Abstract
ICSI may face fertilization failure, prompting the use of assisted oocyte activation (AOA) techniques. While AOA is implemented in infertility clinics, its target patients and definitive application remain uncertain. This systematic review adheres to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to assess reproductive outcomes in ICSI-AOA cycles compared to conventional ICSI and evaluate AOA effectiveness in various infertility disorders. A literature search encompassed PubMed, Web of Science, EMBASE, and Scopus databases until December 2023 for relevant English studies. Included studies compared ICSI-AOA with conventional ICSI in couples with prior fertilization failure, utilizing diverse AOA methods. Control groups consisted of sibling oocytes, previous cycles of the same couples, or couples undergoing conventional ICSI. Evaluated outcomes included fertilization, cleavage, embryo quality, implantation, pregnancy, and live birth rates. Article screening and data extraction were performed by two authors, with risk of bias assessed by another investigator. Out of 3088 initially identified articles, 30 studies were included, focusing on fertilization failure (n = 10), female infertility (n = 3), PLCζ defects (n = 4), poor sperm quality (n = 4), Globozoospermia (n = 4), and surgically retrieved sperm (n = 8). Most studies concluded that AOA could overcome fertilization failure, but success rates varied based on sperm-related or oocyte-related factors in ICSI-AOA cycles. Due to differences in patient inclusion criteria and sample sizes, most studies were not sufficiently similar for pooled analysis, limiting robust conclusions. There is insufficient evidence, particularly from randomized controlled trials (RCTs), to determine the efficacy or safety of ICSI-AOA as a treatment strategy. Registration number is PROSPERO, CRD42024551221.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The improvements in assisted reproductive technology (ART) have significantly increased success rate in the treatment outcomes of infertile couples worldwide. Applying the technique known as intracytoplasmic sperm injection (ICSI) provides the direct injection of a single spermatozoon into a mature oocyte. This procedure is commonly utilized in cases of severe male factor infertility and also in previous unsuccessful attempts of in vitro fertilization (IVF) cycles [1]. ICSI has a fertilization rate of approximately 70%. Nonetheless, full or almost total fertilization failure (TFF) is still estimated to occur in 1–5% of all ICSI cycles and has the potential of recurring in subsequent cycles [2, 3]. However, infertile couples, particularly those with having a low number of available oocytes and abnormal semen characteristics, may experience multiple instances of ICSI failure. One of the prominent reasons leading to ICSI failure is oocyte activation deficiency (OAD), which is believed to be caused by inadequate production of certain molecules, including phospholipase C-zeta (PLCζ), by the sperm. These molecules are crucial for generating the necessary calcium ion (Ca+2) oscillations for oocyte activation. Collectively, OAD, regardless of whether it originates from the sperm or the oocyte, is the main factor contributing to TFF [4,5,6]. It is believed that the anticipated rise in rates of TFF and OAD might be attributed to the growing occurrence of ICSI cycles, as shown by previous studies [7,8,9]. Significant efforts have been devoted to elucidate the molecular mechanisms underlying oocyte activation and fertilization failure, as well as the development of artificial oocyte activation (AOA) techniques for treatment in the cases of OAD.
In order to overcome OAD and ICSI failure, researchers have introduced the use of stimulants, including Ca+2 ionophore A23187, calcimycin, ionomycin, and strontium chloride, as well as other stimulators such as electrical activation [10]. These compounds are employed alongside natural activators to enhance the activation process. Calcium, in particular, is recognized as a pivotal signaling molecule for successful oocyte activation and the initiation of cleavage step [11]. Throughout the early stages of development, both in vivo and in vitro, there are fluctuations in Ca2+ levels until the zygote undergoes the first cell division. In fact, when a spontaneous Ca2+ signal arises, triggering the onset of cleavage and resulting in the formation of the two-cell stage. Evidence suggests a significant correlation between cell division and the availability of Ca2+ during subsequent embryonic development. In addition to the primary role of Ca2+ as a trigger, a growing body of data indicates the involvement of fluctuating proteins levels in the mitosis process [12] (Fig. 1). The current focus of extensive research is to gain a comprehensive understanding of the role of PLCζ in male fertility/infertility [13,14,15,16]. However, it is clear that the absence or disruption of the normal pattern of Ca2+ release during oocyte activation is a significant factor contributing to male infertility and abnormal embryonic development [2]. In this context, the aim of AOA is to artificially raise the levels of intracellular Ca2+ in order to initiate the resumption of meiosis in the oocyte [17, 18].
Schematic representation of Ca2+ release involvement during oocyte activation After sperm-specific phospholipase C zeta (PLCζ) is delivered to the oolemma during or after oocyte-sperm membrane fusion, the fertilizing sperm triggers Ca2+ release. Through its interaction with an unidentified oocyte-borne component, PLCζ facilitates the hydrolysis of PIP2 into DAG and IP3. This, in turn, causes the release of Ca2+ from intracellular storage, therefore mitigating the MII arrest. The process that has been suggested inhibits CSF (Emi2) and releases APC through cortical granules exocytosis, MAPK inactivation, pronuclei production, and CaMKII activation. In the maturation promoting factor (MPF) complex, which is made up of CDK1 and Cyclin B1, this lowers the levels of Cyclin B1, inactivating MPF and freeing the oocyte from MII-arrest (Created with BioRender.com). Abbreviations: APC, anaphase-promoting complex/cyclosome; CaM/CaMKII, calcium/calmodulin-dependent protein kinase II; CSF, cytostatic factor; CNB1, cyclin B1; CDK1, cyclin-dependent kinase 1; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; MAPK, mitogen-activated protein kinase; PKC, protein kinase C
AOA can be triggered by mechanical, electrical, chemical, or a combination of these stimuli (Fig. 2), and each of which has its potential risks and advantages [19,20,21]. Typically, electrical activation entails directly introducing a voltage current, which causes charged lipid bilayer proteins movement and the formation of pores in the membrane. This allows external Ca2+ to influx into the oolemma [21,22,23]. The mechanical activation process requires piercing the oolemma using micromanipulation techniques, followed by the forceful suction of the cytoplasm using a modified ICSI method. This action induces a Ca2+ influx, typically followed by the execution of ICSI, associated with release of internal storages of Ca2+ [24,25,26,27]. Another mechanical approach for activation involves the microinjection of CaCL2 directly into the oocyte [27, 28]. Nevertheless, the standardization of such approaches is expected to provide challenges similar to other physical techniques. Additionally, it is important to note that these methods can induce a singular rise in Ca2+ levels [2].
Schematic illustration of sperm-related natural oocyte activation compared with the three most commonly applied methods of artificial oocyte activation In the natural activation procedure, the acrosome reaction allows the fusion of the sperm membrane with the oocyte and continues to penetrate. Phospholipase C zeta (PLCζ) is released into the ooplasm, triggering the Ca+2 oscillations via IP3. In the artificial oocyte activation, different strategies following ICSI could trigger Ca+2 oscillations: Mechanical activation usually involves a disruption of the plasma membrane with or without PLCζ microinjection, leading to an elevation of Ca+2 within the oocyte due to influx of Ca+2 and/or disruption of Ca+2 store membranes such as the endoplasmic reticulum, Electrical activation involves the generation of pores within the oocyte membrane via application of varying electrical fields, allowing extracellular Ca+2 influx into the oolemma, and Chemical activation mechanisms vary on the type of agent applied, but usually involve the facilitated transport of extracellular Ca+2 into the oocyte either directly or via transport channels (Created with BioRender.com). Abbreviations: PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor
On the other hand, the chemical activation process involves the use of Ca2+ ionophores. These ionophores are molecules that possess the capacity to transport Ca2+ across the oolemma. They achieve this by enhancing the permeability of Ca2+ and inducing the influx of extracellular Ca2+. Additionally, they stimulate the release of stored Ca2+ from intracellular Ca2+ stores [29, 30]. The clinical applications of these methods have remained controversial and at present, according ESHRE, the routine clinical use of AOA is not recommended [31].
In this regard, we systematically review the findings related to the clinical utilization of different AOA methods in couples with various reproductive problems. Therefore, by systematically evaluating and categorizing the available clinical evidence, in the next step, it can be accurately formulated and applied these methods more confidently in the clinical settings, according to the success rate of each AOA method.
Materials and Methods
The search strategy was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [32]. The review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42024551221).
Data Sources and Search Strategy
A systematic search was conducted using online databases: PubMed, Web of Science, EMBASE, and Scopus. The search strategy combined the following keywords and medical subjects heading terms alone or in combination by using “AND” or “OR”: “AOA: assisted oocyte activation”; “AOA: artificial oocyte activation”; “artificial eggs activation”; “assisted eggs activation”; “AOD: oocyte activation deficiency”; “infertility”; “sterility”; “female infertility”; “male infertility”; “ART: assisted reproductive technology”; “ICSI: intra-cytoplasmic sperm injection”; “TFF: total fertilization failure”; “low fertilization failure”; “Ca+2: Calcium”; “Calcium ionophore”; “ionophores”; “calcimycin”; “ionmycin”; “Sr+2: strontium”; “A23187”; “CICR: calcium-induced calcium-release”; “SOAFs: spermatozoon-released oocyte-activating factors”; “PLC: phospholipase C; PLCζ: phospholipase C-zeta”; “PAWP: post-acrosomal WW-binding domain protein”. Furthermore, we manually searched the reference lists of review articles and relevant studies to identify any articles not captured by electronic database searches. We included the relevant English-language journal articles in this systematic review from 2000 to December 2023.
The research question was formulated using the PICO strategy (population, intervention, comparison, and outcomes): “Does AOA improve TTF or low fertilization in infertile couples with various reproductive problems”? (Table 1).
Study Selection and Data Extraction
Two reviewers (SH. M. and F.M.) will independently review the titles and abstracts of the studies to determine their inclusion in the review. The reviewers will be blinded to each other’s decisions. In case of any disagreement, a consensus will be reached through discussion. If consensus cannot be reached, another author (N.A.) will be consulted for resolution.
Initially, articles were screened by title and abstract and irrelevant articles removed. Subsequently, full-text screening of the remaining articles was then conducted. Data from articles were manually extrapolated by two reviewers (N.G. and A.G.) independently. The following data were extracted for each eligible study: date of publication, study design, study population (number of participants, subgroups, age). They also noted AOA method employed (mechanical, electrical, chemical, or a combination), and how oocytes were allocated to control and case groups (comparing oocytes from different patients, sibling oocytes, or previous cycles from the same patients). Additionally, they recorded ART outcomes, relevant results including primary and secondary outcomes (fertilization and cleavage rates, embryo quality, clinical pregnancies, and live births), study conclusions, and reference details.
Following data extraction, the information will be organized and divided into distinct categories based on the various reasons for using AOA. These categories will include instances of fertilization failure, cases of female infertility, situations involving PLCζ defects, issues related to poor sperm quality, occurrences of globozoospermia, and scenarios where surgically retrieved sperm were used. This categorization will help in analyzing the effectiveness of AOA across different fertility challenges. All the extracted data will be tabulated based on these categories (Table 2).
