCO with the lowest loading in comparison to MWNT-NH2 and MWNT-O exhibited more efficiency due to superior grafts with the polymer chains. Among the prepared MMMs, the one with PEBA/MWNT-NCO (0.3 wt%) also showed the best results in terms of selectivity for CO2/N2 and CO2/CH4. DSC and XRD showed that this membrane had the highest Tg for hard segments and melting temperatures (Tm) for soft segments as well as the lowest d-spacing. These results were associated with disrupted chain linkages, interfacial interactions and chain mobility, which affected the free volume and polymer density, resulting in higher selectivity.
For the membranes to maintain their separation properties for long periods of operation, it is important that they are made with thermally stable polymers. Dilshad et al. (2020) used the blend of cross-linked polyvinyl alcohol (PVA) and polyethylene glycol (PEG) as a polymeric matrix to prepare MMMs. The gas separation performance of the PVA/PEG membranes is reported in the literature. However, the incorporation of MWNT-O into this membrane had not yet been investigated. TGA and dynamic mechanical analysis (DMA) showed that adding MWNT-O could improve the overall thermal stability of the membranes. For example, a 75% weight loss of the PVA/PEG membrane occurred at 442.6 °C. The same weight loss was only observed at 449.5, 458.3 and 508.5 °C with the incorporation of 0.25, 0.5 and 0.75 wt% of MWNT-O, respectively. All MMMs had an increase in Tg, with an emphasis on the MMM with 0.75 wt%, for which the Tg was 17 °C higher than that of the PVA/PEG membrane. The gas permeation tests also showed good compatibility between the components of the MMMs. Significant increments were obtained for permeation of CO2 and for selectivity of CO2/N2 and CO2/CH4 for all MMMs. For example, compared to the PVA/PEG membrane, the MMM with the best performance (0.5 wt%) had an increase in CO2 permeation from 22.6 to 115.6 Barrer, and in the selectivity of CO2/N2 from 21 to 203 and CO2/CH4 from 13 to 82. In addition, the long-term stability of this MMM was verified by exposing it to each gas at 20 bar for 96 h.
The incompatibility between the polymeric matrix and the filler is still a challenge for the manufacture of MMMs with ideal morphology. Zhang et al. (2020b) used PI containing hierarchical triptycene units (PI-TP) as a polymeric matrix to control and optimize the polymer-SWNT interfacial interactions. The triptycene units may introduce π–π stacking and supramolecular shape-fitting interactions with SWNT, enabling the formation of ideal interface morphology. MMMs containing raw SWNT (r-SWNT), purified SWNT (p-SWNT) and acid-treated SWNT (SWNT-O) were prepared and characterized. SEM images showed that homogeneous, defect-free and ductile thin films were obtained with all SWNT due to strong π–π interactions between SWNT and the benzene rings of the tripytcene moieties of the polymer matrix. Thermal properties were evaluated for MMMs with r-SWNT. The Tg of the pristine polymer was mantained with addition of 2 wt% r-SWNT, then it increased very slightly with increasing filler content for 5, 10 and 15 wt%. Although the typical behavior of an improvement in the filler–polymer interaction is polymer rigidification and the increase in Tg, the results indicated that the interfacial interaction only restricted polymer chain mobility locally. In other words, π–π stacking and supramolecular shape-induced nano-confinement between triptycene units and SWNT simultaneously caused two effects: (i) an increase in interfacial affinity and (ii) largely retaining the flexibility of the polymer chains. As a result, the CO2 permeability of MMMs incorporated with r-SWNT increased with very slight change in the selectivity of CO2/N2 and CO2/CH4 over the entire filler concentration range. This membrane exhibited the best separation performance at 2 wt% filler. However, the selectivities of MMMs incorporated with p-SWNT and SWNT-O also were superior at 2 wt% filler. Probably, the carboxylic groups on the SWNT-O surface improved the interfacial affinity with the polymeric matrix.
Sarfraz and Ba-Shammakh (2018) showed that combining MWNT-O and ZIF can be another way to obtain MMMs with high performance for CO2 separation and high thermal stability. In PSF, MWNT-O acted to increase CO2 permeability due to their relatively large pore diameter and smooth inner surfaces, while ZIF-301 fillers increased CO2/N2 selectivity due to the chemical structure. Therefore, the synergistic effect of adding MWNT-O and ZIF-301 fillers increased the CO2 permeability and CO2/N2 selectivity of the MMMs. The best separation performance (maximum CO2 permeability and maximum CO2/N2 selectivity) among all MMMs was achieved with 6 wt% MWNT-O and 18 wt% ZIF-301. The good interaction between the polymer matrix and the fillers improved the rigidity of MMMs, resulting in thermal disintegration of MMMs at higher temperatures and an increase of Tg with filler concentration. Table 6 shows the effect of CNTs on the thermal properties of MMMs.
Effect of CNTs on mechanical properties
CNTs have very high mechanical properties (see Table 4), which, when transferred to the polymeric matrix, can increase the mechanical resistance of MMMs. However, CNTs must be homogeneously dispersed in the polymer and present good interfacial morphology between the phases; otherwise, the formation of agglomerates and the polymer-filler incompatibility can reduce mechanical stability of MMMs (Dai and Sun 2016). The degree of adhesion between the phases can be qualitatively assessed in MMMs through stress–strain tests to obtain the parameters that determine their mechanical behavior, including strain to break (ε) and Young’s modulus (E).
Cong et al. (2007) investigated the effect of incorporating CNTs (rSWNT and rMWNT) on the mechanical properties of brominated poly (2,6-diphenyl-1,4-phenylene oxide) (BPPO). With 5 wt% of CNTs, the break elongation was reduced and Young’s modulus increased compared to the pure BPPO membrane. CNTs restrict the mobility of polymer chains, therefore reducing the percent elongation of the polymer matrix, making CNTs-MMM more fragile. On the other hand, due to good interfacial interactions, the polymeric matrix transfers part of the stresses to the CNTs, resulting in an increase in Young’s modulus (Yetgin 2019). Another important observation in the work by Cong et al. (2007) is that rSWNT made MMMs more fragile and more resistant to deformation when compared to MMMs prepared with the same rMWNT concentration. Yu et al. (2013) observed the same behavior of mechanical properties with Pebax incorporated with rMWNT and rSWNT. The possible cause may be associated with the difference between the aspect ratio of rMWNT and rSWNT. Molecular simulations using poly(methyl methacrylate) (PMMA) incorporated with CNTs showed that CNTs/polymer interfacial bonds became stronger with increasing aspect ratio (l/d), resulting in increased stress transfer between CNTs and the polymer, which in turn leads to higher composite stiffness and strength (Arash et al. 2014).
Experimental studies have shown that two important parameters for the improvement of mechanical properties are the functionalization and the concentration of CNTs. Ahmad et al. (2014) observed that, with the incorporation of 0.1 wt% of rMWNT, the mechanical properties of the CA matrix suffered a drastic reduction. On the other hand, with MWNT-β-CD incorporated in the same concentration (0.1 wt%), the CA membrane became more resistant. However, increasing the concentration of MWNT-β-CD to 0.2 wt% dramatically reduced the properties of CA due to the formation of clusters. This indicated the limiting filler concentration that the polymer can incorporate, even though CNTs are functionalized. Zhao et al. (2014b) reported significant improvements in Young’s modulus of Pebax incorporated with high concentrations of MWNT-NH2. With the incorporation of 9 wt% of MWNT-NH2, there was an increase in Young's modulus in relation to the polymeric matrix. By raising the concentration to 23 wt%, the improvements were even more significant. Usually, with an incorporation of the fillers in the MMMs, the mobility of the polymer chains is reduced. This causes an increase in Young’s modulus and reduces elongation. However, some exceptions have been observed and may be related to the way the membranes are prepared. The long evaporation time of the solvent of MMMs prepared by casting can lead to the sedimentation of the fillers. As a consequence, a filler gradient can be created with the two faces (upper and lower) presenting very different mechanical characteristics. At the beginning of the stress–strain test, only the most rigid face supported all the applied stress. In a second stage, after a filler break, the tension was transferred to a more flexible face. This combination results in materials with high Young’s modulus and elongation (Flauzino Neto et al. 2016). Moghadassi et al. (2014) prepared, by casting, MMMs with MWNT-O incorporated in different polymeric matrices: CA, blend of CA and PEG (CA/PEG), and blend of CA and styrene butadiene rubber (SBR) (CA/SBR). The elongation at break and Young’s modulus increased with increasing concentration of MWNT-O in the polymeric matrices. In addition, the groups adapted from PEG and SBR added mobility to the blends CA/PEG and CA/SBR and increased the molecular interactions of polymer chains with MWNT-O. Thus, the CA/PEG/MWNT-O and CA/SBR/MWNT-O membranes have high mechanical properties, superior to the CA and CA/MWNT-O membranes with the same concentration of MWNT-O. Similar effects were also observed when SBR was added to polyvinyl chloride (PVC) incorporated with MWNT-O (Rajabi et al. 2013). Table 7 shows the impact on the mechanical properties of different polymers with the incorporation of CNTs.
