Abstract
Pre-pregnancy obesity was associated with gestational diabetes in observational studies, but whether this relationship is causal remains to be determined. To evaluate whether pre-pregnancy obesity traits causally affect gestational diabetes risk, a two-sample Mendelian randomization (MR) analysis was performed utilizing summary-level statistics from published genome-wide association studies (GWAS). Obesity-related traits included body mass index (BMI), overweight, obesity, obesity class 1, obesity class 2, obesity class 3, childhood obesity, waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), percent liver fat, visceral adipose tissue volume, abdominal subcutaneous adipose tissue volume. Effect estimates were evaluated using the inverse-variance weighting method. Weighted median, MR-Egger, simple mode, and weighted mode were performed as sensitivity analyses. Genetically predicted pre-pregnancy BMI [odds ratio (OR) = 1.68; 95% confidence interval (CI): 1.45–1.95; P = 9.13 × 10–12], overweight (OR = 1.49; 95% CI: 1.21–1.85; P = 2.06 × 10–4), obesity (OR = 1.25; 95% CI: 1.18–1.33; P = 8.01 × 10–13), obesity class 1 (OR = 1.31; 95% CI: 1.17–1.46; P = 1.49 × 10–6), obesity class 2 (OR = 1.26; 95% CI: 1.16–1.37; P = 5.23 × 10–8), childhood obesity (OR = 1.33; 95% CI: 1.23–1.44; P = 4.06 × 10–12), and WHR (OR = 2.35; 95% CI: 1.44–3.83; P = 5.89 × 10–4) were associated with increased risk of gestational diabetes. No significant association was observed with obesity class 3, WC, HC, percent liver fat, visceral adipose tissue volume, or abdominal subcutaneous adipose tissue volume. Similar results were observed in sensitivity analyses. Therefore, genetically predicted pre-pregnancy obesity traits may increase the risk of gestational diabetes. Weight control before pregnancy may be beneficial to prevent gestational diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global epidemics of overweight and obesity are leading health burdens worldwide, with 40% of women overweight (body mass index [BMI] 25–29.99 kg/m2) and 15% obese (BMI ≥ 30 kg/m2) [1]. It is estimated that the total number of adults who are overweight or obese will reach 2.1 billion worldwide [2]. The increasing prevalence of obesity in women of reproductive age will have a significant effect on obstetric outcomes, especially maternal complications such as gestational diabetes [3], which will put further strain on healthcare systems. Prevention and optimal treatment of pre-pregnancy obesity have the potential to significantly minimize negative fetal and maternal pregnancy outcomes. Early identification of females at risk of gestational diabetes is essential so they can be placed under increased surveillance as early as possible.
Although pre-pregnancy obesity may be associated with an increased incidence of gestational diabetes in previous studies using traditional epidemiological methods, the inability to fully adjust for numerous confounders was an important limitation. In some observational studies, the use of maternal BMI based on weight during early pregnancy as a proxy for pre-pregnancy weight may be influenced by weight gain in pregnancy [4, 5]. This could influence the interpretation of the results and should be considered for future studies. In Mendelian randomization (MR), genetic variation significantly correlated with exposure is used as an instrumental variable to infer causality between exposure and outcome. Because the alleles of the parents are randomly assigned to the offspring during the decelerated division and are fixed at conception, MR is also known as a "natural randomized controlled trial", which could avoid the confounding bias and reversed causality common in observational studies.
Against this background, this study aimed to systematically assess the causal associations between pre-pregnancy obesity traits and gestational diabetes using summary statistics of genome-wide association studies (GWAS).
Methods and Materials
Study Design
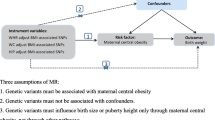
The present study was a two-sample MR study using publicly available genetic data obtained from GWAS. Three basic assumptions had to be met to draw causal conclusions from this MR study: (A) the genetic variants were significantly associated with obesity traits; (B) the genetic variants were not linked with any potential confounders; (C) the genetic variants were not associated with gestational diabetes except via the way of obesity traits.