The studies included in the analysis focused on infertile couples, as defined by World Health Organization (WHO) criteria, who underwent AOA following ICSI to address OAD. The underlying causes of OAD in these studies could be attributed to male factors, female factors, or unexplained reasons. To be eligible, these studies had to incorporate a control group using one of the following methods: 1) splitting sibling oocytes between an intervention group and a control group. 2) using results from a previous ICSI cycle with TTF or low fertilization rates as a control, compared to the current cycle where intervention was applied. 3) employing a control group with the same disorder, rather than using fertile couples as controls. Studies were excluded from the analysis based on the following criteria: studies conducted using discarded oocyte samples, studies involving IVM-ICSI cycles, cases where the AOA method was not clearly specified, studies lacking essential data on ART outcomes and relevant results, studies reporting only pregnancy and neonatal outcomes without other relevant data, research where AOA was applied to unfertilized oocytes rather than after the ICSI procedure, studies conducted without a control group, research incorporating additional interventions (such as cytokine factors) alongside AOA, various types of review articles and short communications, studies involving infertile couples with multiple causes where outcomes were not reported separately for each cause, research using fertile couples as the control group, studies conducted using animal models, abstracts and posters, editorials and opinions.
Outcome Measures
The main purpose of this systematic review was to evaluate the efficacy of the AOA technique in combination with ICSI compared with the traditional ICSI process in infertile couples with a history of fertilization failure due to male/female factors or unexplained reasons. The primary and secondary ART outcomes were searched in the included studies as follows: Fertilization rate (defined as the percentage of fertilized oocytes with detectable zygote formation), Cleavage rate (defined as the number of embryos formed on the 3rd day of culture), Embryo quality (the assessment of embryo quality, categorized as either top quality or poor quality), Implantation rate (defined as the number of gestational sacs observed per the number of embryos transferred), Biochemical pregnancy (defined as positive β-hCG measurement two weeks after embryo transfer), Clinical pregnancy (defined as the presence of a visible pregnancy confirmed through ultrasound after 12 weeks of gestation), Undefined pregnancy (defined as biochemical or clinical pregnancy), Live birth (defined as the birth of a live child after 24 weeks of gestation).
Quality Assessment
The quality of included studies was independently assessed by two authors (N.G. and A.G.) by applying the “Cambridge Quality Checklists” [33]. Possible inconsistencies were resolved through common agreement or discussion with another researcher (N.A.). The quality was categorized as poor quality (0–5 points), fair quality (6–10 points), or good quality (11–15 points). All studies were included in this review regardless of the quality score. The three Cambridge Quality Checklists are used to identify quality of studies, including correlates, risk factors and causal risk factors for systematic reviews and meta-analyses. In total, the checklists include 15 score, consist of the correlate score (sum out of 5), risk Factor score (out of 3), and causal risk factor score (out of 7).
Results
Identification and Selection of Articles
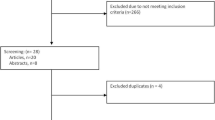
The PRISMA flowchart of the search strategy, identification and selection process is presented in Fig. 3. The initial search identified a total of 3088 articles, including PubMed (n = 595), Embase (n = 807), Web of sciences (n = 693), and Scopus (n = 993). Duplicate publications were removed using Endnote Desktop version 21.3. The remaining 1501 articles were screened for title and abstract, which were excluded 1275 records. 226 records were evaluated for depth analysis of abstracts and 140 articles excluded. With further analysis, we selected 86 records to evaluate the full texts for eligibility, which 56 articles didn’t meet our inclusion criteria. Eventually 30 articles met the inclusion criteria and classified into six categories based on the various reasons for using AOA, including Fertilization failure (n = 10), Female infertility (n = 3), PLCζ defects (n = 4), Poor sperm quality (n = 4), Globozoospermia (n = 4), surgically retrieved sperm (n = 8); three records involved various types of infertile couples for AOA application with a report of individual results. In the included records, splitting sibling oocytes in 4 studies, using results from a previous ICSI cycle in 12 studies, and using distinct patients in 10 studies were employed as control groups. This systematic review included different agents of AOA, including chemical agents: Calcium ionophore (A23187) (n = 10), Ionomycin (n = 7), Strontium/SrCl2 (n = 2), Calcimycin/GM508 (n = 4), Ionomycin plus A23187 (n = 3), other combinations of chemical agents (n = 3), and method of Electrical activation (n = 2). Totally, the quality of the studies identified in this systematic literature search was fair. Five of the 30 studies were categorized as poor quality, 18 studies were categorized as fair quality and seven studies were good quality.
Main Text of Review
AOA and Fertilization Failure
The initiation of development and successful reproduction primarily relies on the process of fertilization. Fertilization begins with the binding and penetration of the sperm into the oocyte, followed by various processes such as calcium oscillations, the release of cortical granules, and the completion of the second polar body extrusion [34]. Unsuccessful fertilization can occur due to two main reasons: either the inability of the sperm to activate oocyte or the inability of the oocyte to facilitate sperm decondensation [35]. Several factors contribute to fertilization failure, and these factors can be attributed to both the oocyte and the sperm. Oocyte-related factors include the quality of the oocytes and the presence of chromosomal abnormalities within them. In addition to the factors related to the oocyte, factors associated with sperm are also significant contributors to fertilization failure. These factors include motility, morphology, viability, and DNA conditions, as well as the ability of sperm to undergo decondensation inside the oocyte are worth mentioning. However, studies have shown that the most important reason for fertilization failure is the inability of the sperm to activate the oocyte by factors related to sperm [36]. TFF refers to the absence of fertilization in all oocytes, where neither male nor female pronuclei are formed. Despite higher fertilization rates observed in ICSI compared to conventional IVF, cases of TFF still occur in ICSI cycles. Although the main cause of TFF has been attributed to defects in oocyte activation, the exact underlying mechanism of oocyte activation failure has not yet been fully elucidated [37].
The utilization of AOA methods has led to initial success in overcoming TFF. These methods attempt to increase calcium levels following fertilization [37]. The results of meta-analysis study by Murugesu et al. (2017) showed that AOA using calcium ionophore after ICSI leads to a significant improvement in the fertilization, cleavage, blastulation, and implantation rates, as well as overall pregnancy and live-birth rates [38].
In 2010, a prospective controlled trial was conducted to assess the effectiveness of ICSI followed by piezoelectric stimulation for oocyte activation in couples struggling with infertility and a history of TFF. The study involved 71 patients, who were divided into two groups: group I (n = 21) with a single occurrence of TFF, and group II (n = 50) with multiple occurrences of TFF. In group I, the oocytes from the patients’ siblings were randomly assigned to two subgroups: in subgroup IA, 123 out of 211 (58.2%) mature oocytes underwent ICSI with piezoelectric stimulation, while in subgroup IB, 88 out of 211 (41.8%) mature oocytes were subjected to the standard ICSI procedure as the control group. In group II, all 612 mature oocytes received piezoelectric activation. The results revealed that the combined ICSI with piezoelectric stimulation yielded significantly higher fertilization rates of 62% and 48.3%, compared to the standard ICSI procedure’s rate of 12%. Moreover, there was an increase in the proportion of higher quality embryos, as well as improvements in implantation and pregnancy rates. These findings indicate that incorporating piezoelectric stimulation may enhance the outcomes of IVF for patients with a history of fertilization failure [39].
Studies investigating the combination of electrical methods with ICSI have been limited, while chemical methods have been more extensively explored for oocyte activation. In a case series study conducted by Juan Chen et al., the effectiveness of ICSI combined with AOA using strontium was evaluated. The study included six couples who had experienced complete fertilization failure or low fertilization rates in previous ICSI cycles. In the most recent ICSI cycles, AOA treatment with strontium was combined with ICSI to improve the clinical outcomes. The results showed a significant improvement in fertilization rates in the AOA group (78.8%) compared to the previous ICSI cycles without AOA treatment (ranging from 0% to 16.7%; mean = 7.7%). Additionally, after culturing the embryos for 5 days, a high-quality embryo rate of 41.5% (17/41) was observed. Ultimately, four clinical pregnancies were achieved, resulting in the birth of four healthy babies. This study suggests that the incorporation of AOA using strontium in ICSI cycles can lead to improved fertilization rates and the development of high-quality embryos, ultimately increasing the chances of successful pregnancies and the birth of healthy infants [40].
Furthermore, another case series study involving eight participants demonstrated that strontium chloride (SrCl2) was effective in overcoming fertilization failure or low fertilization rates following conventional ICSI or calcium ionophore (A23187) treatment cycles. The study found a significant increase in fertilization rates when SrCl2 was used compared to conventional ICSI or ICSI with calcium ionophore treatment (61.7% vs. 20.0% or 25.3%, respectively). Additionally, there was a notable increase in the proportion of good-quality cleaved embryos in the SrCl2-treated group compared to the other two groups (50.0% vs. 0% or 12.5%, respectively). The study observed enhanced blastocyst development in oocytes treated with SrCl2 in comparison to those treated with ICSI alone or ICSI with calcium ionophore (25.7% vs. 0% or 9.1%, respectively). Finally, in the SrCl2 treatment group, five successful pregnancies resulted in the birth of eight healthy children. Based on these findings, the authors suggested that SrCl2 treatment can be considered an effective method for overcoming fertilization failure. These results highlight the potential of SrCl2 as a promising approach to address fertilization challenges, offering improved fertilization rates, increased formation of high-quality embryos, enhanced blastocyst development, and ultimately leading to successful pregnancies and healthy births [41].
A split ICSI-AOA cycle using sibling oocytes can help differentiate between a molecular oocyte-related activation deficiency and previous technical or other biological failures. In a prospective case series study involving 14 patients who had experienced fertilization failure or low fertilization following conventional ICSI, ICSI-AOA was performed using ionomycin on half of the sibling metaphase II oocytes, while conventional ICSI was performed on the remaining oocytes in a "split ICSI-AOA cycle" during the next treatment cycle. The results indicated a significant improvement in fertilization rates in the ICSI-AOA group compared to conventional ICSI (74.2% vs. 43.5%). Further analysis revealed a cumulative pregnancy rate of 35.7% and a live birth rate of 14.3% per treatment cycle. These findings suggest that ICSI-AOA is highly effective in patients who have a suspected deficiency in oocyte-related activation and have experienced fertilization failure after conventional ICSI. However, the study also noted that caution should be exercised when applying ICSI-AOA in cases of previous low fertilization from a different center. The efficiency of ICSI-AOA should be tested on half of the sibling oocytes in such cases because ICSI-AOA is not always beneficial for patients with a history of low fertilization and suspected oocyte-related activation deficiency [42]. In contrast, several studies with a greater sample size reported more promising results. In a prospective multicenter study, Ebner et al. investigated the use of AOA-ICSI using calcium ionophore (A23187) for 101 patients who were faced with fertilization failure. The fertilization rate was 48% in the activation group, while it was 25% in the control group, with a significant difference observed between the two groups. In this study, the fertilization rate in all treatment groups, including those with TFF and low to moderate fertilization rates, was increased with the use of calcium ionophore treatment [43].
