Abstract
Preeclampsia (PE) is a pregnancy-specific disorder and a major contributor to maternal and fetal morbidity and mortality. Role of oxidative stress in early pregnancy with the pathophysiology of the disorder is unclear. The current study aims to analyse maternal levels of oxidative stress markers (MDA and protein carbonyl) longitudinally across gestation and placental levels of oxidative stress markers (MDA, protein carbonyl and 8-oxo-2'-deoxyguanosine) in women with PE and compare them with non-PE women. 324 pregnant women (216 non-PE and 108 PE women) were longitudinally followed during pregnancy. Women with preeclampsia were stratified as early onset preeclampsia (EOP) and late onset preeclampsia (LOP) Maternal blood at four time points across gestation (11–14 weeks, 18–22 weeks, 26–28 weeks, and at delivery) and placenta were collected. Maternal and placental levels of oxidative stress markers were assessed using commercially available kits. Maternal plasma MDA and protein carbonyl levels were comparable between the PE and non-PE group at all timepoints across gestation. Maternal plasma MDA were significantly higher levels at 26–28 weeks in EOP women when compared to non-PE women (p < 0.05). Placental 8-oxo-dG levels were lower in the EOP group as compared to non-PE (p < 0.05). Elevated plasma MDA levels were positively associated with birth length at 18–22 weeks and 26–28 weeks in the PE group (p < 0.05 for both). Maternal plasma MDA levels were positively associated with systolic blood pressure at 18–22 weeks. Oxidative stress in early pregnancy is not associated with risk of PE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is a multifactorial disorder and chief contributor to maternal and fetal morbidity and mortality with a worldwide prevalence of 3–12% [1]. PE can be classified according to the onset-time of clinical symptoms as early-onset PE (EOP) or late-onset PE (LOP) [2] both of which are different entities associated with different clinical features and pathophysiology [3]. EOP is believed to be associated with abnormal placentation and impaired spiral artery remodeling [4]. The impaired spiral artery remodeling leads to uteroplacental hypoxia/reoxygenation triggering oxidative stress and placental apoptosis [5]. On the other hand, LOP results due to a mismatch between the metabolic demands of the fetus and the maternal supply [6]. The progressive mismatch between maternal perfusion and fetoplacental demands induces oxidative changes in LOP placentae [3].
Physiological levels of reactive oxygen species (ROS) are required for normal progression of placental and fetal growth and development. A balance between oxidants and antioxidants is crucial for fertilization, implantation and placental growth [7]. Increased oxidative stress, induced by ROS arises from an imbalance between the generation of oxidants and their clearance by antioxidants [8,9,10]. Excess production of oxidants may lead to distorted signaling and damage to cellular/molecular structures like lipids, proteins and DNA [8, 11].Common oxidative stress markers include lipid peroxidation product malondialdehyde (MDA), protein oxidation product such as protein carbonyls and DNA damage products such as 8-Oxo-2'-deoxyguanosine (8-oxo-dG) [12]. Earlier studies by us and a few others have demonstrated increased levels of maternal MDA in women with PE at the end of pregnancy [13,14,15,16,17,18,19]. Similarly, several authors demonstrate elevated levels of protein carbonyls in the third trimester of pregnancy and at the time of labor in PE pregnancies as compared to healthy pregnancies [20,21,22,23,24,25]. However, these findings were observed after the progression of the disorder making it difficult to understand the involvement of oxidative stress in the pathophysiology of the disorder. There are limited studies that have investigated the levels of oxidative stress markers in pregnancies complicated with preeclampsia throughout the gestation but these studies report conflicting results [26,27,28]. However, these studies are limited by small sample size and did not examine the changes in oxidative stress indices in subtypes of PE.
In view of this, the current study analyzed maternal levels of oxidative stress markers (MDA and protein carbonyl) at four different timepoints across gestation in women preeclampsia and their subtypes and compares them with non-PE women. Moreover, the study also analyzed placental levels of oxidative stress markers (MDA, protein carbonyl and 8-oxo-dG) in the above women.