Data Sources
In the present MR study, the exposure variable was pre-pregnancy obesity traits including BMI, overweight, obesity, obesity class 1, obesity class 2, obesity class 3, childhood obesity, waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), percent liver fat, visceral adipose tissue volume, and abdominal subcutaneous adipose tissue volume. Due to all data being from the European population, the definition of overweight or obesity was based on the degree of BMI (kg/m2) in adults as follows: healthy weight: 18.5–24.9, overweight: 25–29.9, obesity class 1: 30–34.9, obesity class 2: 35–39.9, and obesity class 3: ≥ 40 [6]. The outcome variable was gestational diabetes. Summary-level data for BMI was obtained from the Genetic Investigation of ANthropometric Traits (GIANT) Consortium conducted by Yengo L and colleagues [7]. Summary-level data for overweight, obesity, obesity class 1, obesity class 2, and obesity class 3 were obtained from the GIANT Consortium conducted by Berndt SI and colleagues [8]. Summary-level data for childhood obesity was obtained from the Early Growth Genetics (EGG) Consortium conducted by Bradfield JP and colleagues [9]. Summary-level data for WC, HC, and WHR were obtained from the GIANT Consortium conducted by Shungin D and colleagues [10]. Summary-level data for percent liver fat, visceral adipose tissue volume, and abdominal subcutaneous adipose tissue volume were analyzed by Liu Y and colleagues [11]. Summary-level data for gestational diabetes was obtained from the FinnGen Biobank (www.finngen.fi/en). Detailed information about the included GWAS can be found in Supplementary Table 1. All data used in this study were summary-level data from publicly available GWAS. As informed consent and ethical approval were obtained in the original study, they were not required in the present MR analysis.
Instrumental Variable Selection
The instrumental variable was selected according to the following criteria: (A) all genetic variants were significantly associated with obesity traits (P < 5 × 10–8); (B) all the enrolled genetic variants were clumped based on the linkage disequilibrium (R2 < 0.001 and clumping window > 10,000 kb); (C) the strength of genetic variants was evaluated by the F statistics, with an F statistic above 10 representing a sufficiently strong instrument. Genetic variants with an F statistic of less than 10 will be deleted. We harmonized all instrumental variables to guarantee that an SNP’s effect on exposure and outcome corresponded to the same allele. The baseline characteristics of selected SNPs for BMI (n = 475), overweight (n = 13), obesity (n = 27), obesity class 1 (n = 16), obesity class 2 (n = 11), obesity class 3 (n = 2), childhood obesity (n = 5), WC (n = 63), HC (n = 74), WHR (n = 29), percent liver fat (n = 10), visceral adipose tissue volume (n = 5), abdominal subcutaneous adipose tissue volume (n = 2) can be found in Supplementary Table 2. For exposures with the number of instrumental variables less than 10, sensitivity analyses were performed by setting the P value below P < 5 × 10–6.
Statistical Analysis
A multiplicative random-effects inverse-variance weighting (IVW) method, which synthesizes the effect derived from each instrument by using the inverse variance, was used for the primary MR analysis to estimate the main effects of obesity traits on gestational diabetes. Other MR analyses including weighted median, MR-Egger, simple mode, and weighted mode were performed as sensitivity analyses. The weighted median model was used to check invalid instrument bias. It can provide consistent estimates as long as more than 50% of the weight comes from valid SNPs [12]. MR-Egger regression allowed for horizontal pleiotropic effects and produced unbiased causal effect estimates. If possible horizontal pleiotropy existed, the MR-Egger intercept was nonzero with statistical significance (P < 0.05) [13]. Funnel plots can also detect horizontal pleiotropy if asymmetry exists. The Cochrane’s Q statistic was used to test for heterogeneity. Leave-one-out analysis was performed to examine the effect of each instrument on the overall effect. MR analysis was performed by using the R software (version 4.3.1) and the R package “TwoSampleMR” version 0.5.7.