In a retrospective observational study conducted by Alberto Tejera et al., the impact of AOA was investigated in 66 patients who had experienced fertilization issues in previous attempts. The study evaluated the use of calcium ionophore (A23187) for AOA treatment. The results revealed that AOA treatment with calcium ionophore significantly improved fertilization rates compared to previous attempts (51% vs. 13.1%). Additionally, there was an increase in ongoing pregnancy rates (47% vs. 21.7%), implantation rates (31.1% vs. 13.1%), and a reduced likelihood of cycle cancellation (22.7% vs. 69.3%) when AOA was implemented. Notably, no adverse effects on obstetric and perinatal outcomes were observed following the use of AOA. The study concluded that the application of AOA resulted in a significant increase in reproductive success. This was attributed to an increased number of embryos available for selection, thereby enhancing the chances of achieving successful pregnancy outcomes. The findings suggest that AOA can be a valuable technique to improve fertilization rates and overall success rates in patients with previous fertilization issues [44].
In a large-scale multi-center retrospective study conducted in Japan, the effectiveness of AOA treatment using Ca2+ ionophore (A23187 or ionomycin) following ICSI was assessed in 198 couples who had previously undergone ICSI cycles with unexplained low fertilization rates. The oocytes used for both the intervention and control groups were retrieved from different cycles of the same pool of couples. The evaluation of primary and secondary outcomes demonstrated a significant improvement in the ICSI-AOA group compared to conventional ICSI. The ICSI-AOA group exhibited higher fertilization rates (53.7% vs. 20.8%), biochemical pregnancy rates (35.4% vs. 11.8%), clinical pregnancy rates (28.2% vs. 11.8%), and live birth rates (18% vs. 4.7%) compared to the conventional ICSI group. Furthermore, the study conducted further analysis based on female age, dividing the patients into two groups: those below 35 years of age and those above 35 years of age. The comparison between these subgroups revealed that the clinical pregnancy and live birth rates were higher in the younger women (32.1% and 19.1%, respectively) in the ICSI-AOA group compared to the conventional ICSI subgroup (5.9% and 0.0%, respectively). However, no significant differences were observed in the older subgroup. The rates of miscarriage, preterm delivery, and fetal congenital malformations were similar between the two groups. Overall, the study indicated that AOA treatment using Ca2+ ionophore following ICSI significantly improved fertilization rates, pregnancy rates, and live birth rates compared to conventional ICSI in couples with unexplained low fertilization rates. The benefits of AOA treatment were particularly pronounced in younger women, while no significant differences were observed in older women. Additionally, the rates of adverse obstetric and perinatal outcomes were similar between the ICSI-AOA and conventional ICSI groups [45].
Several studies have been conducted to compare the effectiveness of various compounds of AOA. In a comparison study, the results of a retrospective cohort study by Jia et al. in 2023 also revealed that AOA using ionomycin compared to A23187 led to a higher fertilization rate in cases of TFF (46.9% vs. 28.4%) and low fertilization (67.7% vs. 49.2%) groups. There was no significant differences in rates of day 3 good-quality embryos, implantation, clinical pregnancy, live birth, and the cumulative live birth, between the two groups [46]. Despite the effective role of AOA in fertilization success, some couples still face fertilization failure after AOA using conventional ionophores. Wang et al. (2021) conducted their research with focus on the use of cycloheximide along with ionomycin for AOA in six couples who experienced TFF using ionomycin alone. They observed that cycloheximide plus ionomycin had a superior effect compared to ionomycin only or conventional ICSI and led to an effective improvement in the fertilization rate. Aneuploidy analysis of embryos developed from novel AOA revealed that this approach not only rescued fertilization failure in patients with severe fertilization deficiencies, but also allowed for normal cleavage and development of embryos into good quality blastocysts during extended culture. The authors noted that the novel AOA method demonstrated a potential therapeutic effect for couples experiencing TFF, even after conventional AOA techniques had been attempted. This suggests that the novel AOA method may be effective in overcoming severe fertilization deficiencies in patients who have a history of repeated low fertilization rates or TFF. The findings of this study highlighted the potential of this novel AOA method as a therapeutic approach for couples facing fertilization challenges. By rescuing fertilization failure and supporting normal embryo development, this technique offers hope for improved outcomes in patients with severe fertilization deficiencies. Further research and validation of this novel AOA method are necessary to establish its efficacy and broaden its clinical applications [47].
The mouse oocyte activation test (MOAT) is used as a diagnostic method to assess the oocyte-activating capacity of human spermatozoa. Based on the MOAT result, individuals are recognized with sperm-related OAD, reduced oocyte-activating capacity of the sperm, or suspected oocyte-related OAD. In a 17-year retrospective cohort study involving 122 couples who experienced fertilization failure or low fertilization after ICSI, the effectiveness of ICSI-AOA cycles was compared to standard ICSI cycles. The study aimed to assess the oocyte-activating capacity of sperm in all patients using the MOAT before AOA. The results of the MOAT categorized the patients into three groups: MOAT group 1 (19 patients) with a sperm-related OAD, MOAT group 2 (56 patients) with a diminished sperm-related oocyte-activating capacity, and MOAT group 3 (47 patients) with a suspected oocyte-related OAD. Significant differences in fertilization rates were observed after AOA compared to control groups in each MOAT group: MOAT group 1 (70.1% vs. 9.7%), MOAT group 2 (63.0% vs. 14.8%), and MOAT group 3 (57.3% vs. 17.7%). Patients who underwent diagnostic testing before AOA showed significant improvements in clinical outcomes compared to their previous cycles. Furthermore, significant differences in pregnancy rates (49.0% vs. 29.4%) and live birth rates (41.2% vs. 22.1%) were observed between MOAT group 1 and MOAT group 3. The study concluded that ionomycin treatment for AOA significantly improved fertilization, pregnancy, and live birth rates compared to previous ICSI attempts. The authors emphasized that AOA treatment should be reserved for patients with clear OADs. By identifying patients with specific OADs through diagnostic testing, AOA can be targeted to those who are likely to benefit from it, leading to improved clinical outcomes in terms of fertilization, pregnancy, and live birth rates [9].
Ferrer-Buitrago et al. (2018) investigated whether human oocyte calcium analysis (H-OCA) could be used to predict fertilization success following AOA in patients with fertilization failure. Their findings showed that both mouse oocyte calcium analysis (M-OCA) and H-OCA methods could predict the response to AOA; however, H-OCA determined the defect in activation with more sensitivity. They also determined that the AOA method was not beneficial for patients who exhibited normal calcium oscillatory in the H-OCA method to overcome fertilization failure, suggesting that other factors may be contributing to their fertilization failure. On the other hand, AOA was found to be beneficial for those who have abnormal calcium oscillatory in the H-OCA method, indicating that AOA could address the activation issues in these cases [18]. Therefore, it is essential to further investigate the underlying reasons for fertilization failure in order to determine the most appropriate approach for improving fertilization outcomes.
AOA and Female Infertility
Almost 13% of women are infertile [48], and in some cases, these individuals can be suitable candidates for AOA. Diminished ovarian reserve (DOR) is a situation, whereby these patients exhibit low numbers of oocytes after ovum pick up in treatment cycles of ICSI or IVF; in most cases, these patients face TFF or low fertilization rates. In a prospective, randomized, controlled study involving 296 patients with diminished ovarian reserve, the effect of calcium ionophore solution on fertilization rates was analyzed. The patients were randomly divided into two groups. One group consisted of 148 patients who received AOA immediately after ICSI, while the other group of 148 patients did not receive AOA and served as the control group. The study’s data did not show any significant differences in the main outcomes between the ICSI-AOA group and the control group. This included fertilization rates (60.7% vs. 55.4%), implantation rates (12.8% vs. 10.7%), pregnancy rates (21% vs. 12.8%), and ongoing pregnancy rates (10.9% vs. 6.1%). Therefore, the authors concluded that the application of calcium ionophore (GM508) solution for AOA did not result in increased embryological and clinical outcomes in patients with diminished ovarian reserve. Based on these findings, the study suggests that utilizing AOA through the use of a calcium ionophore solution may not be effective in improving the fertilization and pregnancy outcomes in patients with diminished ovarian reserve [49].
In a retrospective cohort study for the first time with a large sample size of couples (ICSI alone and ICSI-AOA), conducted by Mingrong Lv et al., (2020) investigated the effects of AOA using calcium ionophore (A23187). They included the patients with various reproductive problems including oligoasthenoteratozoospermia (OAT) and individuals with female factor etiology such as PCOS (n = 43), POI (primary ovarian insufficiency) (n = 30), advanced age (n = 93), unexplained infertility (n = 141). The results showed that fertilization rate was no significant difference in all subgroups of female factor. The results obtained for embryo development (cleavage, blastulation, high-quality blastocyst) indicated only the high-quality blastocyst was statistically significant in infertile couples with PCOS and unexplained infertility cases, while there were no significant differences in individuals with advanced age or POI. In all groups treated with AOA, the analysis of implantation data was determined a statistically significant difference compared with controls. In evaluation of clinical pregnancy rate, between all subgroups followed by AOA (except for POI subgroup) compared to controls groups, there were found statistically significant differences [50]. POI is one of the most important causes of female infertility, which refers to the cessation of menstrual cycles before the age of 40 [51].
In a RCT study (2018), the effects of calcimycin and SrCl2 were compared for the first time in 135 cases with previous cycles of low fertilization as unexplained low fertilization or as low fertilization associated with impaired oocyte morphology, with normal sperm parameters. Both SrCl2 and calcimycin AOA have been found to significantly enhance embryological and clinical outcomes compared to ICSI alone. In detail, the use of SrCl2 and calcimycin resulted in higher rates of fertilization (77% and 54% vs. 33%), top-quality Day 3 embryos (86% and 49% vs. 51%), blastocyst formation (63% and 42% vs. 33%), implantation (41% and 34% vs. 19%), clinical pregnancy (70% and 40% vs. 35%), ongoing pregnancy (57% and 36% vs. 30%), and live birth (57% and 31% vs. 21%) compared to ICSI alone. A significant improvement was observed in clinical pregnancy, ongoing pregnancy, and live birth for the couples in SrCl2 group (and not calcimycin). These findings suggested that both SrCl2 and calcimycin AOA methods offer potential therapeutic benefits for improving outcomes in assisted reproductive techniques, surpassing the limitations associated with ICSI alone [52].
AOA and Sperm Oocyte Activating Factors
Perinuclear theca (PT) is a covering of the cell skeleton that is located on the sperm nucleus and contains oocyte activating factors. Oocyte activation is associated with the release of sperm oocyte activating factors (SOAF) from the PT region of the sperm head such as PLCζ/ PLCZ1 and postacrosomal sheath WW domain binding protein (PAWP), which are responsible for triggering the activation process in the oocyte [53]. Additionally, truncated form of the kit receptor (TR-KIT) has been identified as a potential candidate for oocyte activation. However, there is limited information available regarding its specific role and mechanism in the activation process.