Materials and Methods
Study Subjects
The current work is a part of a large ongoing study funded by the Indian Council of Medical Research as a Centre of Advanced Research (ICMR-CAR) – “Investigating Mechanisms leading to Preeclampsia” at IRSHA, Bharati Vidyapeeth University, Pune (5/7/1069/13-RCH, dated 31–03-2017). Bharati Vidyapeeth Medical College Institutional Ethical Committee approved the study protocol (IEC/2015/37, dated 03.10.2015). Pregnant women visiting Bharati Medical College and Hospital, and Gupte Hospital and Research Centre, Pune, for their first antenatal visit were approached by the counselor and their participation was solicited, those who agreed to participate and were ready to deliver at the above hospitals were recruited in the study and followed up throughout their gestation till delivery. Written informed consent was obtained from the women. Each woman’s personal, obstetric, clinical and family history were recorded. The detailed study design and recruitment criteria has been discussed earlier [29].
Briefly, pregnant women were recruited at first antenatal visit and followed up at four different timepoints of gestation i.e., 11–14 weeks of gestation (V1), 18–22 weeks (V2), 26–28 weeks (V3) and at delivery (V4). All women were followed until delivery and categorized as PE if they developed hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on at least two measurements taken at least 4 h apart) after 20 weeks of gestation along with new onset of one of the followings: proteinuria thrombocytopenia, impaired liver function, development of renal insufficiency, pulmonary edema, or new-onset cerebral or visual disturbances. PE women were categorized as EOP if they developed PE before 34 weeks of gestation whereas if the women developed PE later than 34 weeks of gestation they were categorized as LOP.
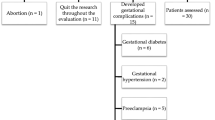
A total of 1154 women were recruited in the study at 11–14 weeks of gestation, among whom 1096 had singleton pregnancies and 58 had twin pregnancies. Of the 1096 singleton pregnancies, 108 developed preeclampsia. We selected control and cases in a ratio of 2:1. For each case of preeclampsia, 2 non-PE women delivering at the same gestational age (± 4 gestational weeks) were randomly selected using “Select Cases” in SPSS. Therefore, the current study investigated changes in oxidative stress markers in 324 women included in the study (108 who developed Preeclampsia and 216 non-Preeclampsia women).
Sample Collection and Processing
Maternal blood was collected into vacutainers containing EDTA at four different timepoints (V1, V2, V3 and at delivery) for each participant. The blood was processed to separate serum, plasma, erythrocyte and lymphocyte fractions of blood that were stored at -80 °C until further analysis.
Fresh placental tissue was obtained immediately after delivery. The fetal membrane was trimmed off and the umbilical cord cut 2 cm away from the insertion point. One-third radius area around cord insertion site was considered the region of interest and cut. From this region, basal and chorionic plate were cut and discarded. Small tissue pieces were made from this region. The small pieces were then placed in cryovials, snap frozen in liquid nitrogen and then stored at -80 °C [29].
Preparation of Placental Tissue Homogenate and Estimation of Total Protein
For MDA analysis, 1 ml of 1X phosphate buffered saline (PBS) was used to homogenize 300 mg of placental tissue. Protease inhibitors (leupeptin, aprotinin and phenyl methane sulphonyl fluoride) were added to the buffer. To lyse the cell membrane in the homogenized tissue two freeze–thaw cycles were given. The homogenate was then centrifuged at 5590 × g for 10 min at 4ºC and the supernatant was collected and stored at -80ºC.
For protein carbonyl estimation, 1 ml of cold buffer was used to homogenize 300 mg of placental tissue {cold buffer: 50 mM PBS [sodium chloride (NaCl) 8.76 g; potassium dihydrogen phosphate (KH2PO4) 3.22 g; dipotassium hydrogen phosphate (K2HPO4) 4.58 g; deionized water up to 1L; pH 6.7] with 1 mM EDTA}. The homogenate was then centrifuged at 5590 × gfor 15 min at 4ºC and the supernatant was collected and stored at -80ºC.
The supernatant was then assayed for total protein content using Pierce™ BCA Protein Assay Kit (Cat. No: 23225). The oxidative stress marker levels were adjusted to total protein concentration in the samples.
Biochemical Estimation
Maternal MDA level
MDA levels were measured from maternal plasma and placenta using Aoxre LLC Oxis Research™ Bioxytech® MDA-586™ Spectrophotometric Assay (Cat. No: 21044). Maternal plasma MDA was expressed as µM and placental MDA was expressed as µM/mg.