Results
The scatter plot of the association between genetically predicted obesity traits and gestational diabetes is shown in Fig. 1. Positive associations were observed for BMI, overweight, obesity, obesity class 1, obesity class 2, obesity class 3, childhood obesity, and WHR, whereas no significant associations were found for WC, HC, percent liver fat, visceral adipose tissue volume, or abdominal subcutaneous adipose tissue volume. According to the IVW results in Fig. 2, genetic predisposition to high BMI [odds ratio (OR) = 1.68; 95% confidence interval (CI): 1.45–1.95; P = 9.13 × 10–12], overweight (OR = 1.49; 95% CI: 1.21–1.85; P = 2.06 × 10–4), obesity (OR = 1.25; 95% CI: 1.18–1.33; P = 8.01 × 10–13), obesity class 1 (OR = 1.31; 95% CI: 1.17–1.46; P = 1.49 × 10–6), obesity class 2 (OR = 1.26; 95% CI: 1.16–1.37; P = 5.23 × 10–8), childhood obesity (OR = 1.33; 95% CI: 1.23–1.44; P = 4.06 × 10–12), WHR (OR = 2.35; 95% CI: 1.44–3.83; P = 5.89 × 10–4) were associated with increased risk of gestational diabetes. Similar results were observed using other MR methods in sensitivity analyses (Table 1). No significant association was observed for obesity class 3 (OR = 1.18; 95% CI: 1.00–1.38; P = 0.053), WC (OR = 1.18; 95% CI: 0.90–1.54; P = 0.24), HC (OR = 0.85; 95% CI: 0.69–1.04; P = 0.12), percent liver fat (OR = 1.00; 95% CI: 0.78–1.30; P = 0.99), visceral adipose tissue volume (OR = 1.51; 95% CI: 0.68–3.37; P = 0.31), or abdominal subcutaneous adipose tissue volume (OR = 2.26; 95% CI: 0.91–5.61; P = 0.08).
Scatter plot of the associations between genetically predicted obesity traits and gestational diabete. A, Body mass index; B, Overweight; C, Obesity; D, Obesity class 1; E, Obesity class 2; F, Obesity class 3; G, Childhood obesity; H, Waist circumference; I, Hip circumference; J, Waist-to-hip ratio; K, Percent liver fat; L, Visceral adipose tissue volume; M, Abdominal subcutaneous adipose tissue volume
The MR-Egger intercept was nonzero without statistical significance, revealing no evidence of horizontal pleiotropy (Table 2). The results were also corroborated by the funnel plot in Fig. 3. Significant heterogeneity was observed for BMI (P < 0.01), overweight (P = 0.04), WC (P = 0.01), HC (P = 0.02), WHR (P < 0.01), percent liver fat (P < 0.01), and visceral adipose tissue volume (P = 0.01, Table 2). The leave-one-out sensitivity analysis indicated that the overall estimate was not influenced by a single SNP (Supplementary Fig. 1).
Funnel plot of the associations between genetically predicted obesity traits and gestational diabetes. A, Body mass index; B, Overweight; C, Obesity; D, Obesity class 1; E, Obesity class 2; F, Obesity class 3; G, Childhood obesity; H, Waist circumference; I, Hip circumference; J, Waist-to-hip ratio; K, Percent liver fat; L, Visceral adipose tissue volume; M, Abdominal subcutaneous adipose tissue volume
After setting the P value below 5 × 10–6 for exposures with the number of instrumental variables less than 10, the number of instrumental variables for obesity class 3, childhood obesity, visceral adipose tissue volume, and abdominal subcutaneous adipose tissue volume was 10, 14, 35, and 34, respectively. The results in Table 3 indicated that genetic predisposition to obesity class 3 and childhood obesity were associated with increased risk of gestational diabetes. No significant association was observed for visceral adipose tissue volume or abdominal subcutaneous adipose tissue volume. The scatter plot in Supplementary Fig. 2 revealed similar results. The funnel plot (Supplementary Fig. 3) and leave-one-out sensitivity analysis (Supplementary Fig. 4) were concordant with the original results.