AOA and Defects in PLCζ
Phospholipase Cζ (PLCζ) is one of the main known SOAFs, whose deletion of this gene leads to defects in oocyte activation [54]. Studies have shown that some mutations in the coding sequence of the PLCζ gene can lead to a negative effect on calcium fluctuations and oocyte activation. Among these mutations, including p.H398P, p.H233L, and p.I489F mutations in the PLCζ gene have been seen in patients with fertilization failure [55,56,57]. In a study by Torra-Massana and et al. (2019), 37 cases identified with TFF due to defects in oocyte activation or other reasons were examined. They predicted that the main cause of TFF is a change in the PLCζ gene and disruption of oocyte activation. By sequencing the gene, they identified 6 mutations related to PLCζ, 4 of which were novel [58]. These findings highlight the importance of PLCζ in oocyte activation and suggest that mutations in the PLCζ gene can contribute to fertilization failure and TFF. Further research on the role of PLCζ and the impact of specific mutations is necessary to better understand the mechanisms and potential clinical implications of these genetic variations in oocyte activation and fertility.
In the RCT study conducted by Nazarian et al. in 2019, the expression level of PLCζ and its correlation with oocyte activation and fertilization rates was investigated in 26 couples who were candidates for ICSI. Semen samples were categorized into two groups based on PLCζ evaluation: a healthy group and a PLCζ deficient group. The PLCζ deficient group was also divided into two subgroups: one that underwent AOA and one that did not. The three groups were compared in terms of fertilization rate, cleavage rate, and embryo quality. The findings showed the fertilization rates in the group without AOA was lower compared to both the control group (healthy group) and the group with AOA (41.9% vs. 78.1% and 69.5%, respectively). However, the cleavage rate and embryos quality were no significant differences between the groups. Based on this, it is suggested that PLCζ could be serve as a potential factor for evaluating the ability of oocyte activation, and further studies are needed to investigate the use of PLCζ as a biomarker clinical practice [59].
The potential of AOA as a therapeutic approach in cases where fertilization failure is attributed to mutations in the PLCζ gene is of interest. In a study, researchers identified five novel mutations in the PLCζ gene from four independent patients using whole-exome sequencing and Sanger sequencing methods. To understand the impact of these mutations on oocyte activation, they injected mutant PLCζ cRNAs (complementary RNAs) and wild-type (WT) PLCζ cRNAs into mouse MII (metaphase II) oocytes. The results showed that the mutant PLCζ cRNAs induced significantly lower rates of pronucleus formation compared to the WT, indicating impaired function of the PLCζ protein caused by the identified mutations. Based on the observed impaired effects of the identified mutations on oocyte activation, the researchers then applied AOA treatment using ionomycin in all four families. The outcomes of this treatment indicated that AOA could successfully rescue the fertilization failure in these families and ultimately resulted in live births. This study provides evidence that the identified mutations in the PLCζ gene negatively affect oocyte activation. However, the use of AOA treatment, specifically using ionomycin, was able to overcome the fertilization failure caused by these mutations, leading to successful pregnancies and live births in the affected families [60]. Some studies have suggested that using recombinant PLCζ for AOA could be a suitable treatment option [61]. Currently, some in IVF clinics employ PLCζ protein testing for diagnostic purposes. However, further investigation of this protein, as therapeutic agent, seems necessary for its reliable use to rule out its possible cytotoxic effects during embryonic development and to confirm the overall safety of recombinant PLCζ in the subsequent offspring.
In a case series study conducted by Xin Meng et al., the researchers investigated the usefulness of PLCζ analysis in guiding the clinical decision-making process for infertile males undergoing ART treatments. The study included 5 couples who were experiencing fertilization failure in previous in IVF cycles. The eligible patients in the study underwent ICSI-AOA using calcimycin (GM508). The results demonstrated a significant improvement in fertilization rates, with an increase from 18.6% in previous ICSI cycles to 56.8% in ICSI-AOA cycles across all five patients. Furthermore, the pregnancy rate in the ICSI-AOA cycles showed a substantial improvement (60% vs. 7.7%) compared to previous cycles. The clinical pregnancy rate and live birth rate were both 40% per initiated cycle. Based on these findings, the researchers concluded that PLCζ analysis is a valuable diagnostic tool for determining patient eligibility for subsequent AOA treatment. By identifying patients with deficiencies in PLCζ, who are likely to benefit from AOA, clinicians can improve fertilization rates and enhance the likelihood of achieving pregnancy and live births in these individuals [15].
In contrast to the studies mentioned, a retrospective cohort study conducted by M. Martínez et al. in 2021 assessed the effectiveness of ICSI-AOA. The study included an experimental group consisting of 41 embryos obtained from 7 ICSI-AOA cycles. In these cases, AOA was performed due to the identification of at least one potentially pathogenic variant in the PLCζ1 gene in the sperm DNA after fertilization failure in a previous ICSI cycle. The control group comprised 100 embryos obtained from 18 ICSI cycles, which were cultured under the same conditions. The study compared fertilization rates and embryo morphological scores between the ICSI-AOA group and the control group. The results showed no significant difference in fertilization rates between the two groups, with rates of 66.2% in the ICSI-AOA group and 83.5% in the control group. Additionally, the use of ionomycin for AOA did not improve the morphological scores of embryos on day 3 in terms of their morphokinetic pattern of development. The study also evaluated other reproductive outcomes, including biochemical pregnancy, clinical pregnancy, ongoing pregnancy, and live birth, but no significant differences were observed between the ICSI-AOA group and the control group in these parameters. Based on these findings, it indicates that AOA with ionomycin may not provide additional benefits in these specific cases of fertilization failure associated with PLCζ1 gene variants [62].
Among the other candidate proteins involved in oocyte activation is the PAWP protein, which is located exclusively in the perinuclear region of sperm and is expressed during the elongating phase of the spermatid. Then, upon fertilization, PAWP is released from the sperm and spreads in the ooplasm [61, 63]. In a study conducted by Aarabi et al. (2014), the level of PAWP in men’s sperm and its relationship with the outcomes of ICSI was investigated. This study showed that there is a statistically significant positive relationship between PAWP expression level and both the fertilization rate and embryonic development [64].
The results of the studies investigating the role of PAWP in oocyte activation are not consistent, and further investigations are necessary to find out its therapeutic applications. In the study of Nomikos et al. (2014), it was found that recombinant mouse PAWP protein was unable to hydrolyze phosphatidyl and its injection into mouse oocytes did not stimulate calcium fluctuations in in vitro [65]. In the following, in another study examining the function of PLCζ and PAWP in inducing calcium fluctuations, the obtained results showed that while PLCζ was capable of causing Ca2+ oscillations, oocyte activation, and embryo development; recombinant human PAWP and different constructs of human PAWP cRNA could not have such an effect. Moreover, the PAWP-derived PPGY peptide could not inhibit Ca2+ oscillations induced by sperm. These findings suggested that the importance of PLCζ and PAWP is not the same and human PAWP was not sufficient or necessary to cause Ca2+ oscillations [66]. It has been shown that the expression of both PLCζ and PAWP proteins was lower in sperm from patients with TFF compared to controls [67]. Researchers observed a homozygous mutation in PLCζ of two infertile brothers with TFF and sperm inability to induce oocyte activation, despite having normal sperm morphology. However, no defect in PAWP expression was observed. These findings suggest that the lack of PLCζ alone is sufficient to prevent oocyte activation, and the presence of PAWP protein alone does not lead to the induction of calcium fluctuations and oocyte activation [55]. Based on these studies, some researchers still cannot confirm a consistent positive relationship between PAWP expression and Ca2+ oscillations. This indicates that further investigation is necessary to determine the exact role of PAWP and its potential therapeutic applications in AOA. Also understanding the precise contributions of PLCζ and PAWP in oocyte activation, both individually and in conjunction, is crucial for developing effective therapeutic strategies. Further research is needed to explore the complex interplay between these proteins and their significance in male infertility, particularly in cases of TFF and AOA.
AOA and High DFI of Sperm
Based on clinical documents, it was found that even when sperm parameters appear normal, more detailed examination of sperm, in particular, DNA integrity can be valuable in cases of couples with TFF [68]. Studies have shown that high sperm DNA fragmentation Index (DFI) is associated with a decrease in fertilization rate and a high risk of TFF [53, 69, 70]. Although the oocyte can repair the DNA damage in sperm, this repair process depends on the oocyte quality and the amount of sperm DFI. If the level of DNA damage exceeds the repair capacity of the oocyte, it may result in failed oocyte activation and fertilization failure [53].
There are limited studies specifically focusing on the correlation between DFI and AOA. However, the relationship between DFI and activators factors involved in oocyte activation has been shown in some studies. PAWP and PLCζ are among the factors that have a direct role in oocyte activation. In the study of Tavalaee et al. (2017), a statistically significant negative relationship was detected between sperm DFI and the expression of PAWP and PLCζ [53]. This suggests that as sperm DFI increases, there is a significant decrease in the levels of PAWP and PLCζ factors. This correlation indicated that impaired sperm DNA integrity may be associated with deficiencies in factors necessary for successful oocyte activation. Also, reactive oxygen species (ROS) have been implicated as an important factor in causing DNA damage and increasing sperm DFI [53]. Park et al. (2015) observed that ROS has harmful effects on the expression of PLCζ [71]. Based on this findings, ROS may reduce the RNA expression and protein of PLCζ through DNA damage [53], potentially compromising its function in oocyte activation. Consequently, AOA may be a beneficial approach for the cases with high DFI to help overcome deficiencies in sperm function and improve fertilization rates.
AOA and Poor Sperm Quality
Studies have shown that AOA is an effective method in improving the clinical results of male infertility. Regarding the relationship between PLCζ and sperm parameters, several studies have investigated this association. It has been found that the level of PLCζ protein is generally higher in sperm with normal parameters compared to sperm with abnormal parameters [14, 72]. Therefore, the expression of PLCζ may be associated with sperm health and functionality. In this way, it has been revealed that the expression of PLCζ protein is lower in sperm from men with teratozoospermia, which refers to abnormal sperm morphology. This suggests a potential link between PLCζ expression and sperm morphology abnormalities [73]. Furthermore, in men with OAT, which is characterized by low sperm count, poor motility, and abnormal morphology, lower expression of both PLCζ and PAWP proteins has been detected in sperm cells compared to men with normozoospermia. A presentation of altered localization patterns of PLCζ have been demonstrated in OAT men [74].
In a retrospective study of 69 infertile men diagnosed with OAT and a history of no or low fertilization in standard ICSI cycles, the effectiveness of AOA was evaluated. These men returned for another ICSI cycle in which AOA was performed. The study found that the use of calcium ionophore (A23187) during AOA significantly improved the fertilization rate in the ICSI-AOA cycle compared to the previous cycle without AOA (control group). The fertilization rate increased from 38.2% in the control group to 74.6% in the intervention group. Furthermore, the study observed significant improvements in other reproductive outcomes in the intervention group. The cleavage of embryos, the production of high-quality embryos, implantation rates, and clinical pregnancy rates were all significantly increased compared to the control group. AOA appears to be a beneficial intervention in these cases, potentially increasing the chances of successful embryo development, implantation, and ultimately, clinical pregnancy [75]. Promising results were reported by a retrospective study with a large sample size (n = 374), examining the effect of AOA in patients with OAT infertility. The study demonstrated that AOA significantly increased the fertilization rate in OAT patients, along with higher rates of blastulation and high-quality blastocysts. There was a significant improvement from the cleavage stage to blastocyst transfers in the ICSI-AOA cycles compared to control cycles. Furthermore, ICSI-AOA cycles showed significantly increased rates of implantation, clinical pregnancy, and live births (43.8% vs. 10.9%) compared to previous control cycles. Calcium ionophore (A23187) treatment during AOA also resulted in a significant decrease in miscarriage rates. In summary, the study showed that AOA with calcium ionophore improved fertilization, embryo development, and reproductive outcomes in OAT patients. It also suggested that calcium ionophore treatment during AOA may reduce the risk of miscarriage [50]. These findings suggest that AOA can be beneficial for patients with OAT infertility, potentially overcoming some of the sperm-related challenges associated with this condition. While AOA has shown positive results in certain cases, its effectiveness may be limited in others. Further research is necessary to optimize its application and identify the most suitable patient populations for this approach.