Effect of CNTs on transport properties
As the permeability is very sensitive to changes in the structure of the membranes, gas permeation experiments are a convincing method to certify the observations made through morphological, thermal and mechanical analyses.
Permeability increase mechanism
The mechanism for increasing gas permeability can be understood by assessing the contributions of the solubility and diffusivity coefficients. Some studies have shown that the incorporation of CNTs in the polymeric matrix does not significantly affect the solubility of gases, including CO2, N2 and CH4 (Kim et al. 2006, 2007; Cong et al. 2007; Khan et al. 2013; Yu et al. 2013). For example, Kim et al. (2006) fabricated MMMs consisting of rSWNT embedded in a poly(imide siloxane) (PDMS) and evaluated their transport properties. After incorporation of 2 wt% rSWNT into the polymer, diffusivity coefficients for CO2, O2, N2 and CH4 increased by 13%, 11%, 29% and 18%, respectively. Despite the even more significant increase in the diffusivity of gases at 10 wt% CNTs loading, which reached 79% for N2, the solubility decreased slightly or remained unchanged. Therefore, in these cases it is mainly the increase in the diffusion coefficient of the gases that results in the increased permeability of the CNTs-MMMs.
The way in which the gases diffuse may depend on the system formed by the polymeric matrix and the CNTs. For MMMs prepared with Pebax/rSWNT and Pebax/rMWNT, the results obtained by Yu et al. (2013) indicated that the incorporation of CNTs could generate more free volume by modifying the packaging of polymer chains and that the gas molecules could be transported through its interior, which resulted in an increase in gas diffusion. On the other hand, for the system prepared by Cong et al. (2007) with BPPO and different CNTs (rSWNT, rMWNT, SWNT-O), diffusion probably increased due to the formation of interfacial voids, since the BPPO permeability and selectivity were not altered with the incorporation of SWNT-O, despite better adhesion to the polymeric matrix. Ge et al. (2011a) conducted experimental and theoretical studies with MMMs prepared with MWNT-O and MWNT modified with metals (MWNT-Ru and MWNT-Fe) incorporated into the polymeric matrix of PES. The results indicated that the gases would be more likely to be transported through the polymer-CNTs interface than through the CNTs channels.
In other studies, the results of the solubility coefficient (Weng et al. 2009; Ge et al. 2011b; Wang et al. 2014) and the permeability coefficient (Zhao et al. 2014a; Soleymanipour et al. 2016; Sun et al. 2017) obtained for different MMMs indicated that the solubility of gases in the polymeric matrix can be modified with the incorporation of CNTs. In these cases, the effects of sorption and diffusion can work together to improve the transport properties of CNTs-MMMs. According to Soleymanipour et al. (2016), CNTs can function as high diffusion paths and also as capillaries that facilitate the permeation of more condensable gases. As a result, for example, CH4 may have a higher permeability compared to N2 with an increase in the concentration of CNTs, despite its larger size.
Plasticization and physical aging
It is essential to test the membrane under mixed gas conditions as well as high pressures to observe the plasticization phenomenon. If the membrane is not resistant, the gas permeation properties will be severely impaired, as the dissolution of CO2 in the polymer increases due to its higher partial pressure, making polymer chains more flexible and causing selectivity loss (Dilshad et al. 2020).
Asghari and Afsari (2018) prepared Pebax/PEG/CNTs MMMs with CNTs concentrations ranging from 2 to 8 wt% and up to 50 wt% of PEG. The results showed that the incorporation of CNTs improved the gas separation performance of the membranes and PEG improved the permeability of CO2. The best performing MMM was prepared with 8 wt% of CNTs and 50 wt% of PEG. The experiments with pure gas for this membrane showed permeability to CO2 of 302 Barrer and selectivity to CO2/CH4 of 45. On the other hand, permeation with a gas mixture induced the effect of plasticization and competitive interactions between gases. Thus, its permeability to CO2 was drastically reduced to 193 Barrer, while the permeability of CH4 had a significant increase, resulting in a drop in the selectivity of CO2/CH4 to 19. Even so, its performance was superior to that of a pure Pebax membrane under the same operating conditions, indicating that CNTs contributed to some extent to reduce the plasticization effect.
Murali et al. (2010) used Pebax as a base polymer for physicochemical modification through incorporation of MWNT-O and cross-linking by 2,4-toluylene diisocyanate (TDI). FFV calculations based on density measurements showed that the free volume of the membranes increased with increasing MWNT-O concentration. As a result, CO2 permeability and CO2/N2 selectivity were improved. In addition, the results obtained at high pressures (10–30 bar) showed that MMMs prepared with 2 wt% of filler did not present any changes in selectivity, indicating the absence of plasticization. On the other hand, in cross-linked Pebax (X-Pebax) membranes incorporating 2 wt% MWNT-O, the CO2 solubility and permeability in the MMM was high and was enhanced with increasing pressure due to plasticization.
Khan et al. (2013) prepared MMMs by dispersing rMWNT and MWNT functionalized with PEG (MWNT-PEG) in polymers with intrinsic microporosity (PIM). The pure gas permeation experiments (CO2, CH4 and N2) showed that MMMs incorporated with MWNT-PEG performed better than those incorporated with rMWNT. In addition, they observed that the CO2 permeability of PIM/MWNT-PEG decreased with a pressure increase from 5 to 30 bar, as was expected for the typical behavior of a glassy polymer without the effect of plasticization.
Koschine et al. (2015) used PALS to investigate changes in the free volume of PIM subjected to aging for 300 days. For purposes of comparison on physical aging, PIM was incorporated with 2 wt% MWNT-PEG. Figure 4 shows the life of the o-Ps versus the implantation energy, which can be converted into an average implantation depth. After use, PIM membranes incorporated with MWNT-PEG had significantly longer useful lives. These results clearly indicated that the incorporation of MWNT-PEG reduced the aging of PIM. The gas permeance (N2, O2, and CO2) with fresh and aged membranes (PIM and MMMs) confirmed that the aged pristine PIM membrane showed the highest declines in permeance of 46%, 43%, and 38% for N2, O2, and CO2, respectively. However, the aged MMMs showed 23%, 19%, and 17% relative declines in gas permeance under the same operating conditions. For membranes that were not subjected to aging, MWNT-PEG hardly affected the free volume of MMMs.
o-Ps lifetime versus the implantation energy for samples with and without MWNT-PEG before and after usage. Reproduced from Koschine et al. (2015), Copyright (2021), with permission from John Wiley and Sons
CNTs-MMMs with symmetric dense structure
Most studies have been carried out with CNTs-MMMs with a dense symmetrical structure, because, despite not having a commercial demand, their preparation is simpler. In addition, they are important for the selection of materials with the potential to be used as a dense layer in asymmetric composite membranes.
Generally, considering the same testing and preparation conditions, the transport properties of MMMs incorporated with functionalized CNTs are better than those using unmodified CNTs (Aroon et al. 2010b; Khan et al. 2013; Rajabi et al. 2013; Moghadassi et al. 2014; Sun et al. 2017), as observed for the morphological, thermal and mechanical properties. For example, rMWNT and MWNT-O can increased the permeability and selectivity of CA matrices (Moghadassi et al. 2014). However, with 0.65 wt% rMWNT, CO2 permeability and CO2/CH4 selectivity were 13.41 Barrer and 6.44, respectively. With the same concentration of MWNT-O, the values obtained were 21.81 Barrer and 13.74 for selectivity. Aroon et al. (2010b) prepared MMMs with rMWNT that had an increase in the selectivity of CO2/CH4 by 60.6% (from 10.9 to 17.5); however, the permeability was lower than that of polymeric PI membranes. On the other hand, MMMs incorporated with MWNT-CS, when compared with the PI membrane, increased the permeability to CO2 from 16.83 to 37.31 Barrer, and the selectivity of CO2/CH4 increased by 51.4% (from 10.9 to 16.5).
Sun et al. (2017) observed that, in addition to the functionalization of CNTs, the method of preparing CNT-MMMs was also important. MMMs incorporated with MWNT-O and prepared by in-situ polymerizition had a higher gas separation performance than MMMs prepared by the solution mixing method. Considering 3 wt% of MWNT-O, there was a 48.1% increase in CO2 permeability, from 6.12 Barrer with MMMs using the solution mixing method to 9.06 Barrer with MMMs using in-situ polymerizition, and a 29.5% increase in CO2/N2 selectivity (from 29.14 to 37.74). Due to in-situ polymerizition (i.e., solution-casting followed by subsequent imidization), the interfacial interactions between the phases became stronger and the MWNT-O dispersed more homogeneously in PI.
Wang et al. (2014) prepared three-component MMMs by simple physical mixing of the Pebax solution and the MWNT containing PEG-based polymer solutions of different molecular weight (PEG20000, PEG10000, PEG2000, PEG600, PEG400, Triton X-100, and poly(ethylene glycol) dimethylether (PEGDME)). SEM images showed improved MWNT dispersion within the polymeric matrix because of the hydrophilic modification of the PEG-based polymers. Pure gas permeation experiments showed that incorporating high molecular weight PEG-based polymers favored CO2/CH4 separation, while incorporating low molecular weight PEG-based polymers favored CO2/N2 separation. The incorporation of MWNT increased CO2/N2 selectivity by increasing the amorphous content. On the other hand, it decreased CO2/CH4 selectivity due to enhanced chain mobility.