Discussion
Leveraging GWAS summary data, we explored the causal association between pre-pregnancy obesity traits and gestational diabetes (pregnancy-induced). Our two-sample MR study demonstrated that increased BMI may increase the risk of gestational diabetes. Meantime, other obesity traits including overweight, obesity, obesity class 1, obesity class 2, childhood obesity, and WHR were also significantly associated with gestational diabetes. This potentially impacts public health policy around targeting education, preventing, and controlling obesity in women of reproductive age.
Consistent with our findings, some previous observational studies also found that obesity was significantly correlated with gestational diabetes risk. In a cohort study with 14451 singleton pregnancies from 15 medical centers in Beijing, the prevalence of gestational diabetes increased continuously as the pre-pregnancy BMI increased. Compared with females with normal weight (BMI 18.5–23.9 kg/m2), the risk of gestational diabetes for overweight (BMI 24–27.9 kg/m2) and obesity (BMI 28 kg/m2) was 1.91 (95% CI: 1.72–2.12; P < 0.0001) and 2.55 (95% CI: 2.16–3.00; P < 0.0001), respectively [14]. It is proposed that pre-pregnancy overweight and obesity are the primary healthcare challenges among the identified risk factors for gestational diabetes [15].
The pathophysiological mechanisms underlying the significant association between obesity and gestational diabetes remain poorly defined and warrant further research. Gestational diabetes is characterized by insulin resistance and insufficient insulin secretion, resulting in hyperglycemia during pregnancy. The combination of gestational diabetes and overweight or obesity worsens the unfavorable pregnancy outcomes caused by either factor alone. Besides, women with obesity were characterized by the broad attenuated metabolic response to pregnancy. In a secondary analysis of two study cohorts including 741 pregnant women, women with obesity (BMI ≥ 30 kg/ m2) had significantly higher levels of many lipoprotein lipids including all very-low-density lipoprotein subclasses, small high-density particles, cholesterol, and triglycerides in very-low-density lipoprotein, and total triglycerides in comparison to those with normal weight (BMI 18.5–24.99 kg/m2) at median 13, 20, and 28 weeks of gestation [16]. Third, pregnant women with obesity were less flexible in adapting to changes in energy fuel demands, and they displayed higher inflammation marker levels after a test meal [17]. Fourth, the biomarker of chronic inflammation, GlycA, which was associated with type 2 diabetes, was persistently high across pregnancies complicated by obesity [18]. GlycA levels elevated during normal pregnancy and were even higher in obese than in overweight pregnant women [19]. Overall, obesity, metabolic inflexibility, attenuated metabolic response, and inflammation may all interact to produce negative long-term outcomes such as decreased glucose metabolism and insulin resistance [20].
Apart from BMI, body fat distribution has been recognized as a vital factor for health. There is considerable discussion about which risk-prediction measures are most effective. It is suggested that WC outperforms BMI alone in identifying individuals with a high-risk obesity phenotype, and this is explained in part by the capacity of WC to recognize adults with greater visceral fat mass [21]. Visceral adiposity has emerged as an independent predictor of insulin resistance [22]. However, it is unclear if body fat distribution is important in predicting the risk of an adverse pregnancy outcome [23]. Therefore, we aimed to systematically assess the causal associations between pre-pregnancy obesity traits and gestational diabetes. Our results indicated that WHR was also significantly associated with gestational diabetes. A meta-analysis also revealed a significantly increased risk of gestational diabetes for females with higher WC (OR = 1.40; 95% CI: 1.04–1.88) and WHR (OR = 2.73; 95% CI: 1.67–4.45) [24]. In a prospective cohort of 485 women, subcutaneous, visceral, and total adipose tissue depth were measured using ultrasound at 11–14 weeks gestation. After adjusting for potential confounders, higher visceral and total adipose tissue depth were significantly associated with the composite outcome of impaired fasting glucose, impaired glucose tolerance, and gestational diabetes. Similar results were observed for gestational diabetes alone [23]. However, in the present MR analyses, no significant association was observed for visceral adipose tissue volume, which may be explained by the limited number of genetic variants. Although sensitivity analysis was performed to obtain more SNPs, the results remained unchanged. Further studies are required to validate or refute our findings. Additionally, childhood obesity may increase the risk of gestational diabetes, which reinforces the need to address rising childhood overweight and obesity rates, whose adverse consequences in adulthood pose serious challenges to personal and population health [25].