Maternal Protein Carbonyl levels
Plasma protein carbonyl levels were measured using CellBioLabs’ Oxiselect™ Protein Carbonyl Elisa kit (Cat. No: STA-310) and expressed as nmol/mg. Placental protein carbonyl was measured using Cayman Chemical’s (USA) Protein Carbonyl Colorimetric assay kit (Cat. No: 10005020) and expressed as nmol/mg.
Placental 8-oxo-dG levels
Placental 8-oxo-dG levels were measured using 8-hydroxy 2 deoxyguanosine ELISA kit by Abcam, USA (Cat. No: ab201734) and expressed as ng/µg of DNA. DNA from placental tissue was extracted using Qiagen Blood & Tissue Kit following standardized protocol for human tissue. dsDNA was denatured by heat shock to yield ssDNA. ssDNA was digested by nuclease P1 and was then treated with alkaline phosphatase. The treated DNA samples were then assayed for 8-oxo-dG following the manufacturer’s protocol.
Statistical Analysis
SPSS/PC + package software (Chicago, IL, USA) was used to analyze the data (Version 28.0). Continuous variables were expressed as Median [Interquartile range (IQR)] and adjusted for gestational age. Log transformation was used for skewed variables. Univariate associations were tested between oxidative stress markers and potential confounders: maternal age, gestational age, body mass index (BMI), standard of living index (SLI) score, hospital, gestational diabetes mellitus (GDM). Multiple linear regression was used to examine the association of maternal MDA with different parameters after adjusting for maternal age.
Results
Maternal and Neonatal Characteristics
Women with PE had a higher maternal BMI (28.0 ± 5.4 kg/m2) as compared to non-PE women (24.1 ± 4.1 kg/m2) at recruitment (p < 0.01). Systolic [non-PE: (108.6 ± 8.4 mmHg); PE: (116.5 ± 11.4 mmHg)] and diastolic blood pressure [non-PE: (70.3 ± 6.6 mmHg); PE: (75.2 ± 8.4 mmHg)] at recruitment was higher in women with PE as compared to non-PE women (p < 0.01) [30]. Systolic and diastolic blood pressure remained higher in women with PE as compared to non-PE women throughout gestation and has been reported by us earlier [30]. The mean gestational age at which PE was clinically diagnosed was 34.3 weeks. Birth weight was lower in PE (2864.5 ± 422.4 gm) as compared to the non-PE group (2698.4 ± 627.2 gm) (p < 0.01) [30].
Maternal plasma MDA levels
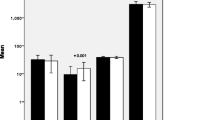
MDA levels in women with PE were comparable to non-PE women (Fig. 1).
When these levels were assessed in subtypes of PE i.e., EOP and LOP showed that MDA levels in women with EOP were higher at V3 timepoint (p < 0.05) than non-PE women (Fig. 1). Maternal plasma MDA levels were not associated with risk of PE in in adjusted and unadjusted model (Table 1).
Maternal Plasma Protein Carbonyl levels
Maternal plasma protein carbonyl levels were higher in preeclampsia group but were not significant (Fig. 2).
Maternal plasma protein carbonyl levels. Boxplot representing maternal plasma protein carbonyl levels across gestation. a Maternal plasma protein carbonyl levels across gestation b Maternal plasma protein carbonyl levels in subtypes of PE. PE: Preeclampsia; EOP: Early Onset Preeclampsia; LOP: Late Onset Preeclampsia; p: the level of significance; *p < 0.05 as compared to non-PE
Plasma protein carbonyl levels were comparable between the subtypes of PE (Fig. 2). Maternal plasma protein carbonyl levels were not associated with risk of PE in in adjusted and unadjusted model (Table 1).
Placental MDA, Protein Carbonyl and 8-oxo-dG levels
Placental oxidative stress markers were comparable between the PE and non-PE groups (Fig. 3). When the levels were assessed in the subtypes of PE, placental 8-oxo-dG levels were significantly lower in the EOP group as compared to non-PE (Fig. 3).