Li et al. (2015) prepared MMMs with the incorporation of MWNT-O and graphene oxide (GO) in a polymeric matrix of PI. The combination of MWNT-O and GO increased the permeability and selectivity of the membranes. The two fillers had different functions: the smooth walls of the CNTs acted as channels, increasing the permeability of CO2, while the hydroxyl and carboxyl groups present on the surface of the GO nanosheets acted as a selective barrier separating the gases by their polarity. The strong steric effect of GO prevented agglomerations of the CNTs and enabled the homogeneous dispersion of the loads in the MMMs in such a way that it was possible to create several efficient routes for CO2 transport. MMMs that contained CNTs combined with GO had better separation performance than those incorporated only with CNTs or GO. In comparison with the pure PI membrane, the membrane prepared with 5 wt% CNTs and 5 wt% GO (PI-CNTs/GO-5/5) increased permeability to CO2 and the selectivity of CO2/CH4 in 331% and 149%, respectively. Permeation experiments with a mixture of CO2/CH4 (30:70 vol%) and CO2/N2 (10:90 vol%) showed that the overall separation performance of all membranes was reduced due to the competitive sorption between gases. However, the MMMs separation factor was higher compared to the pure PI membrane. This indicated that the combination of MWNT-O/GO contributed to some extent to avoid sorption of non-condensable gases.
To develop MMMs with a Pebax matrix, Li et al. (2020) used a hybrid structure MWNT@ZIF-8 as filler, resulting from the combination of zeolitic imidazolate frameworks-8 (ZIF-8) particles in situ inserted with functionalized MWNT, as shown in Fig. 5. The insertion of MWNT was the way to solve the aggregation problems of ZIF-8 particles. Furthermore, the functionalized MWNT can provide rapid transport channels for CO2 molecules between two adjacent ZIF-8 and enhance the CO2 adsorption properties. So, the improvement of performance in CO2 permeability and CO2/N2 selectivity can be achieved. The performance of MMMs with hybrid particles was superior to that of MMMs prepared by mixing the fillers (MWNT/ZIF). The result obtained using the MMM with 8 wt% hybrid filler (Pebax/MWNT@ZIF-8-8) surpassed the 2008 Robeson upper-bound.
Scheme of MMMs with zoom in on the MWNT@ZIF-8 particle (a) and TEM image of MWNT@ZIF-8 (b). Reprinted from Li et al. (2020), Copyright (2021), with permission from Elsevier
Oxygenated CNTs
The incorporation of oxygenated groups on the surface of CNTs through acid treatment allowed the dispersion and good adhesion of CNTs in different polymers, including glassy ones. MMMs developed with glassy polymers commonly show poor interfacial adhesion between the polymer and the fillers, leading to a significant drop in gas selectivity due to voids and interfacial defects (Wang et al., 2016). Rajabi et al. (2013) observed that MMMs prepared with MWNT-O showed better performance when compared to rMWNT when incorporated in a PVC polymer matrix. The selectivity of CO2/CH4 for the PVC membrane increased from 45.0 to 52.2 with the incorporation of 5 wt% of MWNT-O, while, using rMWNT in the same concentration, the selectivity reduced to 39. Also, the addition of MWNT-O allowed a dramatic increase of the CO2 permeability of the PVC membrane from 0.18 to 11.48 Barrer. Weng et al. (2009) incorporated MWNT-O into a polymeric matrix of poly(bisphenol A-co-4-nitrophthalic anhydride-co-1,3-phenylene diamine) (PBNPI), which allowed an increase in CO2 permeability with a slight drop in selectivity of CO2/CH4.
Sun et al. (2017) prepared MMMs with MWNT-O dispersed in PI. Through Fourier transform infrared spectroscopy (FTIR) it was shown that the acid treatment introduced functional oxygen groups such as hydroxyl (-OH) and carboxylic acid (–COOH) on the surface of the MWNT-O. The permeation experiments showed that CO2 permeability increased 292% with 3 wt% of MWNT-O incorporated in PI, and there was a 145% increase in CO2/N2 selectivity and 144% in CO2/CH4 selectivity. The significant increase in properties was attributed to the rapid gas diffusion through the MWNT-O and the strong interaction of groups inserted in the MWNT-O with CO2. In fact, Ge et al. (2011b), using PES as a polymeric matrix, showed that the CO2 solubility coefficient gradually increased with the incorporation of MWNT-O, while the N2 solubility remained constant. This indicated a greater affinity of the oxygenated groups with CO2, resulting in increased selectivity solubility. On the other hand, the diffusion coefficients of the gases also increased, but they varied practically in the same proportion, resulting in little change in the selectivity of diffusivity.
Aminated CNTs
The good affinity of amines for CO2 is already well known in the absorption processes. Thus, after oxygenated groups, amines are the most explored functional groups for superficial modification of CNTs. Zhao et al. (2017) used three different types of CNTs (rMWNT, MWNT-O and MWNT-NH2) incorporated into Pebax to prepare MMMs. Despite the formation of some clusters, the dispersion of MWNT-O and MWNT-NH2 were more uniform than of rMWNT, indicating that functionalizations contributed to some extent to dispersion. MMMs prepared with MWNT-NH2 showed the highest permeability to CO2. Then, MWNT-NH2 were incorporated into MMMs prepared with Pebax and glycerol triacetate (GTA), an additive recognized for increasing CO2 solubility. GTA also functioned as a plasticizer, improving the dispersion of MWNT-NH2 in Pebax, in addition to increasing the mobility of the polymer chains and the free volume of the MMMs. Thus, the diffusivity and solubility of the gases increased with the increase in the composition of GTA. The CO2 permeability for MMMs prepared with the mass ratios of Pebax, MWNT-NH2 and GTA of 10: 1: 25 was almost ten times greater than Pebax. In addition, the separation of CO2 from other gases, including N2 and CH4, has been significantly improved by MWNT-NH2 and GTA. Table 8 summarizes the transport properties of the CNTs-MMMs with symmetric dense structure.
CNTs-MMMs with asymmetric structure
The dense and thick structure of symmetric MMMs can offer a high resistance to mass transfer; therefore, the incorporation of fillers may not contribute to significant improvements in permeability (Junaidi et al. 2014). In addition, it is assumed that the thin and dense selective layers (~ 100 nm) on the surface of asymmetric membranes have the same permeability as dense and thick membranes (~ 20 μm or more). However, this assumption is not always valid, as the gas permeability of thin films is not only a function of thickness, but also a function of time (Xia et al. 2012). As a result, data from symmetric MMMs may not provide real perspectives on material performance as gas separation membranes. Thus, the development and research of MMMs with an asymmetric structure it is also very important.
An integrally skinned asymmetric membrane consists of a very thin and dense skin layer (0.1–1 µm) overlaying on a thick and highly porous sublayer (100–200 µm), where both layers are composed of the same material and formed in a single operation (Ismail and Yean 2003). Since gas permeance is inversely proportional to the dense-layer thickness, the skin can offer the required selectivity property for membranes with high productivity (permeance or permeability). On the other hand, the effects of the support on separation properties are negligible due to its high porosity. Thus, the main function of the porous support is to provide mechanical resistance for the membrane. In this type of structure, incorporating inorganic fillers into polymeric matrices increases the permeation properties and reinforces the porous structure, reducing the compaction phenomena that can happen during high pressure operations and can lead to deformation of the porous structure and flux decline (Kamal et al. 2020).
Wong et al. (2021) fabricated MMMs using a polymer blend of PEG/PES as the polymeric matrix and MWNT-β-CD as filler through a wet phase-inversion technique. Results showed that MWNT-β-CD were able to enhance gas separation performance of MMMs. Flores and Figueiredo (2020) also developed MMMs using a phase inversion technique by immersion precipitation. Membranes with 0, 1, 3 and 5 wt% of MWNT-O in PSf were prepared. The best results were found for the 1 wt% MWNT-O, with increase in CO2 permeability and ideal selectivity for CO2/N2 compared with pure PSf. The MMMs also exhibited good thermal stability for gas separation and DSC and SEM results revealed that the addition of 1 wt% of the filler caused changes in chain arrangements and membrane morphology.
Aroon et al. (2018) used the immersion precipitation technique to prepare asymmetric MMMs containing different concentrations of CNTs incorporated in the polymeric PES matrix. The rMWNT were purified by acid mixture (HNO3/H2SO4; v/v = 1:3) and then pMWNT were functionalized with LMWC (pMWNT-LMWC). SEM images of MMMs prepared with 0, 1 and 3 wt% pMWNT-LMWC showed that the asymmetric structure of the membranes was formed by a thin dense layer supported by a thick, porous sublayer. In addition, pMWNT-LMWC were well dispersed in the polymer matrix and there was no evidence of agglomeration. The permeation experiments showed that the permeability of CO2 was favored with the increase of the load concentration. With 1 wt% pMWNT-LMWC, compared to the pure PES membrane, CO2 permeability increased from 8.0 to 17.3 GPU and the selectivity of CO2/CH4 raised from 29.6 to 55.8. On the other hand, with 1 wt% of rMWNT, the transport properties were lower than that of pure PES; with the same concentration of pMWNT, there was an improvement only in selectivity. Therefore, the combination of acid treatment purification and functionalization was important to develop high-performance asymmetric CNTs-MMMs. In another study, Aroon et al. (2010a) demonstrated that the PI/rMWNT MMMs prepared by the immersion precipitation technique increased the selectivity of CO2/CH4 more than two fold compared to pure PI by reducing gas permeability.