The clinical implications of our findings are linked to the need to enhance education among women of childbearing age about the hazards of obesity and overweight, as well as the beneficial impact of appropriate physical activity and proper diet. According to a prospective randomized clinical trial conducted in 300 Chinese singleton pregnancies who met the criteria for overweight/obese status (BMI 24–28 kg/m2), women randomized to the exercise group (cycling exercise initiated early in pregnancy and performed at least 30 min, three times per week) had a significantly lower incidence of gestational diabetes than those in the control group (22.0% vs 40.6%; P < 0.001). Furthermore, exercise did not increase the risk of preterm birth or reduce the mean gestational age at birth. [26] The Polish guidelines recommend four to five meals per day, with calorie consumption increasing by 360 and 475 kcal/day in the second and third trimesters, respectively [27]. The recommendations from the Institute of Medicine (IOM) of 2009 suggest that the optimal weight gain depends on the pre-pregnancy BMI. For underweight, normal weight, overweight, and obese females, the optimal weight gain is 12.5–18, 11.5–16, 7–11.5, and 5–9 kg, respectively [28]. However, a national study of maternity units in England revealed that 40% have not implemented guidelines to screen all women with a BMI above 30 kg/m2 for gestational diabetes, owing to a lack of capacity given the high prevalence of maternal obesity [29].
Our findings highlight the need to investigate how useful adiposity measures, other than BMI, are in predicting risk in pregnancy to allocate care to women in most need. Meanwhile, there is a strong need for education and lifestyle intervention programs designed to help women before and during pregnancy, even as early as childhood period, to gain weight within the recommended guidelines.
Strengths and Limitations
The main strength of this study is the use of two-sample MR analysis, which allowed us to account for unmeasured or unknown potential confounders and alleviate the impact of reverse causality. Several sensitivity analyses also confirmed the robustness of our results. Nonetheless, this study has some limitations that deserve our attention. First, the included GWAS were based on individuals of European descent, thus limiting its generalizability to other descents. However, this restriction reduced bias from population stratification. Besides, analysis based on Chinese populations also indicated that the risk for gestational diabetes was higher for those who were overweight or obese before becoming pregnant (P < 0.05) [30]. Second, the nonsignificant association between obesity class 3 and abdominal subcutaneous adipose tissue volume may be explained by a limited number of genetic variants. Sensitivity analysis was therefore performed by setting P < 5 × 10–6 to obtain more SNPs for obesity traits with the number of SNPs below 10. The results indicated that genetic predisposition to obesity class 3 and childhood obesity were associated with increased risk of gestational diabetes. No significant association was observed for visceral adipose tissue volume or abdominal subcutaneous adipose tissue volume. Further studies are required to validate or refute our findings. Third, as individual-level data were not available, we were unable to determine whether there was a non-linear relationship between BMI and gestational diabetes in the current MR analysis. However, according to a cohort study conducted on 14,451 singleton pregnancies, there is a positive-linear relationship between pre-pregnancy BMI and the prevalence of gestational diabetes [14].