Placental levels of oxidative stress markers. Boxplot representing placental levels of oxidative stress markers. a Placental MDA levels (PE vs. Non-PE) b Placental MDA levels in subtypes of PE c Placental protein carbonyl levels (PE vs. Non-PE) d Placental protein carbonyl levels in subtypes of PE e Placental 8-oxo-dG levels (PE vs. Non-PE) f Placental 8-oxo-dG levels in subtypes of PE. PE: Preeclampsia; EOP: Early Onset Preeclampsia; LOP: Late Onset Preeclampsia; p: the level of significance; *p < 0.05 as compared to non-PE
Association of Maternal Oxidative Stress Markers with Blood Pressure and Birth Outcome
Maternal plasma MDA levels were positively associated with systolic blood pressure at V2 (p < 0.01) timepoint. However, there was no association between systolic blood pressure and plasma MDA levels at V1, V3 and V4 timepoints (Table 2).
There was no association between diastolic blood pressure and plasma MDA levels at any timepoint (Table 2).
There was no association between systolic and diastolic blood pressure and plasma protein carbonyl levels at all timepoints (Table 2).
Maternal plasma MDA levels at V1 and V3 and placental levels were positively associated with birth length (p < 0.05) (Table 3).
There was no association of protein carbonyl levels and placental 8-oxo-dG levels with birth weight and birth length (Table 3).
Discussion
The present study for the first time gives an enlarged view of the circulatory oxidative stress measures of pregnant women diagnosed with PE, as it sought to examine the changes across the gestation beginning as early as 11–14 weeks of gestation.
The key findings of the study are: 1) Maternal plasma MDA and protein carbonyl levels were comparable between the PE and non-PE group at all timepoints across gestation. 3) When the levels of OS markers were analyzed in subtypes of PE, maternal plasma MDA were significantly higher at 26–28 weeks in women with early onset PE but not late onset when compared to non-PE women. 4) Maternal plasma MDA levels at 18–22 weeks was associated with systolic blood pressure. 5) None of the markers were associated with the risk of preeclampsia 6) Maternal plasma MDA levels was positively associated with birth length.
The current study demonstrates that maternal plasma MDA levels in early pregnancy were not higher in women with PE as compared to normotensive control women. MDA is a byproduct of lipid peroxidation which is known to induce endothelial dysfunction, damage cell membranes and reduce endothelial vasorelaxation [1]. Defective placentation due to inadequate remodeling of uterine spiral arteries induces endothelial dysfunction in PE. Although emerging evidence implies the involvement of increased oxidative stress in PE, it is still unclear whether this endothelial dysfunction caused due to oxidative stress is implicated in pathogenesis of the disorder or is it merely a malfunction instigated by establishment of the disorder which may lead to increased oxidative stress.
Increased lipid peroxidation has been linked with pathogenesis of PE. To support this hypothesis, many studies have reported increased plasma MDA levels in women with PE compared to normal healthy women at 24–36 weeks [28, 31] and in the third trimester [18]. Additionally, only one study has examined the changes in levels of maternal MDA levels based on severity which suggests that higher blood levels of MDA at delivery correlate with severity of PE [32]. Further few studies report higher maternal blood levels after 20 weeks of gestation in women with PE [18, 33].
The present study reports increased levels of plasma MDA after the onset of PE at 26–28 weeks of gestation but not in early pregnancy. This suggests that the increase in oxidative stress is successive to the disorder. In contrast, a study conducted to examine the levels of MDA from early pregnancy on a small sample size of 21 preeclamptic women and 24 control women has reported higher serum MDA levels between 10–12 weeks and 20–24 weeks of gestation in women with PE compared to normotensive control women [27]. Additionally, our earlier longitudinal study has reported increased plasma MDA levels at 16–20 weeks of gestation in PE pregnancies as compared to control [26]. This discrepancy could be attributed to the difference in the time points as well as socio-economic status of the women studied.
The results of this study show that oxidative damage, which was evaluated by protein carbonyls in plasma, was unchanged in PE. This is in accordance with other studies that have shown that carbonylation in third trimester can be unaltered in PE compared with normal pregnancy [34, 35].
The present study also reports no change in the levels of placental 8-oxo-dG in PE women as compared to women in the non-PE, although it was lower in the EOP group. However, this is contradictory to studies that have reported increased oxidative DNA damage measured by placental 8-oxo-dG levels in women with PE [36,37,38,39] where severe damage was observed in women with EOP [40]. All the above-mentioned studies were conducted on a smaller sample size and have used immunohistochemistry to measure the proportion of oxidative DNA damage. Further studies are needed to clarify the role of placental 8-oxo-dG in the pathophysiology of PE.