In a randomized controlled trial (RCT) conducted by M. Eftekhar et al., the efficacy of calcium ionophore (A23187) as an oocyte activation method in ICSI-AOA was investigated. The study included 38 women with partners diagnosed with teratozoospermia, a condition characterized by abnormal sperm morphology. The patients were randomly assigned to two groups using a computer-based randomization list. The study found that there were no significant differences between the study group (ICSI-AOA) and the control group in terms of fertilization rate and cleavage rate. The fertilization rates were 95.33% in the study group and 84.4% in the control group, while the cleavage rates were 89.56% and 87.74%, respectively. Regarding implantation rate, the AOA group exhibited a higher rate compared to the control group, but the difference was not statistically significant (17.64% vs. 7.4%). Furthermore, there were no significant differences observed in chemical pregnancy rate and clinical pregnancy rate between the two groups 47.1% vs. 16.7%, and 41.2% vs. 16.7%, respectively. Based on these findings, the study suggests that the use of calcium ionophore as an oocyte activation method in ICSI-AOA for women with partners diagnosed with teratozoospermia did not significantly affect fertilization and cleavage rates and clinical outcomes [76]. Studies with a greater sample size is necessary for confirmation the results obtained from this RCT.
In the comparison of the efficacy of ionomycin and A23187 for oocyte activation from a retrospective cohort study, it was found that the fertilization rate was significantly higher in the ionomycin group compared to the A23187 group (66.4% vs. 31.6%) in OAT patients who had experienced a history of TFF or low fertilization. However, the rate of cleavage, good quality embryos on day 3, clinical pregnancy, implantation and live birth was no significant differences between the two groups [46]. This suggests that while ionomycin may result in higher fertilization rates, it does not necessarily translate into improved clinical outcomes compared to A23187 in this specific patient population. Furthermore, in a RCT study comparing the effects of calcimycin and SrCl2 in couples with male factor infertility, it was observed that calcimycin (but not with SrCl2) was superior to ICSI alone in terms of clinical pregnancy, ongoing pregnancy and live birth rates [52]. These findings highlight the importance of evaluating different agents used for oocyte activation and their impacts on specific clinical outcomes in cases of male factor infertility. The choice of the activation agent may vary depending on the specific needs and characteristics of the patient population, and further research is need to optimize the selection and application of these agents for improved fertility outcomes.
AOA in Cases with Globozoospermia
Among the factors involved in male infertility, there is a rare morphology disorder called “ Globozoospermia,” that was first described by Wolff, Schill, and Moritz (1976) [77]. It is characterized by specific abnormalities in sperm morphology. Generally, the main features of globozoospermia include round-headed spermatozoa without an acrosome, cytoskeleton defects around the nucleus, and the absence of a post-acrosomal sheath [78]. In 1992, Singh classified globozoospermia into type I and type II. Accordingly, in type I globozoospermia, also called as classic or total globozoospermia, is characterized by the presence of small, round-headed spermatozoa without an acrosome. In this type, all spermatozoa exhibit the same round-headed morphology without the acrosomal cap. Since round-headed spermatozoa cannot penetrate the zona pellucida due to the absence of an acrosome, this type of disorder results in primary infertility in males [79]. Contrary to this, men with type II globozoospermia is characterized by sperm cells with large cytoplasmic droplets that restrict the motility of the sperm [80, 81]. In this type of globozoospermia, a varying percentage ranging from 20 to 90% of sperm lack an acrosome was seen; therefore, it is also known as partial globozoospermia [80, 81]. Round-head spermatozoa are lack of the oocyte activation factor and acrosome. Sperm specific antigen 2 (SSFA2) plays a vital role in the formation of acrosomes in spermatogenesis. Loss-of-function mutations in SSFA2 have been identified as a cause of male infertility with globozoospermia [82]. Infertile globozoospermia individuals also express lower levels of the genes PLCζ, PAWP, and TR-KIT compared to fertile men [59, 83]. These genes play crucial roles in sperm function, including oocyte activation. Furthermore, the deletion of the DPY19L2 gene has been reported as a major genetic cause of infertility with globozoospermia in over 70 percent of cases. Infertile men with DPY19 deletion showed that this disorder also is associated with reduced expression of PLCζ in sperm. Therefore, the application of AOA (ionomycin) techniques can improve fertilization rates and increase the chances of achieving live birth rates following ICSI in these cases [84].
AOA techniques, specifically the use of calcium ionophores, have shown promising results in enhancing fertilization rates and live birth rates in individuals with globozoospermia. A case report documented a couple with a 17-year history of male infertility due to total globozoospermia. Despite multiple standard ICSI attempts in the past, they experienced either fertilization failure or low fertilization rates. In their seventh attempt, calcium ionophore (A23187) was employed in combination with ICSI. The introduction of AOA led to a significant improvement in the fertilization rate, which increased to 38.46% compared to previous cycles. Subsequent follow-up showed enhanced embryo development, and ultimately, two blastocysts on day 5 were transferred. This resulted in a successful and healthy delivery [85]. These findings highlight the potential of AOA techniques, particularly the use of calcium ionophores, in overcoming fertilization challenges associated with globozoospermia. By bypassing the need for natural sperm-oocyte interaction, AOA can improve fertilization rates and increase the chances of achieving a successful pregnancy and live birth in individuals with this condition. The successful clinical outcomes with healthy singleton were reported by Kochhar et.al in two cases with total globozoospermia, when calcium ionophore (GM508) was used post ICSI compared to previous traditional ICSI cycles [86].
Embryo development may be affected in ICSI-AOA cycles. In a study conducted by Tejera et al., the treatment of oocytes (n = 9) from a globozoospermic case with calcium ionophore (ionomycin) was found to significantly improve the fertilization rate compared to sibling oocytes (n = 14) that did not receive the treatment. The oocytes treated with AOA achieved a higher fertilization rate compared to untreated oocytes (55.6% vs. 35.7%). Furthermore, the study observed that AOA had a positive impact on the development and quality of the embryos. The untreated embryos exhibited slower development, poor quality, and a high incidence of multinucleated blastomeres (60%). In contrast, the embryos that underwent AOA did not show any multinucleation (0%) and demonstrated better cleavage rates on days 2 and 3. Two embryos that had undergone AOA were transferred into the uterus on day 3, resulting in the birth of a healthy newborn [87].
According to the comparison results between oocytes injected with round-headed sperm followed by calcium ionophore and oocytes injected with donor sperm (without calcium ionophore), the fertilization rates were 3 out of 6 for injected round-headed sperm oocytes, and 5 out of 6 for injected donor sperm oocytes. The rate of aneuploidies and blastocyst formation was equal between the both groups in the resulting embryos. This indicates that the use of injected round-headed sperm, despite its specific morphology abnormalities, did not significantly affect the occurrence of aneuploidies or the development of blastocysts compared to injected donor sperm. Also calcium ionophore treatment may not affect chromosome integrity or embryonic development [88].
The potential adverse effects of ionophores on human oocytes or embryos in clinical ICSI procedures have not been adequately evaluated. Therefore, the use of these drugs for activation needs further investigation to ensure their safety and effectiveness. To address this concern, nonchemical activation methods like electrical stimulation can be considered as an alternative method. By exploring electrical stimulation, the use of insufficiently tested drugs such as ionophores can be avoided. This approach requires additional research to assess its efficacy and safety in reproductive treatments, aiming to prioritize patient well-being and minimize potential risks [89, 90]. In a specific case report, the initial ICSI attempt did not result in fertilization of any of the injected oocytes. However, in a subsequent ICSI attempt, a different approach was employed. Human oocytes were subjected to electrical activation using a single square direct current pulse immediately after microinjection. This method successfully led to the fertilization of all seven injected oocytes. As a result, two healthy embryos at the eight-cell stage were transferred on day 3 of development. The transferred embryos resulted in a successful pregnancy and the birth of a live baby. This case highlights the effectiveness of electrical activation in promoting fertilization and achieving a positive outcome in the treatment of infertility [91].
In patients with partial globozoospermia, where both round-headed and normal-shaped spermatozoa are present in the semen sample, using of the intracytoplasmic morphologically selected sperm injection (IMSI) method has been observed to be superior to chemical AOA methods [92]. Contrary to the mentioned studies, a normal live birth was reported after ICSI in a globozoospermic patient without oocyte activation. Approximately 12 live births have been reported after ICSI of round-headed spermatozoa without the use of AOA. These results suggest that ICSI without AOA may be useful in cases of partial Globozoospermia [93,94,95].
AOA and Surgically Retrieved Sperm-Related Studies
Surgically retrieved sperm combined with ICSI is a pioneering infertility treatment modality that can be used in patients with azoospermia [96]. In 1997, calcium ionophore was used to activate oocytes by injecting spermatids. In a study, results suggested that SrCl2 may be useful for fertilizing oocytes after ICSI using frozen-thawed testis sperm in cases of repeated failures of complete fertilization. In this case report, fertilization rate 51.6% was reported compared with 9.1% and 0.0% in previous cycles. One embryo on day 3 was transferred and resulted in pregnancy and live birth. However, further studies are needed to confirm the safety and efficacy of SrCl2 treatment for oocyte activation in clinical applications [97].