Conclusions
Our two-sample MR study suggested that pre-pregnancy obesity traits including BMI, overweight, obesity, obesity class 1, obesity class 2, childhood obesity, and WHR had an important influence on gestational diabetes. The abovementioned obesity traits should be used in targeting, understanding, and reducing the risk of gestational diabetes. Further public health measures on weight control such as diet adjustment and physical activity to reduce obesity traits may be essential in controlling gestational diabetes.
Data Availability
Our data and materials will be shared upon reasonable request by contacting the corresponding author.
References
WHO. Obesity and overweight. Switzerland: WHO; 2016. http://www.who.int/mediacentre/factsheets/fs311/en/
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–81.
Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–6.
Fattah C, Farah N, Barry SC, O’Connor N, Stuart B, Turner MJ. Maternal weight and body composition in the first trimester of pregnancy. Acta Obstet Gynecol Scand. 2010;89(7):952–5.
Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol. 1995;86(2):163–9.
Stegenga H, Haines A, Jones K, Wilding J. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ. 2014;349:g6608.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–9.
Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–12.
Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44(5):526–31.
Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96.
Liu Y, Basty N, Whitcher B, Bell JD, Sorokin EP, van Bruggen N, et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife. 2021;10:e65554.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28(1):30–42.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Wei YM, Yang HX, Zhu WW, Liu XY, Meng WY, Wang YQ, et al. Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. J Matern Fetal Neonatal Med. 2016;29(13):2205–9.
Singh J, Huang CC, Driggers RW, Timofeev J, Amini D, Landy HJ, et al. The impact of pre-pregnancy body mass index on the risk of gestational diabetes. J Matern Fetal Neonatal Med. 2012;25(1):5–10.
Kivelä J, Sormunen-Harju H, Girchenko PV, Huvinen E, Stach-Lempinen B, Kajantie E, et al. Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. 2021;106(11):e4372–88.
Tinius RA, Blankenship MM, Furgal KE, Cade WT, Pearson KJ, Rowland NS, et al. Metabolic flexibility is impaired in women who are pregnant and overweight/obese and related to insulin resistance and inflammation. Metabolism. 2020;104:154142.
Ritchie SC, Würtz P, Nath AP, Abraham G, Havulinna AS, Fearnley LG, et al. The biomarker glyca is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst. 2015;1(4):293–301.
Houttu N, Mokkala K, Laitinen K. Overweight and obesity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clin Nutr. 2018;37(6 Pt A):1955–66.
Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25(5):1027–36.
Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–89.
Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–8.
De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW, et al. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care. 2016;39(1):61–4.
Heslehurst N, Ngongalah L, Bigirumurame T, Nguyen G, Odeniyi A, Flynn A, et al. Association between maternal adiposity measures and adverse maternal outcomes of pregnancy: Systematic review and meta-analysis. Obes Rev. 2022;23(7):e13449.
Censin JC, Nowak C, Cooper N, Bergsten P, Todd JA, Fall T. Childhood adiposity and risk of type 1 diabetes: A Mendelian randomization study. PLoS Med. 2017;14(8):e1002362.
Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51.
Ługowska K, Kolanowski W. The nutritional behaviour of pregnant women in Poland. Int J Environ Res Public Health. 2019;16(22):4357.
Lewandowska M, Więckowska B, Sajdak S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. J Clin Med. 2020;9(6):1980.
Bell R, Hayes L, Pasecinic N, Heslehurst N. Implementation of national screening guidelines for gestational diabetes: A national survey of maternity units in England. Diabetes Res Clin Pract. 2018;146:58–66.
Chen Z, Du J, Shao L, Zheng L, Wu M, Ai M, et al. Prepregnancy body mass index, gestational weight gain, and pregnancy outcomes in China. Int J Gynaecol Obstet. 2010;109(1):41–4.
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFC3605000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, M., Li, X., Wu, J. et al. Phenome-Wide Investigation of the Causal Associations Between Pre-Pregnancy Obesity Traits and Gestational Diabetes: A Two-Sample Mendelian Randomization Analyses. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01577-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01577-w