Mustafa et al. (2012) prepared MMMs with an asymmetric structure using the dry/wet phase inversion method. The polymeric PES matrix was incorporated with modified SWNT and rSWNT. The SWNT were functionalized with chemical modification using Dynasylan Ameo (DA) (SWNT-DA) silane coupling agent. SEM was used to check the asymmetric structure of the membranes. The nanogaps were observed in MMMs prepared with rSWNT, resulting in a drop in the selectivity of CO2/CH4 in relation to the pure PES membrane. On the other hand, the incorporation of SWNT-DA in the same concentration increased the thermal and transport properties of PES. The PES/SWNT-DA membrane (1 wt%) showed a CO2 permeability of 22.18 GPU and a selectivity of CO2/CH4 of 30.42.
However, it is difficult to obtain CNTs/MMMs with an integral asymmetric structure with the dense layer free from defects and with high selective properties. Immersion precipitation is the most widely used technique for preparing asymmetric membranes. In this technique, the polymer plus solvent (polymer solution) are cast on a proper supporting layer and then submerged in a coagulation bath containing a nonsolvent, usually water. To prepare MMMs, CNTs are generally functionalized to improve their dispersion in the polymeric matrix. On the other hand, functionalization increases the hydrophilic character of CNTs and can modify the morphology and performance of membranes due to the competition between hydrophilic and viscous effects (Choi et al. 2006). The incorporation of functionalized fillers in the polymeric matrix can accelerate the diffusion rate from the nonsolvent to the polymeric film, which results in more porous and less selective structures (Shahmirzadi and Kargari 2018).
Therefore, the construction of composite membranes consisting of one or multi-thin layers on top of porous substrates becomes the potential solution to maximize the selectivity. To prepare these membranes, the thin layer is deposited on top of the substrates in two or more stages. Compared with conventional integrally skinned asymmetric membranes, the composite membranes use a smaller amount of material to form the thin selective layer that is deposited on top of porous substrates and this allows for optimization of the membrane material and morphology in each layer independently, according to the product requirements (Liang et al. 2019).
Khan et al. (2012) used a polymeric PIM matrix incorporated with rMWNT and MWNT-PEG to prepare the dense layer of asymmetric composite membranes. The MMMs were manufactured by the dip-coating method using a microporous polyacrylonitrile (PAN) membrane as a support. The gas permeation experiments showed that the incorporation of CNTs increased the permeability of the gases (O2, N2, CO2, CH4) compared to pure PIM. In addition, the PIM/MWNT-PEG membranes performed better in terms of selectivity compared to all developed membranes, with values that reached 37.8 for the separation of CO2/N2 with 3 wt% of MWNT-PEG. MMMs also showed high stability in the long term of operation, indicating a promising alternative for practical application.
Habibiannejad et al. (2016) incorporated MWNT-O, MWNT-NH2 and MWNT modified with Triton X-100 (MWNT-X100) to improve the gas separation abilities of Pebax. The porous PES membrane as support layer was prepared by phase separation via immersion precipitation technique. The active dense layer of the composite membranes was prepared by phase separation via the solvent evaporation technique. Polymer solution with pure Pebax and CNTs was cast on the PES substrate using a stainless-steel casting knife with a polytetrafluoroethylene (PTFE) blade. For CO2/N2 separation, the MMM incorporated with MWNT-NH2 showed a 125% enhancement in selectivity without reduction in the permeability of CO2, compared to the neat membrane, and the MMM containing MWNT-X-100 overcame Robeson’s upper bound. Moreover, the good interfacial interaction between polymer and MWNTs contributed towards suitable load transfer characteristics, resulting in enhancing the Young’s modulus of the Pebax layer.
Sun et al. (2020) combined the ultra-permeable characteristics of a novel bis(phenyl)fluorene-based PIM (Cardo-PIM-1) with CNTs to prepare the dense layer, by casting it onto a PAN substrate membrane. They showed through SEM images that MWNT-NH2 and MWNT-O were well dispersed in the polymer matrix. On the other hand, with rMWNT, the MMMs exhibited heterogeneous agglomeration and cluster formation of rMWNT, due to the interactions between the rMWNT (π–π interaction) being stronger than with the polymer matrix. The best gas separation performance was obtained with MMM incorporated with MWNT-NH2, because -NH2 groups have strong affinity with the CO2 molecules, which enhances CO2 permeability and selectivity. The permeability of CO2 increased from 1.3 × 104 Barrer with Cardo-PIM-1 to 2.9 × 104 Barrer with 7.5 wt% MWNT-NH2 loading, and CO2/N2 selectivity increased from 14.6 to 24.4, crossing the trade-off line for CO2/N2 separation. The effect of CNTs on the transport properties of the asymmetric membranes, including some composite and integrally skinned asymmetric membranes, are summarized in Table 9.
Overview CNTs-MMMs
From the data presented in Tables 8 and 9, it can be seen that, generally, the permeability increases with the increase in the concentration of CNTs until reaching an ideal loading point. This improvement may be associated with changes in the free volume of the polymeric matrix, with an increase in gas diffusion through the interior of CNTs or through the polymer-CNTs interface, and with an increase in solubility of gases on the surface of CNTs, especially for the more condensable ones. Above the ideal loading point, the permeability can increase dramatically due to agglomeration effects, or in other cases, it can be reduced due to the tortuous effect of CNTs. On the other hand, the relationship between the increase in the concentration of CNTs and selectivity is not well defined, and probably depends on the interfacial morphology that the CNTs-MMMs assume. Selectivity can remain practically constant, increase, or even be reduced with the incorporation of CNTs in MMMs. In any event, for the three situations, there is a loading point where selectivity drops dramatically, indicating the limit of CNTs that the polymeric matrix can incorporate without intense agglomeration effects.
The overall separation performance for CNTs-MMMs is compared with Robeson’s upper bounds in Fig. 6. In Fig. 6a and b, it can be seen that, despite the majority of CNTs-MMMs being located below the upper limit of Robeson, the incorporation of CNTs improved the overall performance of polymeric membranes. Some successful strategies for separating CO2/CH4 are shown in Fig. 6c, and for CO2/N2 in Fig. 6d. These results showed that the polymer matrix had its performance improved when incorporated with functionalized CNTs. Another strategy that proved to be very interesting was the combination of CNTs with other fillers. For example, MWNT-O mixed with GO, as explored by Li et al. (2015), or the preparation of a hybrid filler formed by MWNT and ZIF-8 (MWNT@ZIF-8) studied by Li et al. (2020).
Other important observations are shown in Fig. 7. Despite the development of new materials with high permeability to CO2 and high selectivity for CO2/CH4, the membrane material installed in essentially every natural gas processing plants is still CA, because in real operating conditions, such as high pressure and gas mixtures, it manages to maintain separation stability (Baker and Low 2014). Thus, considering the excellent results of transport and mechanical properties obtained by Moghadassi et al. (2014) using CA incorporated with MWNT-O, MMMs can have a great potential for industrial application. Another highlight is the PVC used by Rajabi et al. (2013), that after the incorporation of MWNT-O showed an increase of up to 46 times in the FP of CO2, up to two times in the FS of CO2/CH4 and three times in the FS of CO2/N2. Regarding the structure of the CNTs-MMMs, it is clear that the performance of the symmetrical membranes is superior compared to that of asymmetric membranes, for which most results showed a poor suitability of the CNTs for the separation of CO2/CH4 (Fig. 7a) and fair suitability of the CNTs for CO2/N2 separation (Fig. 7b). Probably, defects in the dense skin prevent the increments in the properties of asymmetric membranes with the incorporation of CNTs from being as high as those recorded for symmetrical membranes. In addition, Figs. 6 and 7 showed that CNTs-MMMs have a better applicability for separating CO2 from N2. This can be verified by the value of Ep. When CNTs are incorporated into the polymeric matrix, the EP of gases increase. However, this increase occurs more intensely for CO2 than for non-condensable gases (Habibiannejad et al. 2016). As verified by Zhao et al. (2014a), N2 has the smallest increase in the value of Ep compared to CH4. Thus, CNTs-MMMs have greater selectivity for CO2/N2 separation than for CO2/CH4 separation.