To address the issue of AOA effectiveness in patients with different sources of spermatozoa, several clinical studies were conducted. A clinical study evaluated the effects of AOA with calcium ionophore (A23187) on ICSI cycles using sperm from various sources, including ejaculated, epididymal, and testicular sperm. Three groups of 314 couples were evaluated based on the origin of the sperm; ejaculated (n = 92), epididymal (n = 82), and testicular (n = 140). Each group was further divided into experimental subgroups based on whether AOA was performed or not. Based on the results of evaluating the cycles, the percentage of high-quality embryos (74.5 vs. 53.0%) and implantation rate (19.3 vs. 10.5%) increased when AOA was performed with ejaculated; and the percentage of high-quality embryos increased (64.4 vs. 50.3%) with epididymal spermatozoa. According to these findings, both sperm maturity and oocyte quality are important factors in the activation of oocytes [98]. Similarly, in a study involving 204 couples, the participants were categorized into three groups based on the source of sperm used for injection during ICSI. The groups consisted of patients with non-obstructive azoospermia who underwent testicular sperm aspiration (TESA-NOA, n = 58), patients with obstructive azoospermia who also underwent testicular sperm aspiration (TESA-OA, n = 48), and patients with obstructive azoospermia who underwent percutaneous epididymal sperm aspiration (PESA-OA, n = 98). The study aimed to assess the impact of AOA treatment using calcium ionophore (A23187) on ICSI outcomes for the different types of azoospermia in patients undergoing TESA. The results indicated that AOA treatment did not improve ICSI outcomes for either type of azoospermia in patients who underwent TESA. However, interestingly, when the sperm used for injection was retrieved from the epididymis (PESA-OA group), a statistically significant increase in the number of high-quality embryos was observed when AOA was performed. This suggests that AOA may have a beneficial effect on embryo development when the sperm is obtained from the epididymis in cases of obstructive azoospermia. The observed differences in the effectiveness of AOA between testicular and epididymal sperm may be explained by the stage of maturity of the male germ cells. According to one hypothesis the oocyte activation sperm factor, PLCζ, may be present at a different concentration or may have impaired activation capacity in immature spermatozoa, which could lead to reduced efficacy of AOA [99].
In contrast, a retrospective study showed different results regarding TESA-ICSI-AOA, involving 44 patients who experienced fertilization failure or low fertilization due to male infertility in previous cycles. Sperm samples were surgically retrieved through TESA. To address the issue of fertilization failure, calcium ionophore (A23187) was used for post-ICSI AOA in the study cycle. The study found significant differences between the ICSI-AOA cycles and the previous standard ICSI cycles in various parameters. The rate of fertilization was significantly higher in the ICSI-AOA cycles compared to the previous cycles (76.3% vs. 37.4%). Additionally, there were improvements in embryo quality, implantation rates, and clinical pregnancy rates in the ICSI-AOA cycles compared to the previous cycles. It is worth noting that the study included patients with different sources of spermatozoa, including ejaculated normal, ejaculated with OAT, or TESA. The study observed that the origin of the spermatozoa did not affect the clinical outcomes of AOA. Significant improvements were observed in all groups, suggesting that AOA can benefit patients regardless of the source of sperm used [75].
One of the major causes of TFF in patients with surgically sperm retrieved sperm is immobility in ICSI cycles. To stimulate motility pentoxifylline was known as the most common inducer of sperm motility in IVF lab. AOA was administered following pentoxifylline treatment in situations where there had been total fertilization failure or low fertilization in previous cycles or in cases of severe male factor infertility with non-motile spermatozoa. In this regard, Hee Jung Kang et al. conducted a retrospective study and included patients with TESE-ICSI cycles (TESE-ICSI without AOA: control n = 480 and TESE-ICSI with AOA: case group n = 29). The AOA group was divided into subgroups based on sperm motility following pentoxifylline (PF) treatment. Specifically, there were two subgroups: the motile sperm-injected group (n = 12) and the non-motile sperm-injected group (n = 17). The PF/AOA group exhibited lower rates of good embryo development (52.3% vs. 66.9%), pregnancy (20.7% vs. 52.1%), and successful delivery (10.3% vs. 40.8%) compared to the control group. However, when analyzing the impact of restoring sperm motility after PF treatment on clinical outcomes, there were no significant differences observed in the fertilization rate (66.6% vs. 64.7% for non-motile and motile sperm, respectively), pregnancy rate (17.6% vs. 33.3%), or delivery rate (5.9% vs. 16.7%) between the two groups. The findings of the study suggest that AOA is a successful approach for achieving fertilization in TESE-ICSI cycles, regardless of whether sperm motility is restored after pentoxifylline therapy [100].
Micro-TESE has become increasingly popular due to its high sperm retrieval rate and minimal tissue damage. In 2023, Xi Zhang and colleagues performed a retrospective study on 235 NOA patients who underwent micro-TESE for ICSI. The study divided the retrieved sperms into three groups: motile sperm with AOA, motile sperm without AOA (control), and immotile sperm with AOA. The findings revealed that AOA using ionomycin significantly enhanced fertilization rates in AOA groups compared to non-AOA (72.77% and 78.56% vs. 67.59%). However, it did not improve embryo quality or live birth outcomes. The researchers concluded that for NOA patients with only immotile sperm, AOA could achieve satisfactory fertilization rates and live birth outcomes. Therefore, the study suggests that AOA is recommended for patients with NOA when only immotile sperm are available for injection during ICSI. This technique can improve fertilization rates and increase the chances of achieving a live birth in such cases [101]. Despite the promising results reported in earlier studies, in a retrospective cohort study (n = 75), the inclusion of calcium ionophore (A23187) in TESE-ICSI cycles (n = 27) from patients with NOA or severe OAT did not lead to improvements in fertilization rates (60.8% vs. 66.9%) or clinical outcomes compared to TESE-ICSI cycles (n = 48) without AOA. As a result, the use of AOA should be limited to cases where there has been a history of low or total fertilization failure [102].
In a retrospective cohort study with a larg sample size (n = 365), cumulative live birth rate, as primary outcome, in surgically retrieved sperms in male factor infertility was evaluated following treatment with P–149 Ca2+ ionophores (GM508) as an aid. Study subjects (n = 365 cycles) were categorized into 4 groups: Microdissection Testicular Sperm Extraction (M-TESE) (n = 143), NOA-TESA (n = 38), OA-TESA (n = 62) and non-azoospermia (Testicular) (n = 92). Subgroups were further divided into cases where AOA was performed and controls where conventional ICSI without AOA was used. The application of AOA resulted in a significant improvement in the cumulative live birth rate in AOA-M-TESE (55.8% vs 33.3%), TESA for AOA-Testicular and AOA-NOA-TESA (55.55% vs 15%), and AOA-NOA-TESA (55.55% vs 15%) procedures. However, AOA did not demonstrate a significant improvement in the live birth rate for OA-TESA cases. A higher fertilization rate was significantly observed in AOA-M-TESE (81 ± 0.84 vs 64 ± 0.97), AOA-NOA-TESA (86 ± 0.76 vs 64 ± 0.13) and AOA-Testicular (72 ± 0.12 vs 57 ± 0.11). Similarly, cleavage rates and clinical pregnancy rates also exhibited significant differences, while miscarriage rate was comparable across these groups. However, in OA-TESA group, no significant differences were detected in any of the measured outcomes between case and controls, including cumulative live birth rate (51.6% vs 41.9%), fertilization rate (0.77 ± 0.14 vs 0.75 ± 0.11), clinical pregnancy and miscarriage rate, p- value > 0.05. A hypothesis can be proposed that in cases of NOA or Testicular, surgically obtained sperm might have reduced or absent capacity to induce Ca2+ oscillations. This could be due to deficient or inadequate levels of PLCζ or potential chromatin abnormalities within these sperm. Consequently, the control group, where AOA was not performed, exhibited lower fertilization rates and produce fewer high-quality embryos [103].
AOA and Epigenetic Modifications
The AOA procedure, being an additional manipulation in ART, has the potential to impact the cell metabolism and embryo development [3]. It is possible that side effects of AOA may become evident in future generations [104]. Importantly, during the AOA procedure, inducing an elevation in calcium levels does not fully replicate the effects of natural calcium oscillations observed under normal physiological conditions [21]. Therefore, the primary scientific obstacle hindering the application of AOA in clinical settings is the non-physiological approach of triggering the release of Ca2+ through the use of most AOA agents [105].
During fertilization, the occurrence of rising calcium coincides with active and rapid demethylation in the paternal genome, followed by passive DNA demethylation in the maternal genome. These Ca2+ fluctuations play a crucial role in coordinating and regulating these processes, which are essential for the proper development and reprogramming of the embryonic genome. Therefore, there is a possibility that an altered pattern of calcium signaling during fertilization could potentially have irreversible effects on the genomic imprinting process during this crucial period, which leads to long-lasting effects on gene expression and development [106]. There is limited research available regarding the potential side effects of AOA procedures on the developmental stages of embryos, particularly in relation to the disruption of subsequent gene expression during pre- and post-implantation events. The complex process of gene expression regulation plays a crucial role in normal embryonic development, and any disturbances caused by AOA procedures could potentially have consequences on the proper progression and outcome of these events.
In a study, Mingru Yin et al. investigated the effects of oocyte activation (OA) procedures on DNA methylation and gene expression changes in mouse embryos (pre and post implantation) generated through ICSI with additional AOA treatment. The study included several groups: ICSI, ICSI with AOA, oocyte activation-deficient ICSI (dICSI), and dICSI with AOA treatment. The findings of the study revealed lower rates of fertilization and blastocyst formation in the oocyte activation-deficient sperm injection group (dICSI) compared to the ICSI group. Subsequent analysis of the study detected AOA treatment can indeed influence the expression and methylation states of the imprinted gene Igf2r in mouse pre- and post-implantation embryos. The regulation of Igf2r is mediated by the imprinted Airn gene. In details, Mingru Yin et al. identified a total of 133 differentially expressed genes (DEGs) between the ICSI-AOA group and the ICSI group. These DEGs represent genes that showed significant differences in expression levels between the two groups, potentially indicating the impact of AOA treatment on gene expression patterns. Similarly, the study also identified 266 DEGs between the dICSI-AOA group (oocyte activation-deficient ICSI with AOA treatment) and the ICSI group. These DEGs reflect genes that exhibited distinct expression patterns between the dICSI-AOA group and the ICSI group [107].
The effects of AOA on imprinted genes during ART treatment were examined by Rong Liang et al. They specifically evaluated the methylation levels of imprinted genes, including H19, PEG3 (paternally expressed gene 3), and SNRPN (small nuclear ribonucleoprotein polypeptide N), using the pyrosequencing method. The samples analyzed in the study included cleavage stage embryos, blastocysts, and placenta. The analysis of three key imprinted genes (H19, PEG3, and SNRPN) in patients with and without AOA treatment revealed different methylation levels specifically in cleavage stage embryos. The observed variation in methylation levels was more pronounced in cleavage embryos compared to blastocysts and placenta tissues. In embryos that underwent AOA treatment, higher levels of methylation were detected in the SNRPN gene, while lower levels of methylation were observed in the H19 gene, in comparison to embryos without AOA treatment. However, no significant differences in methylation levels of the three genes were found in the other groups, including placenta tissue and blastocysts, irrespective of AOA treatment. Given these findings, the study suggests that transferring blastocysts rather than cleavage stage embryos may be a more suitable approach for patients undergoing AOA treatment during ART procedures. This recommendation is based on the understanding that the imprinting methylation levels of H19 and SNRPN genes, which play important roles in embryonic development, were not significantly affected at the blastocyst stage or in placenta tissues [108]. It is important to note that the specific consequences and implications of these differential methylation levels on gene expression and embryonic development would require further investigation.
Conclusion, Limitations, and Future Prospective
In summary, while AOA may hold promise as a method to improve fertilization and reproductive outcomes in certain situations, further research is needed to establish its efficacy and potential risks. Factors such as the underlying cause of infertility, the specific patient characteristics, and the overall treatment goals should be taken into account when making decisions about the use of AOA. Although, Advancements in this field of research can potentially lead to improved diagnostic techniques and tailored treatment strategies, this approach is not recommended for general population of ICSI. A cautious approach is warranted, and clinical decisions regarding AOA should be made on a case-by-case basis, considering the available evidence, individual patient factors, and a thorough assessment of the potential benefits and drawbacks associated with its use. However, it is important to note that further research and larger studies are needed to validate these findings and establish standardized protocols for the use of AOA in clinical practice.