Therefore, CNTs-MMMs can provide a promising alternative for flue gas separation. However, to make a more accurate assessment, tests with high temperatures are more suitable. For example, Li et al. (2020) chose the Pebax/MWNT-O@ZIF-8 MMMs with the best gas separation performance to carry out experiments to study operating temperature and time effects on the gas separation performance. MMMs were subjected to temperatures of 25–65 °C. Although pure Pebax was evaluated only at a temperature of 35 °C, the permeability of CO2 and the selectivity of CO2/N2 of the MMMs were superior even though they were subjected to the most severe temperatures, which allowed them to state that the gas separation performance of MMMs was better than that of pure Pebax under different temperatures. In addition, the structural stability of MMMs was investigated by carrying out the operation test for 7 days. The MMMs exhibited high and stable separation performance with average CO2 permeability of 188.0 Barrer and CO2/N2 selectivity of 61.2. Similar results were obtained by Zhao et al. (2014a) at temperatures up to 75 °C with Pebax/MWNT-NH2 MMMs. These results show the potential of CNTs-MMMs for CO2/N2 separation.
Membranes prepared with Pebax, PI and PSF presented good results in pilot plants developed for CO2 capture (Scholes 2020). This is very important, considering that successful pilot plant tests demonstrate the technology's ability to operate under industrial conditions. Therefore, because CNTs can improve the properties of these polymers, CNTs-MMMs can have even more promising results.
Conclusions
Membrane-based technology is more favorable in terms of energy and environment compared to the traditional processes used for CO2 separation. However, inorganic membranes are expensive, while polymeric membranes have a trade-off between productivity (permeability) and efficiency (selectivity). Thus, the development of MMMs through the incorporation of inorganic fillers in the polymeric matrix has been extensively explored. In this approach, the combination of the processability of the polymer with the high thermal and chemical stability of the filler can result in a material with superior mechanical, thermal and transport properties compared to the individual materials.
The fillers play a fundamental role for MMMs to achieve high permeability with better selectivity for gas separation. Among a variety of materials, CNTs stand out due to the great potential for chemical adaptation and good mechanical and thermal properties that, if transferred to the polymeric matrix, can result in a high performance MMM. In this sense, this review addressed the main advances made with CNTs-MMMs and discussed their possible application in CO2 separation.
Generally, studies have shown that the functionalization of CNTs improves their interfacial interaction with the polymer and effectively reduces the formation of voids in CNTs-MMMs, resulting in an interfacial morphology closer to the ideal. In addition, the thermal stability, mechanical strength and transport properties of MMMs prepared with modified CNTs were superior compared to MMMs prepared with unmodified CNTs or the respective polymeric matrices.
Under real operating conditions, membranes can suffer from plasticization and physical aging. The permeation results obtained with mixtures of gases or a single gas at high pressures, as well as measurements of free volume by PALS, showed that the incorporation of CNTs in the polymeric matrices can reduce these problems.
Therefore, the results presented in this review are quite encouraging and indicate that some CNTs-MMMs can be used for industrial applications. Polymer-CNT systems grouped in regions C and D or that exceed the Robeson limit can be selected to prepare MMMs according to the specifics of each process. The greatest application potential of CNTs-MMMs is for CO2/N2 separation.
However, it should be noted that most of the work was carried out with CNTs-MMMs prepared with dense symmetrical structures. On the other hand, industrial demands require asymmetric membranes to obtain greater productivity. In these membranes, the probability of defects in the selective layer can increase with the incorporation of CNTs, which decreases the selectivity compared to the polymeric matrix. As a result, for asymmetric CNTs-MMMs, most of the CO2/CH4 separation factors are found in the trade-off region (poor efficiency) and most of the CO2/N2 separation factors are found in the A region (fair efficiency). Thus, some efforts still need to be made to improve the preparation of these membranes and make them commercially attractive.
Finally, the performance of CNTs-MMMs is generally examined using a single gas, low pressure and room temperature. Purifications of a mixture of gases under real operating conditions are rarely reported. Under real operating conditions, problems such as competitive sorption between gases, plasticization, compaction and physical aging can dramatically affect membrane performance. For future work, performance tests of CNTs-MMMs using real operating conditions should be considered.
References
Adewole JK, Ahmad AL, Ismail S, Leo CP (2013) Current challenges in membrane separation of CO2 from natural gas: a review. Int J Greenh Gas Control 17:46–65. https://doi.org/10.1016/j.ijggc.2013.04.012
Ahmad AL, Jawad ZA, Low SC, Zein SHS (2014) A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J Memb Sci 451:55–66. https://doi.org/10.1016/j.memsci.2013.09.043
Ahmad NNR, Mukhtar H, Mohshim DF et al (2016) Surface modification in inorganic filler of mixed matrix membrane for enhancing the gas separation performance. Rev Chem Eng 32:181–200. https://doi.org/10.1515/revce-2015-0031
Alcheikhhamdon Y, Hoorfar M (2017) Natural gas purification from acid gases using membranes: a review of the history, features, techno-commercial challenges, and process intensification of commercial membranes. Chem Eng Process Process Intensif 120:105–113. https://doi.org/10.1016/j.cep.2017.07.009
Alqaheem Y, Alomair A, Vinoba M, Pérez A (2017) Polymeric gas-separation membranes for petroleum refining. Int J Polym Sci 2017:1–20. https://doi.org/10.1155/2017/4250927
Amirkhani F, Mosadegh M, Asghari M, Parnian MJ (2020) The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym Test 82:1–14. https://doi.org/10.1016/j.polymertesting.2019.106285
Anastasiou S, Bhoria N, Pokhrel J et al (2018) Metal-organic framework graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater Chem Phys 212:513–522. https://doi.org/10.1016/j.matchemphys.2018.03.064
Anderson TR, Hawkins E, Jones PD (2016) CO2, the greenhouse effect and global warming: from the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 40:178–187. https://doi.org/10.1016/j.endeavour.2016.07.002
Ansaloni L, Deng L (2017) Advances in polymer-inorganic hybrids as membrane materials. In: Recent developments in polymer macro, micro and nano blends: preparation and characterisation. Elsevier Ltd, pp 163–206
Arash B, Wang Q, Varadan VK (2014) Mechanical properties of carbon nanotube/polymer composites. Sci Rep 4:1–8. https://doi.org/10.1038/srep06479
Aroon MA, Ismail AF, Montazer-Rahmati MM, Matsuura T (2010a) Effect of raw multi-wall carbon nanotubes on morphology and separation properties of polyimide membranes. Sep Sci Technol 45:2287–2297. https://doi.org/10.1080/01496395.2010.484007
Aroon MA, Ismail AF, Montazer-Rahmati MM, Matsuura T (2010b) Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multi-walled carbon nanotubes mixed matrix flat sheet membranes. J Memb Sci 364:309–317. https://doi.org/10.1016/j.memsci.2010.08.023
Aroon MA, Ismail AF, Matsuura T (2013) Beta-cyclodextrin functionalized MWCNT: a potential nano-membrane material for mixed matrix gas separation membranes development. Sep Purif Technol 115:39–50. https://doi.org/10.1016/j.seppur.2013.04.025
Aroon MA, Beheshti H, Barzin J, Shariaty-Niassar M (2018) Purified and functionalized MWCNTs: application in CO2/CH4 separation using mixed matrix membranes. Int J Nanosci Nanotechnol 14:251–266
Asghari M, Afsari M (2018) Effect of ethylene oxide functional groups in PEBA-CNT membranes on CO2/CH4 mixed gas separation. J Membr Sci Res 4:34–40. https://doi.org/10.22079/jmsr.2017.61016.1131
Awad S, Chen HM, Grady BP et al (2012) Positron annihilation spectroscopy of polystyrene filled with carbon nanomaterials. Macromolecules 45:933–940. https://doi.org/10.1021/ma202458c
Baker RW, Lokhandwala K (2008) Natural gas processing with membranes: an overview. Ind Eng Chem Res 47:2109–2121. https://doi.org/10.1021/ie071083w
Baker RW, Low BT (2014) Gas separation membrane materials: a perspective. Macromolecules 47:6999–7013. https://doi.org/10.1021/ma501488s
Baker RW, Freeman B, Kniep J et al (2018) CO2 capture from cement plants and steel mills using membranes. Ind Eng Chem Res 57:15963–15970. https://doi.org/10.1021/acs.iecr.8b02574
Bastani D, Esmaeili N, Asadollahi M (2013) Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications : a review. J Ind Eng Chem 19:375–393. https://doi.org/10.1016/j.jiec.2012.09.019
Borhani TNG, Azarpour A, Akbari V et al (2015) CO2 capture with potassium carbonate solutions: a state-of-the-art review. Int J Greenh Gas Control 41:142–162. https://doi.org/10.1016/j.ijggc.2015.06.026
British Petroleum (2020) Statistical Review of World Energy. London. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf?utm_source=BP_Global_GroupCommunications_UK_external&utm_medium=email&utm_campaign=11599394_Statistical%20Review%202020%20-%20on%20the%20day%20reminder&dm_i=1PGC%2C6WM5E%2COV0LQ4%2CRQW75%2C1. Accessed 21 Jan 2021
Cangialosi D, Boucher VM, Alegría A, Colmenero J (2013) Physical aging in polymers and polymer nanocomposites: recent results and open questions. Soft Matter 9:8619–8630. https://doi.org/10.1039/c3sm51077h
Cao Q, Yu Q, Connell DW, Yu G (2013) Titania carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technol Environ Policy 15:871–880. https://doi.org/10.1007/s10098-013-0581-y
Casado-Coterillo C (2019) Mixed matrix membranes. Membranes (Basel) 9:1–5. https://doi.org/10.3390/membranes9110149
Castro VG, Costa IB, Lopes MC et al (2017) Tailored degree of functionalization and length preservation of multiwalled carbon nanotubes by an optimized acid treatment process. J Braz Chem Soc 28:1158–1166. https://doi.org/10.21577/0103-5053.20160274
Choi JH, Jegal J, Kim WN (2006) Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J Memb Sci 284:406–415. https://doi.org/10.1016/j.memsci.2006.08.013
Cohen-Tanugi D, Grossman JC (2014) Mechanical strength of nanoporous graphene as a desalination membrane. Nano Lett 14:6171–6178. https://doi.org/10.1021/nl502399y
Cong H, Zhang J, Radosz M, Shen Y (2007) Carbon nanotube composite membranes of brominated poly(2,6-diphenyl-1,4-phenylene oxide) for gas separation. J Memb Sci 294:178–185. https://doi.org/10.1016/j.memsci.2007.02.035
Cormos A-M, Dragan S, Petrescu L et al (2020) Techno-economic and environmental evaluations of decarbonized fossil-intensive industrial processes by reactive absorption & adsorption CO2 capture systems. Energies 13:1–16. https://doi.org/10.3390/en13051268
Crespo JG, Brazinha C (2015) Fundamentals of pervaporation. In: Basile A, Figoli A, Khayet M (eds) Pervaporation, vapour permeation and membrane distillation. Woodhead Publishing, Oxford, pp 3–17
Dai L, Sun J (2016) Mechanical properties of carbon nanotubes-polymer composites. In: Berber M (ed) Carbon nanotubes—current progress of their polymer composites. Intech, London, pp 155–194
Dai Z, Ansaloni L, Deng L (2016) Recent advances in multi-layer composite polymeric membranes for CO2 separation: a review. Green Energy Environ 1:102–128. https://doi.org/10.1016/j.gee.2016.08.001
Dalane K, Dai Z, Mogseth G et al (2017) Potential applications of membrane separation for subsea natural gas processing: a review. J Nat Gas Sci Eng 39:101–117. https://doi.org/10.1016/j.jngse.2017.01.023
Dalane K, Svendsen HF, Hillestad M, Deng L (2018) Membrane contactor for subsea natural gas dehydration: model development and sensitivity study. J Memb Sci 556:263–276. https://doi.org/10.1016/j.memsci.2018.03.033
Dalane K, Hillestad M, Deng L (2019) Subsea natural gas dehydration with membrane processes: simulation and process optimization. Chem Eng Res Des 142:257–267. https://doi.org/10.1016/j.cherd.2018.12.027
Dilshad MR, Islam A, Haider B et al (2020) Novel PVA/PEG nano-composite membranes tethered with surface engineered multi-walled carbon nanotubes for carbon dioxide separation. Microporous Mesoporous Mater 308:1–13. https://doi.org/10.1016/j.micromeso.2020.110545
Dixon-Garrett SV, Nagai K, Freeman BD (2000) Ethylbenzene solubility, diffusivity, and permeability in. J Polym Sci B Polym Phys 38:1461–1473
Flauzino Neto WP, Mariano M, da Silva ISV et al (2016) Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr Polym 153:143–152. https://doi.org/10.1016/j.carbpol.2016.07.073
Flores MC, de Figueiredo KC (2020) Carbon dioxide/nitrogen separation through oxygenated carbon nanotubes-containing polysulfone membranes. Macromol Symp 394:1–5. https://doi.org/10.1002/masy.202000058
Friedlingstein P, Jones MW, O’Sullivan M et al (2019) Global carbon budget 2019. Earth Syst Sci Data 11:1783–1838. https://doi.org/10.5194/essd-11-1783-2019
Galizia M, Chi WS, Smith ZP et al (2017) 50th anniversary perspective: polymers and mixed matrix membranes for gas and vapor separation: a review and prospective opportunities. Macromolecules 50:7809–7843. https://doi.org/10.1021/acs.macromol.7b01718
Gallucci F, Fernandez E, Corengia P, van Sint AM (2013) Recent advances on membranes and membrane reactors for hydrogen production. Chem Eng Sci 92:40–66. https://doi.org/10.1016/j.ces.2013.01.008
Gallucci F, Medrano JA, Fernandez E et al (2017) Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purifcation and production. J Membr Sci Res 3:142–156. https://doi.org/10.22079/jmsr.2017.23644
Ge L, Zhu Z, Li F et al (2011a) Investigation of Gas Permeability in carbon nanotube (CNT)-polymer matrix membranes via modifying CNTs with functional groups/metals and controlling modification location. J Phys Chem C 115:6661–6670. https://doi.org/10.1021/jp1120965
Ge L, Zhu Z, Rudolph V (2011b) Enhanced gas permeability by fabricating functionalized multi-walled carbon nanotubes and polyethersulfone nanocomposite membrane. Sep Purif Technol 78:76–82. https://doi.org/10.1016/j.seppur.2011.01.024
Grande CA, Roussanaly S, Anantharaman R et al (2017) CO2 capture in natural gas production by adsorption processes. Energy Proc 114:2259–2264. https://doi.org/10.1016/j.egypro.2017.03.1363
Habibiannejad SA, Aroujalian A, Raisi A (2016) Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv 6:79563–79577. https://doi.org/10.1039/c6ra14141b
Harami HR, Amirkhani F, Khadem SA et al (2019a) Mass transfer through PDMS/zeolite 4A MMMs for hydrogen separation: Molecular dynamics and grand canonical Monte Carlo simulations. Int Commun Heat Mass Transf 108:104259. https://doi.org/10.1016/j.icheatmasstransfer.2019.05.005
Harami HR, Fini FR, Rezakazemi M, Shirazian S (2019b) Sorption in mixed matrix membranes : experimental and molecular dynamic simulation and Grand Canonical Monte Carlo method. J Mol Liq 282:566–576. https://doi.org/10.1016/j.molliq.2019.03.047
Hasegawa H, Nishida K, Oguro S, Fujimura Y (2017) Gas separation process for CO2 removal from natural gas with DDR-type zeolite membrane. Energy Proc 114:32–36. https://doi.org/10.1016/j.egypro.2017.03.1143
Ismail AF, Yean LP (2003) Review on the development of defect-free and ultrathin-skinned asymmetric membranes for gas separation through manipulation of phase inversion and rheological factors. J Appl Polym Sci 88:442–451. https://doi.org/10.1002/app.11744
Ismail AF, Rahim NH, Mustafa A et al (2011) Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep Purif Technol 80:20–31. https://doi.org/10.1016/j.seppur.2011.03.031