There exist certain limitations to this systematic review. A portion of the included studies are case reports or have small sample sizes, which renders them of unacceptable quality. It’s also important to consider how uncommon the condition is. The ICSI results followed by AOA intervention are therefore not well-evaluated by high-quality studies. Variations in the control group designs were observed among the included studies. The records mostly included the previous ICSI cycles without AOA as control groups. Moreover, scanty information about the possible diseases of the participants/couples is provided by the studies that are currently available. This could help to explain some of the wide variation observed across studies in terms of other reproductive outcomes and the rate of fertilization. Consequently, it is impossible to avoid the following limitations: imprecise estimates of effect sizes (wider confidence interval), lack of generalizability, increased risk of bias (e.g., selection bias, measurement error) and high heterogeneity (variability in study designs and outcomes can affect the overall conclusions).
While there have been various methods of oocyte activation, chemicals agents have been studied more in-depth for oocyte activation in a clinical context. Because of this, the majority of research involving chemical activation techniques satisfied our inclusion criteria and were taken into account for this analysis. Except for electrical activation, which has two studies, nonchemical activation methods were extremely uncommon and did not meet the inclusion criteria for studies. Although, electrical stimulation can be considered as an alternative method, this method is more complicated. In this method, different protocols have been described to activate oocytes with different voltages and pulses in electrical stimulation. A high rate of oocyte degeneration is detected as disadvantage of this method. This approach requires additional research to optimize the protocol and assess its efficacy and safety in reproductive treatments, aiming to prioritize patient well-being and minimize potential risks.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8.
Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16(6):690–703.
Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril. 2016;106(3):590-6.e2.
Tosti E, Ménézo Y. Gamete activation: basic knowledge and clinical applications. Hum Reprod Update. 2016;22(4):420–39.
Karabulut S, Aksünger Ö, Ata C, Sağıroglu Y, Keskin İ. Artificial oocyte activation with calcium ionophore for frozen sperm cycles. Syst Biol Reprod Med. 2018;64(5):381–8.
Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv Biol Regul. 2018;67:148–62.
Aydinuraz B, Dirican EK, Olgan S, Aksunger O, Erturk OK. Artificial oocyte activation after intracytoplasmic morphologically selected sperm injection: a prospective randomized sibling oocyte study. Hum Fertil (Camb). 2016;19(4):282–8.
Ebner T, Montag M. Artificial oocyte activation: evidence for clinical readiness. Reprod Biomed Online. 2016;32(3):271–3.
Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019;112(2):266–74.
Anifandis G, Michopoulos A, Daponte A, Chatzimeletiou K, Simopoulou M, Messini CI, et al. Artificial oocyte activation: physiological, pathophysiological and ethical aspects. Syst Biol Reprod Med. 2019;65(1):3–11.
Ramadan WM, Kashir J, Jones C, Coward K. Oocyte activation and phospholipase C zeta (PLCζ): diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal. 2012;10(1):12.
Dai W, Bai Y, Hebda L, Zhong X, Liu J, Kao J, et al. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014;21(4):568–81.
Kashir J. Increasing associations between defects in phospholipase C zeta and conditions of male infertility: not just ICSI failure? J Assist Reprod Genet. 2020;37(6):1273–93.
Kashir J, Mistry BV, BuSaleh L, Abu-Dawas R, Nomikos M, Ajlan A, et al. Phospholipase C zeta profiles are indicative of optimal sperm parameters and fertilisation success in patients undergoing fertility treatment. Andrology. 2020;8(5):1143–59.
Meng X, Melo P, Jones C, Ross C, Mounce G, Turner K, et al. Use of phospholipase C zeta analysis to identify candidates for artificial oocyte activation: a case series of clinical pregnancies and a proposed algorithm for patient management. Fertil Steril. 2020;114(1):163–74.
Cheung S, Xie P, Parrella A, Keating D, Rosenwaks Z, Palermo GD. Identification and treatment of men with phospholipase Cζ-defective spermatozoa. Fertil Steril. 2020;114(3):535–44.
Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca(2+) transients vs oscillatory Ca(2+) signaling for assisted oocyte activation: limitations and benefits. Reproduction. 2018;155(2):R105–19.
Ferrer-Buitrago M, Dhaenens L, Lu Y, Bonte D, Vanden Meerschaut F, De Sutter P, et al. Human oocyte calcium analysis predicts the response to assisted oocyte activation in patients experiencing fertilization failure after ICSI. Hum Reprod. 2018;33(3):416–25.
Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: clinical update and topical developments. Adv Biol Regul. 2016;61:58–67.
Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94(2):520–6.
Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online. 2014;28(5):560–71.
Yanagida K, Fujikura Y, Katayose H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol. 2008;7(3):133–42.
Egashira A, Murakami M, Haigo K, Horiuchi T, Kuramoto T. A successful pregnancy and live birth after intracytoplasmic sperm injection with globozoospermic sperm and electrical oocyte activation. Fertil Steril. 2009;92(6):2037.e5-9.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002;78(3):619–24.
Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19(8):1837–41.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9(3):511–8.
Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014;55(1):24–37.
Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online. 2008;17(5):662–8.
Yoshida S, Plant S. Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J Physiol. 1992;458:307–18.
Vanden Meerschaut F, Nikiforaki D, De Roo C, Lierman S, Qian C, Schmitt-John T, et al. Comparison of pre- and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum Reprod. 2013;28(5):1190–8.
ESHRE Add-ons working group, Lundin K, Bentzen J, Bozdag G, Ebner T, Harper J, et al. Good practice recommendations on add-ons in reproductive medicine. Human Reproduction. 2023; 38(11):2062–104.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2020;2021:372.
Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: The Cambridge Quality Checklists. J Exp Criminol. 2009;5:1–23.
Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, et al. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102(4):649–57.
Nasr-Esfahani MH, Razavi S, Javdan Z, Tavalaee M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril. 2008;90(6):2231–7.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Artificial Oocyte Activation: Methods and Clinical Efficiency after Intra-Cytoplasmic Sperm Injection. J Isfahan Med Sch. 2013;31(240):851–66.
Sun B, Yeh J. Calcium oscillatory patterns and oocyte activation during fertilization: a possible mechanism for total fertilization failure (TFF) in human in vitro fertilization? Reprod Sci. 2021;28:639–48.
Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertility and Sterility. 2017;108(3):468–82. e3.
Baltaci V, Ayvaz ÖÜ, Ünsal E, Aktaş Y, Baltacı A, Turhan F, et al. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril. 2010;94(3):900–4.
Chen J, Qian Y, Tan Y, Mima H. Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilisation failures during previous intracytoplasmic sperm injection treatment. Reprod Fertil Dev. 2010;22(5):852–5.
Kim J-W, Kim S-D, Yang S-H, Yoon S-H, Jung J-H, Lim J-H. Successful pregnancy after SrCl2 oocyte activation in couples with repeated low fertilization rates following calcium ionophore treatment. Syst Biol Reprod Med. 2014;60(3):177–82.
Vanden Meerschaut F, Nikiforaki D, De Gheselle S, Dullaerts V, Van den Abbeel E, Gerris J, et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012;27(7):1977–84.
Ebner T, Montag M, Montag M, Van der Ven K, Van der Ven H, Ebner T, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30(4):359–65.
Tejera A, Ferri LA, Izquierdo PG, Torregrosa DB, Remohí JA, Escrivá MM. Treatment with Calcium Ionophore Improves The Results in Patients with Previous Unsuccessful Attempts at The Fertilization: A Cohort Study. Int J Fertil Steril. 2021;15(4):286.
Akashi K, Yamada M, Jwa SC, Utsuno H, Kamijo S, Hirota Y, et al. Artificial oocyte activation using Ca2+ ionophores following intracytoplasmic sperm injection for low fertilization rate. Front Endocrinol. 2023;14:1131808.
Jia L, Chen P, Su W, He S, Guo Y, Zheng L, et al. Artificial oocyte activation with ionomycin compared with A23187 among patients at risk of failed or impaired fertilization. Reprod Biomed Online. 2023;46(1):35–45.
Wang M, Zhu L, Liu C, He H, Wang C, Xing C, et al. A novel assisted oocyte activation method improves fertilization in patients with recurrent fertilization failure. Front Cell Dev Biol. 2021;9: 672081.
Barbieri RL. Female infertility. In: Yen and Jaffe’s reproductive endocrinology: Elsevier; 2019. pp. 556–81.e7.
Aytac PC, Kilicdag EB, Haydardedeoglu B, Simsek E, Cok T, Parlakgumus HA. Can calcium ionophore “use” in patients with diminished ovarian reserve increase fertilization and pregnancy rates? A randomized, controlled study. Fertil Steril. 2015;104(5):1168–74.
Lv M, Zhang D, He X, Chen B, Li Q, Ding D, et al. Artificial oocyte activation to improve reproductive outcomes in couples with various causes of infertility: A retrospective cohort study. Reprod Biomed Online. 2020;40(4):501–9.
Demain LA, Conway GS, Newman WG. Genetics of mitochondrial dysfunction and infertility. Clin Genet. 2017;91(2):199–207.
Fawzy M, Emad M, Mahran A, Sabry M, Fetih AN, Abdelghafar H, et al. Artificial oocyte activation with SrCl2 or calcimycin after ICSI improves clinical and embryological outcomes compared with ICSI alone: results of a randomized clinical trial. Hum Reprod. 2018;33(9):1636–44.
Tavalaee M, Kiani-Esfahani A, Nasr-Esfahani MH. Relationship between potential sperm factors involved in oocyte activation and sperm DNA fragmentation with intra-cytoplasmic sperm injection clinical outcomes. Cell Journal (Yakhteh). 2017;18(4):588.
Yoon S-Y, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod. 2012;27(6):1768–80.
Escoffier J, Lee HC, Yassine S, Zouari R, Martinez G, Karaouzène T, et al. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum Mol Genet. 2016;25(5):878–91.
Kashir J, Konstantinidis M, Jones C, Lemmon B, Chang Lee H, Hamer R, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012;27(1):222–31.
Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod. 2011;26(12):3372–87.
Torra-Massana M, Cornet-Bartolomé D, Barragán M, Durban M, Ferrer-Vaquer A, Zambelli F, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019;34(8):1494–504.
Nazarian H, Azad N, Nazari L, Piryaei A, Heidari MH, Masteri-Farahani R, et al. Effect of artificial oocyte activation on intra-cytoplasmic sperm injection outcomes in patients with lower percentage of sperm containing phospholipase Cζ: A randomized clinical trial. Journal of Reproduction & Infertility. 2019;20(1):3.
Mu J, Zhang Z, Wu L, Fu J, Chen B, Yan Z, et al. The identification of novel mutations in PLCZ1 responsible for human fertilization failure and a therapeutic intervention by artificial oocyte activation. Mol Hum Reprod. 2020;26(2):80–7.
Amdani SN, Yeste M, Jones C, Coward K. Sperm factors and oocyte activation: current controversies and considerations. Biol Reprod. 2015;93(2):50.
Martínez M, Durban M, Santaló J, Rodríguez A, Vassena R. Assisted oocyte activation effects on the morphokinetic pattern of derived embryos. J Assist Reprod Genet. 2021;38:531–7.
Kashir J, Nomikos M, Swann K, Lai FA. PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mhr. 2015;21(5):383–8.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril. 2014;102(2):440–7.
Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, et al. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod. 2014;20(10):938–47.
Nomikos M, Sanders JR, Kashir J, Sanusi R, Buntwal L, Love D, et al. Functional disparity between human PAWP and PLCζ in the generation of Ca2+ oscillations for oocyte activation. Mol Hum Reprod. 2015;21(9):702–10.
Azad N, Nazarian H, Nazari L, Ghaffari Novin M, Piryaei A, Heidari MH, et al. Evaluation of PAWP and PLC? Expression in Infertile Men with Previous ICSI Fertilization Failure. Urol J. 2018;15(3):116–21.
Lee HC, Arny M, Grow D, Dumesic D, Fissore RA, Jellerette-Nolan T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet. 2014;31:749–56.
Mirsanei JS, Sheibak N, Zandieh Z, Mehdizadeh M, Aflatoonian R, Tabatabaei M, et al. Microfluidic chips as a method for sperm selection improve fertilization rate in couples with fertilization failure. Arch Gynecol Obstet. 2022;306(3):901–10.
Tang L, Rao M, Yang W, Yao Y, Luo Q, Lu L, et al. Predictive value of the sperm DNA fragmentation index for low or failed IVF fertilization in men with mild-to-moderate asthenozoospermia. J Gynecol Obstet Hum Reprod. 2021;50(6): 101868.
Park JH, Kim SK, Kim J, Kim JH, Chang JH, Jee BC, et al. Relationship between phospholipase C zeta immunoreactivity and DNA fragmentation and oxidation in human sperm. Obstet Gynecol Sci. 2015;58(3):232–8.
Duval C, Joao F, Calvé A, Montjean D, Bélanger M, Benkhlaifa M, et al. O-020 Sperm phospholipase C zeta levels measured by flow cytometry are associated with sperm parameters. Human Reprod. 2022;37:104.
Azad N, Nazarian H, Ghaffari Novin M, Masteri Farahani R, Piryaei A, Heidari MH. Phospholipase C zeta parameters in sperm from polymorphic teratozoospermic men. Ann Anat. 2018;215:63–70.
Azad N, Nazarian H, Ghaffari Novin M, Masteri Farahani R, Piryaei A, Heidari MH, et al. Oligoasthenoteratozoospermic (OAT) men display altered phospholipase C ζ (PLCζ) localization and a lower percentage of sperm cells expressing PLCζ and post-acrosomal sheath WW domain-binding protein (PAWP). Bosn J Basic Med Sci. 2018;18(2):178–84.
Yoon HJ, Bae IH, Kim HJ, Jang JM, Hur YS, Kim HK, et al. Analysis of clinical outcomes with respect to spermatozoan origin after artificial oocyte activation with a calcium ionophore. J Assist Reprod Genet. 2013;30:1569–75.
Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: A randomized clinical trial. Iran J Reprod Med. 2013;11(11):875.
Wolff H, Schill W, Moritz P. Round-headed spermatozoa: a rare andrologic finding (“globe-headed spermatozoa”, “globozoospermia”). Der Hautarzt; Zeitschrift Fur Dermatologie, Venerologie, Und Verwandte Gebiete. 1976;27(3):111–6.
Han F, Liu C, Zhang L, Chen M, Zhou Y, Qin Y, et al. Globozoospermia and lack of acrosome formation in GM130-deficient mice. Cell Death Dis. 2018;8(1):e2532.
Singh G. Ultrastructural features of round-headed human spermatozoa. Int J Fertil. 1992;37(2):99–102.
Dam A, Feenstra I, Westphal J, Ramos L, Van Golde R, Kremer J. Globozoospermia revisited. Hum Reprod Update. 2007;13(1):63–75.
Dam AH, Ramos L, Dijkman HB, Woestenenk R, Robben H, van den Hoven L, et al. Morphology of partial globozoospermia. J Androl. 2011;32(2):199–206.
Huang G, Zhang X, Yao G, Huang L, Wu S, Li X, et al. A loss-of-function variant in SSFA2 causes male infertility with globozoospermia and failed oocyte activation. Reprod Biol Endocrinol. 2022;20(1):1–13.
Tavalaee M, Nasr-Esfahani M. Expression profile of PLC ζ, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology. 2016;4(5):850–6.
Tavalaee M, Nomikos M, Lai FA, Nasr-Esfahani MH. Expression of sperm PLCζ and clinical outcomes of ICSI-AOA in men affected by globozoospermia due to DPY19L2 deletion. Reprod Biomed Online. 2018;36(3):348–55.
Karaca N, Akpak YK, Oral S, Durmus T, Yilmaz R. A successful healthy childbirth in a case of total globozoospermia with oocyte activation by calcium ionophore. J Reprod Infertil. 2015;16(2):116.
Kochhar PK, Ghosh P. Intracytoplasmic sperm injection with assisted oocyte activation resulting in successful pregnancies and live birth in couples with globozoospermia: a report of two cases. J Hum Reprod Sci. 2018;11(1):72.
Tejera A, Mollá M, Muriel L, Remohí J, Pellicer A, De Pablo JL. Successful pregnancy and childbirth after intracytoplasmic sperm injection with calcium ionophore oocyte activation in a globozoospermic patient. Fertil Steril. 2008;90(4):1202.e1-1202.e5.
Niu X, Ruan Q, Witz CA, Wang W. Comparison of human oocyte activation between round-headed sperm injection followed by calcium ionophore treatment and normal sperm injection in a patient with globozoospermia. Front Endocrinol. 2020;11:183.
Yanagida K, Katayose H, Yazawa H, Kimura Y, Sato A, Yanagimachi H, et al. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod. 1999;14(5):1307–11.
Mansour R, Fahmy I, Tawab NA, Kamal A, El-Demery Y, Aboulghar M, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009;91(1):133–9.
Egashira A, Murakami M, Haigo K, Horiuchi T, Kuramoto T. A successful pregnancy and live birth after intracytoplasmic sperm injection with globozoospermic sperm and electrical oocyte activation. Fertil Steril. 2009;92(6):2037.e5-2037.e9.
Kuentz P, Vanden Meerschaut F, Elinati E, Nasr-Esfahani M, Gurgan T, Iqbal N, et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum Reprod. 2013;28(4):1054–61.
Stone S, O’Mahony F, Khalaf Y, Taylor A, Braude P. A normal livebirth after intracytoplasmic sperm injection for globozoospermia without assisted oocyte activation: case report. Hum Reprod. 2000;15(1):139–41.
Dirican EK, Isik A, Vicdan K, Sozen E, Suludere Z. Clinical pregnancies and livebirths achieved by intracytoplasmic injection of round headed acrosomeless spermatozoa with and without oocyte activation in familial globozoospermia: case report. Asian J Androl. 2008;10(2):332–6.
Zhang ZQ, Long SG, Huang ZH, Xin CL, Wu QF. Different outcomes after intracytoplasmic sperm injection without oocyte activation in two patients with different types of globozoospermia. Andrologia. 2016;48(1):116–20.
Akbal C, Mangır N, Tavukçu HH, Özgür Ö, Şimşek F. Effect of testicular sperm extraction outcome on sexual function in patients with male factor infertility. Urology. 2010;75(3):598–601.
Kim J-W, Choi J-L, Yang S-H, Yoon S-H, Jung J-H, Lim J-H. Live birth after SrCl 2 oocyte activation in previous repeated failed or low fertilization rates after ICSI of frozen-thawed testicular spermatozoa: case report. J Assist Reprod Genet. 2012;29:1393–6.
Borges E Jr, Braga DPdAF, de Sousa Bonetti TC, Iaconelli A Jr, Franco JG Jr. Artificial oocyte activation using calcium ionophore in ICSI cycles with spermatozoa from different sources. Reprod Biomed Online. 2009;18(1):45–52.
Borges E Jr, Braga DPdAF, de Sousa Bonetti TC, Iaconelli A Jr, Franco JG Jr. Artificial oocyte activation with calcium ionophore A23187 in intracytoplasmic sperm injection cycles using surgically retrieved spermatozoa. Fertil Steril. 2009;92(1):131–6.
Kang HJ, Lee S-H, Park Y-S, Lim CK, Ko DS, Yang KM, et al. Artificial oocyte activation in intracytoplasmic sperm injection cycles using testicular sperm in human in vitro fertilization. Clin Exp Reprod Med. 2015;42(2):45.
Zhang X, Li L, Zhang W, Luo Y, Mao Y, Du H, et al. Embryo development and live birth resulted from artificial oocyte activation after microdissection testicular sperm extraction with ICSI in patients with non-obstructive azoospermia. Front Endocrinol. 2023;14:1123541.
Sdrigotti A, Nieto FL, Dacharry L, Valzacchi GR. Artificial oocyte activation in ICSI cycles using testicular spermatozoa. Fertil Steril. 2016;106(3): e188.
Sahu A, Singh S, Varghese A, Ashraf R, Majiyd N, Singh S, et al., editors. Calcium ionophores as an aid to surgically retrieved sperms in male factor infertility for increasing cumulative live birth rate. Human Reproduction; 2021: Oxford Univ Press
Santella L, Dale B. Assisted yes, but where do we draw the line? Reprod Biomed Online. 2015;31(4):476–8.
Kashir J, Ganesh D, Jones C, Coward K. Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application. Human Reprod Open. 2022;2022(2):hoac003.
Nikiforaki D, Meerschaut FV, De Roo C, Lu Y, Ferrer-Buitrago M, De Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798-806.e2.
Yin M, Yu W, Li W, Zhu Q, Long H, Kong P, et al. DNA methylation and gene expression changes in mouse pre-and post-implantation embryos generated by intracytoplasmic sperm injection with artificial oocyte activation. Reprod Biol Endocrinol. 2021;19:1–14.
Liang R, Fang F, Li S, Chen X, Zhang X, Lu Q. Is there any effect on imprinted genes H19, PEG3, and SNRPN during AOA? Open Medicine. 2022;17(1):174–84.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
N.A. created the concept and design of the study; SH.M., N.G., F.M., A.G., M.M. participated in data collection and writing of the draft of the manuscript; N.A and SH.M critically revised the manuscript; K.M reviewed English language editing and revised the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors gave consent for publication.
All authors agree to the order of authors.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43032_2024_1671_MOESM1_ESM.docx
Supplementary file1 Supplementary Table 1 Evaluation of studies quality using “The Cambridge Quality Checklists” Supplementary. (DOCX 22 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehdinejadiani, S., Goudarzi, N., Masjedi, F. et al. Clinical Applications of Assisted Oocyte Activation in Couples with Various Reproductive Problems: A Systematic Review. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01671-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01671-z