Jeon I-Y, Chang DW, Kumar NA, Baek J-B (2011) Functionalization of carbon nanotubes. In: Yellampalli S (ed) Carbon nanotubes—polymer nanocomposites. InTech, Shanghai, pp 91–110
Junaidi MUM, Leo CP, Ahmad AL et al (2014) Carbon dioxide separation using asymmetric polysulfone mixed matrix membranes incorporated with SAPO-34 zeolite. Fuel Process Technol 118:125–132. https://doi.org/10.1016/j.fuproc.2013.08.009
Kamal N, Ahzi S, Kochkodan V (2020) Polysulfone/halloysite composite membranes with low fouling properties and enhanced compaction resistance. Appl Clay Sci 199:1–20. https://doi.org/10.1016/j.clay.2020.105873
Karimi M, Solati N, Amiri M et al (2015) Carbon nanotubes part I: preparation of a novel and versatile drug-delivery vehicle. Expert Opin Drug Deliv 12:1071–1087. https://doi.org/10.1517/17425247.2015.1003806
Khalilpour R, Mumford K, Zhai H et al (2015) Membrane-based carbon capture from flue gas: a review. J Clean Prod 103:286–300. https://doi.org/10.1016/j.jclepro.2014.10.050
Khan MM, Filiz V, Bengtson G et al (2012) Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res Lett 7:1–12. https://doi.org/10.1186/1556-276X-7-504
Khan MM, Filiz V, Bengtson G et al (2013) Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J Memb Sci 436:109–120. https://doi.org/10.1016/j.memsci.2013.02.032
Kim S, Pechar TW, Marand E (2006) Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 192:330–339. https://doi.org/10.1016/j.desal.2005.03.098
Kim S, Chen L, Johnson JK, Marand E (2007) Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: theory and experiment. J Memb Sci 294:147–158. https://doi.org/10.1016/j.memsci.2007.02.028
Kobayashi N, Izumi H, Morimoto Y (2017) Review of toxicity studies of carbon nanotubes. J Occup Health 59:394–407. https://doi.org/10.1539/joh.17-0089-RA
Koschine T, Rätzke K, Faupel F et al (2015) Correlation of gas permeation and free volume in new and used high free volume thin film composite membranes. J Polym Sci B Polym Phys 53:213–217. https://doi.org/10.1002/polb.23616
Langer E, Bortel K, Waskiewicz S, Lenartowicz-Klik M (2020) Essential quality parameters of plasticizers. In: Langer E, Bortel K, Lenartowicz-Klik M, Waskiewicz S (eds) Plasticizers derived from post-consumer PET. William Andrew Publishing, Netherlands, pp 45–100
Li X, Ma L, Zhang H et al (2015) Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for effi cient CO2 separation. J Memb Sci 479:1–10. https://doi.org/10.1016/j.memsci.2015.01.014
Li X, Yu S, Li K et al (2020) Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep Purif Technol 248:1–9. https://doi.org/10.1016/j.seppur.2020.117080
Liang CZ, Chung TS, Lai JY (2019) A review of polymeric composite membranes for gas separation and energy production. Prog Polym Sci 97:1–18. https://doi.org/10.1016/j.progpolymsci.2019.06.001
Lock SSM, Lau KK, Shariff AM et al (2018) Mathematical modelling of thickness and temperature dependent physical aging to O2/N2 gas separation in polymeric membranes. RSC Adv 8:30265–30279. https://doi.org/10.1039/c8ra05323e
Madani SY, Tan A, Dwek M, Seifalian AM (2012) Functionalization of single-walled carbon nanotubes and their binding to cancer cells. Int J Nanomed 7:905–914. https://doi.org/10.2147/IJN.S25035
Mallakpour S, Soltanian S (2016) Surface functionalization of carbon nanotubes: fabrication and applications. RSC Adv 6:109916–109935. https://doi.org/10.1039/c6ra24522f
Mangukiya S, Prajapati S, Kumar S et al (2016) Polysulfone-based composite membranes with functionalized carbon nanotubes show controlled porosity and enhanced electrical conductivity. J Appl Polym Sci 133:1–9. https://doi.org/10.1002/app.43778
Matteucci S, Kusuma VA, Sanders D et al (2008) Gas transport in TiO2 nanoparticle-filled poly(1-trimethylsilyl-1-propyne). J Memb Sci 307:196–217. https://doi.org/10.1016/j.memsci.2007.09.035
Merkel TC, Lin H, Wei X, Baker R (2010) Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Memb Sci 359:126–139. https://doi.org/10.1016/j.memsci.2009.10.041
Minelli M, Sarti GC (2013) Permeability and diffusivity of CO2 in glassy polymers with and without plasticization. J Memb Sci 435:176–185. https://doi.org/10.1016/j.memsci.2013.02.013
Minelli M, Oradei S, Fiorini M, Sarti GC (2019) CO2 plasticization effect on glassy polymeric membranes. Polymer (Guildf) 163:29–35. https://doi.org/10.1016/j.polymer.2018.12.043
Mirqasemi MS, Homayoonfal M, Rezakazemi M (2020) Zeolitic imidazolate framework membranes for gas and water purification. Environ Chem Lett 18:1–52. https://doi.org/10.1007/s10311-019-00933-6
Mittal G, Dhand V, Rhee KY et al (2015) A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J Ind Eng Chem 21:11–25. https://doi.org/10.1016/j.jiec.2014.03.022
Moghadassi AR, Rajabi Z, Hosseini SM, Mohammadi M (2014) Fabrication and modification of cellulose acetate based mixed matrix membrane: gas separation and physical properties. J Ind Eng Chem 20:1050–1060. https://doi.org/10.1016/j.jiec.2013.06.042
Mokhatab S, Poe WA, Mak JY (2019) Natural gas treating. In: Mokhatab S, Poe WA, Mak JY (eds) Handbook of natural gas transmission and processing. Gulf Professional Publishing, pp 231–269
Mukaddam M, Litwiller E, Pinnau I (2016) Gas sorption, diffusion, and permeation in nafion. Macromolecules 49:280–286. https://doi.org/10.1021/acs.macromol.5b02578
Mukhtar A, Saqib S, Mellon NB et al (2020) CO2 capturing, thermo-kinetic principles, synthesis and amine functionalization of covalent organic polymers for CO2 separation from natural gas: a review. J Nat Gas Sci Eng 77:103203. https://doi.org/10.1016/j.jngse.2020.103203
Murai S, Kato Y, Maezawa Y et al (2013) Novel hindered amine absorbent for CO2 capture. Energy Proc 37:417–422. https://doi.org/10.1016/j.egypro.2013.05.126
Murali RS, Sridhar S, Sankarshana T, Ravikumar YVL (2010) Gas permeation behavior of pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind Eng Chem Res 49:6530–6538. https://doi.org/10.1021/ie9016495
Mustafa A, Kusworo TD, Busairi A et al (2012) Increasing the performance of PES-CNTs mixed matrix membrane using carbon nanotubes (CNTs) functionalization. Int J Waste Resour 2:22–24. https://doi.org/10.4172/2252-5211.1000109
Norahim N, Yaisanga P, Faungnawakij K et al (2018) Recent membrane developments for CO2 separation and capture. Chem Eng Technol 41:211–223. https://doi.org/10.1002/ceat.201700406
Ohs B, Falkenberg M, Wessling M (2019) Optimizing hybrid membrane-pressure swing adsorption processes for biogenic hydrogen recovery. Chem Eng J 364:452–461. https://doi.org/10.1016/j.cej.2019.01.136
Oko E, Wang M, Joel AS (2017) Current status and future development of solvent-based carbon capture. Int J Coal Sci Technol 4:5–14. https://doi.org/10.1007/s40789-017-0159-0
Ong YT, Ahmad AL, Hussein S et al (2010) A review on carbon nanotubes in an environmental protection and green engineering perspective. J Chem Eng 27:227–242. https://doi.org/10.1590/S0104-66322010000200002
Rajabi Z, Moghadassi AR, Hosseini SM, Mohammadi M (2013) Preparation and characterization of polyvinylchloride based mixed matrix membrane filled with multi walled carbon nano tubes for carbon dioxide separation. J Ind Eng Chem 19:347–352. https://doi.org/10.1016/j.jiec.2012.08.023
Ramírez-Santos ÁA, Castel C, Favre E (2018) A review of gas separation technologies within emission reduction programs in the iron and steel sector: current application and development perspectives. Sep Purif Technol 194:425–442. https://doi.org/10.1016/j.seppur.2017.11.063
Rezakazemi M, Ebadi Amooghin A, Montazer-Rahmati MM et al (2014) State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): an overview on current status and future directions. Prog Polym Sci 39:817–861. https://doi.org/10.1016/j.progpolymsci.2014.01.003
Rezakazemi M, Heydari I, Zhang Z (2017) Hybrid systems: combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J CO2 Util 18:362–369. https://doi.org/10.1016/j.jcou.2017.02.006.
Rezakazemi M, Sadrzadeh M, Matsuura T (2018) Thermally stable polymers for advanced high-performance gas separation membranes. Prog Energy Combust Sci 66:1–41. https://doi.org/10.1016/j.pecs.2017.11.002
Ricci E, De Angelis MG (2019) Modelling mixed-gas sorption in glassy polymers for CO2 removal: a sensitivity analysis of the dual mode sorption model. Membranes (Basel) 9:1–26. https://doi.org/10.3390/membranes9010008
Robeson LM (1991) Correlation of separation factor versus permeability for polymeric membranes. J Memb Sci 62:165–185. https://doi.org/10.1016/0376-7388(91)80060-J
Robeson LM (2008) The upper bound revisited. J Memb Sci 320:390–400. https://doi.org/10.1016/j.memsci.2008.04.030
Rowe BW, Robeson LM, Freeman BD, Paul DR (2010) Influence of temperature on the upper bound: theoretical considerations and comparison with experimental results. J Memb Sci 360:58–69. https://doi.org/10.1016/j.memsci.2010.04.047
Roy S, Singha NR (2017) Polymeric nanocomposite membranes for next generation pervaporation process: strategies, challenges and future prospects. Membranes (Basel). https://doi.org/10.3390/membranes7030053
Safari M, Ghanizadeh A, Montazer-Rahmati MM (2009) Optimization of membrane-based CO2-removal from natural gas using simple models considering both pressure and temperature effects. Int J Greenh Gas Control 3:3–10. https://doi.org/10.1016/j.ijggc.2008.05.001
Sanders DF, Smith ZP, Guo R et al (2013) Energy-efficient polymeric gas separation membranes for a sustainable future: a review. Polymer (Guildf) 54:4729–4761. https://doi.org/10.1016/j.polymer.2013.05.075
Sarfraz M, Ba-Shammakh M (2018) Harmonious interaction of incorporating CNTs and zeolitic imidazole frameworks into polysulfone to prepare high performance MMMS for CO2 separation from humidified post combustion gases. Brazilian J Chem Eng 35:217–228. https://doi.org/10.1590/0104-6632.20180351s20150595
Scholes CA (2020) Pilot plants of membrane technology in industry: challenges and key learnings. Front Chem Sci Eng 14:305–316. https://doi.org/10.1007/s11705-019-1860-x
Setiawan WK, Chiang KY (2019) Silica applied as mixed matrix membrane inorganic filler for gas separation: a review. Sustain Environ Res 1:1–21. https://doi.org/10.1186/s42834-019-0028-1
Shahmirzadi MAA, Kargari A (2018) Nanocomposite membranes. Emerging technologies for sustainable desalination handbook. Elsevier Inc, Amsterdam, pp 285–330
Sharif A (2018) Polymeric gas separation membranes: what makes them industrially more attractive? J Membr Sci Res 4:2–3. https://doi.org/10.22079/jmsr.2017.71695.1158
Sodeifian G, Raji M, Asghari M et al (2019) Polyurethane-SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. Chinese J Chem Eng 27:322–334. https://doi.org/10.1016/j.cjche.2018.03.012
Sohaib Q, Muhammad A, Younas M et al (2021) Rigorous non-isothermal modeling approach for mass and energy transport during CO2 absorption into aqueous solution of amino acid ionic liquids in hollow fiber membrane contactors. Sep Purif Technol 254:117644. https://doi.org/10.1016/j.seppur.2020.117644
Soleymanipour SF, Dehaghani AHS, Pirouzfar V, Alihosseini A (2016) The morphology and gas-separation performance of membranes comprising multiwalled carbon nanotubes/polysulfone—Kapton. J Appl Polym Sci 133:1–8. https://doi.org/10.1002/app.43839
Sreenivasulu B, Gayatri DV, Sreedhar I, Raghavan KV (2015) A journey into the process and engineering aspects of carbon capture technologies. Renew Sustain Energy Rev 41:1324–1350. https://doi.org/10.1016/j.rser.2014.09.029
Sun H, Wang T, Xu Y et al (2017) Fabrication of polyimide and functionalized multi-walled carbon nanotubes mixed matrix membranes by in-situ polymerization for CO2 separation. Sep Purif Technol 177:327–336. https://doi.org/10.1016/j.seppur.2017.01.015
Sun H, Gao W, Zhang Y et al (2020) Bis(phenyl)fluorene-based polymer of intrinsic microporosity/functionalized multi-walled carbon nanotubes mixed matrix membranes for enhanced CO2 separation performance. React Funct Polym 147:1–10. https://doi.org/10.1016/j.reactfunctpolym.2019.104465
Swapna VP, Abhisha VS, Stephen R (2020) Polymer/polyhedral oligomeric silsesquioxane nanocomposite membranes for pervaporation. In: Thomas S, George SC, Jose T (eds) Polymer nanocomposite membranes for pervaporation. Elsevier Inc, Amsterdam, pp 201–229
Swati IK, Sohaib Q, Cao S et al (2021) Protic/aprotic ionic liquids for effective CO2 separation using supported ionic liquid membrane. Chemosphere 267:128894. https://doi.org/10.1016/j.chemosphere.2020.128894
Taghizadeh M, Aghili F (2019) Recent advances in membrane reactors for hydrogen production by steam reforming of ethanol as a renewable resource. Rev Chem Eng 35:377–392. https://doi.org/10.1515/revce-2017-0083
Tanh Jeazet HB, Koschine T, Staudt C et al (2013) Correlation of gas permeability in a metal-organic framework MIL-101(Cr)-polysulfone mixed-matrix membrane with free volume measurements by positron annihilation lifetime spectroscopy (PALS). Membranes (Basel) 3:331–353. https://doi.org/10.3390/membranes3040331
Tofighy MA, Mohammadi T (2020) Carbon nanotubes-polymer nanocomposite membranes for pervaporation. In: Thomas S, George SC, Jose T (eds) polymer nanocomposite membranes for pervaporation. Elsevier Inc, Amsterdam, pp 105–133
Vinoba M, Bhagiyalakshmi M, Alqaheem Y et al (2017) Recent progress of fillers in mixed matrix membranes for CO2 separation: a review. Sep Purif Technol 188:431–450. https://doi.org/10.1016/j.seppur.2017.07.051
Wang S, Liu Y, Huang S et al (2014) Pebax-PEG-MWCNT hybrid membranes with enhanced CO2 capture properties. J Memb Sci 460:62–70. https://doi.org/10.1016/j.memsci.2014.02.036
Wang S, Li X, Wu H et al (2016) Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ Sci 9:1863–1890. https://doi.org/10.1039/C6EE00811A
Wang H, Zhang K, Ho Li JP et al (2020) Engineering plasticization resistant gas separation membranes using metal-organic nanocapsules. Chem Sci 11:4687–4694. https://doi.org/10.1039/d0sc01498b
Weng TH, Tseng HH, Wey MY (2009) Preparation and characterization of multi-walled carbon nanotube/PBNPI nanocomposite membrane for H2/CH4 separation. Int J Hydrogen Energy 34:8707–8715. https://doi.org/10.1016/j.ijhydene.2009.08.027
Wong KK, Jawad ZA, Chin BLF (2021) A polyethylene glycol (PEG) – polyethersulfone (PES)/multi-walled carbon nanotubes (MWCNTs) polymer blend mixed matrix membrane for CO2/N2 separation. J Polym Res 28:1–19. https://doi.org/10.1007/s10965-020-02361-5
Xia J, Chung TS, Li P et al (2012) Aging and carbon dioxide plasticization of thin polyetherimide films. Polymer (Guildf) 53:2099–2108. https://doi.org/10.1016/j.polymer.2012.03.009
Xiao Y, Low BT, Hosseini SS et al (2009) The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—a review. Prog Polym Sci 34:561–580. https://doi.org/10.1016/j.progpolymsci.2008.12.004
Xie K, Fu Q, Qiao GG, Webley PA (2019) Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J Memb Sci 572:38–60. https://doi.org/10.1016/j.memsci.2018.10.049
Xue Q, Pan X, Li X et al (2017) Effective enhancement of gas separation performance in mixed matrix membranes using core/shell structured multi-walled carbon nanotube/graphene oxide nanoribbons. Nanotechnology 28:1–10. https://doi.org/10.1088/1361-6528/aa510d
Yang G, Xie Z, Cran M et al (2020) Dimensional nanofillers in mixed matrix membranes for pervaporation separations: a review. Membranes (Basel) 10:1–27. https://doi.org/10.3390/membranes10090193
Yeo ZY, Chew TL, Zhu PW et al (2012) Conventional processes and membrane technology for carbon dioxide removal from natural gas: a review. J Nat Gas Chem 21:282–298. https://doi.org/10.1016/S1003-9953(11)60366-6
Yetgin SH (2019) Effect of multi walled carbon nanotube on mechanical, thermal and rheological properties of polypropylene. J Mater Res Technol 8:4725–4735. https://doi.org/10.1016/j.jmrt.2019.08.018
Younas M, Rezakazemi M, Daud M et al (2020a) Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2020.100849
Younas M, Tahir T, Wu C et al (2020b) Post-combustion CO2 capture with sweep gas in thin film composite (TFC) hollow fiber membrane (HFM) contactor. J CO2 Util 40:101266. https://doi.org/10.1016/j.jcou.2020.101266
Yu B, Cong H, Li Z et al (2013) Pebax-1657 nanocomposite membranes incorporated with nanoparticles/ colloids/carbon nanotubes for CO2/N2 and CO2/H2 separation. J Appl Polym Sci 130:2867–2876. https://doi.org/10.1002/app.39500
Zhang M, Deng L, Xiang D et al (2019) Approaches to suppress CO2-induced plasticization of polyimide membranes in gas separation applications. Processes 7:1–31. https://doi.org/10.3390/pr7010051
Zhang N, Pan Z, Zhang Z et al (2020a) CO2 capture from coalbed methane using membranes: a review. Environ Chem Lett 18:79–96. https://doi.org/10.1007/s10311-019-00919-4
Zhang Q, Li S, Wang C et al (2020b) Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J Memb Sci 598:1–11. https://doi.org/10.1016/j.memsci.2019.117794
Zhao L, Riensche E, Menzer R et al (2008) A parametric study of CO2/N2 gas separation membrane processes for post-combustion capture. J Memb Sci 325:284–294. https://doi.org/10.1016/j.memsci.2008.07.058
Zhao D, Ren J, Li H et al (2014a) Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J Memb Sci 467:41–47. https://doi.org/10.1016/j.memsci.2014.05.009
Zhao Y, Jung BT, Ansaloni L, Ho WSW (2014b) Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J Memb Sci 459:233–243. https://doi.org/10.1016/j.memsci.2014.02.022
Zhao D, Ren J, Wang Y et al (2017) High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J Memb Sci 521:104–113. https://doi.org/10.1016/j.memsci.2016.08.061
Zhou K, Chaemchuen S, Verpoort F (2017) Alternative materials in technologies for biogas upgrading via CO2 capture. Renew Sustain Energy Rev 79:1414–1441. https://doi.org/10.1016/j.rser.2017.05.198
Acknowledgements
Marcelo Flores thanks to the Brazilian governmental agency Coordination of Improvement of Higher Education Personnel (CAPES) for his fellowship. The authors are also grateful to the manuscript reviewers for their important contributions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception. Material preparation, data collection and analysis were performed by MCF, BJAG and KCSF. The first draft of the manuscript was written by MCF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flores, M.C., Gonçalves, B.J.A. & Figueiredo, K.C. CO2 separation by mixed matrix membranes incorporated with carbon nanotubes: a review of morphological, mechanical, thermal and transport properties. Braz. J. Chem. Eng. 38, 777–810 (2021). https://doi.org/10.1007/s43153-021-00165-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00165-8