The current study reports positive association of maternal plasma MDA levels at 11–14 weeks and 26–28 weeks with baby length. A study conducted on Bangladeshi healthy pregnant women aiming to evaluate the associations of oxidative stress markers in late pregnancy with infant size reported that higher levels of lipid peroxidation markers at 14 weeks of gestation were associated with larger infant size (baby length and chest circumference) [41]. Other studies have reported no association of maternal plasma MDA with birth length [26, 42]. A recent study reports no association of newborn birth length with placental MDA levels [43].
The current study demonstrates maternal MDA levels in early pregnancy to be associated with increased systolic BP. A study examining the oxidative stress levels in serum of 50 PE pregnancies also reported a positive association of maternal serum MDA levels in late third trimester with systolic blood pressure [44]. In our study, maternal plasma MDA levels were not associated with risk of PE, despite its association with blood pressure. This could be attributed to MDA being a ubiquitous oxidative stress marker and hence fails to show any specific associations.
Conclusion
Our findings suggest that oxidative stress in early pregnancy does not play a role in the pathophysiology of PE but is more likely a consequence of preeclampsia.
Data Availability
All data supporting the findings of this study are available within the paper.
References
Ferreira RC, Fragoso MBT, Bueno NB, Goulart MOF, de Oliveira ACM. Oxidative stress markers in preeclamptic placentas: A systematic review with meta-analysis. Placenta. 2020. https://doi.org/10.1016/j.placenta.2020.07.023.
Ren Z, Gao Y, Gao Y, Liang G, Chen Q, Jiang S, Yang X, Fan C, Wang H, Wang J, Shi YW, Xiao C, Zhong M, Yang X. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics. 2021. https://doi.org/10.7150/thno.56141.
Marín R, Chiarello DI, Abad C, Rojas D, Toledo F, Sobrevia L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020. https://doi.org/10.1016/j.bbadis.2020.165961.
Staff AC, Redman CWG. The differences between early- and late-onset pre-eclampsia. In: Saito S, editor. Preeclampsia: basic, genomic, and clinical. Comprehensive gynecology and obstetrics. Springer, Singapore. 2018. pp. 151–172. https://doi.org/10.1007/978-981-10-5891-2_10
Hansson SR, Nääv Å, Erlandsson L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front Physiol. 2015. https://doi.org/10.3389/fphys.2014.00516.
Erez O, Romero R, Maymon E, Chaemsaithong P, Done B, Pacora P, Panaitescu B, Chaiworapongsa T, Hassan SS, Tarca AL. The prediction of late-onset preeclampsia: Results from a longitudinal proteomics study. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0181468.
Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative Stress in Preeclampsia and Placental Diseases. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19051496.
Cindrova-Davies T, Fogarty NME, Jones CJP, Kingdom J, Burton GJ. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta. 2018. https://doi.org/10.1016/j.placenta.2018.06.307.
Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017. https://doi.org/10.1146/annurev-biochem-061516-045037.
Golbidi S, Laher I. Antioxidant therapy in human endocrine disorders. Med Sci Monit. 2010. PMID: 20037503.
Gratacós E. Lipid-mediated endothelial dysfunction: a common factor to preeclampsia and chronic vascular disease. Eur J Obstet Gynecol Reprod Biol. 2000. https://doi.org/10.1016/s0301-2115(00)00427-9.
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005. https://doi.org/10.1016/j.numecd.2005.05.003.
Roy S, Sable P, Khaire A, Randhir K, Kale A, Joshi S. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Develop. 2014. https://doi.org/10.1016/j.braindev.2013.03.004.
Kulkarni A, Mehendale S, Pisal H, Kilari A, Dangat K, Salunkhe S, Taralekar V, Joshi S. Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin Nutr. 2011. https://doi.org/10.1016/j.clnu.2010.07.007.
Kulkarni AV, Mehendale SS, Yadav HR, Kilari AS, Taralekar VS, Joshi SR. Circulating angiogenic factors and their association with birth outcomes in preeclampsia. Hypertens Res. 2010. https://doi.org/10.1038/hr.2010.31.
Suhail M, Suhail S, Gupta BK, Bharat V. Malondialdehyde and Antioxidant Enzymes in Maternal and Cord Blood, and their Correlation in Normotensive and Preeclamptic Women. J Clin Med Res. 2009. https://doi.org/10.4021/jocmr2009.07.1252.
Mehendale S, Kilari A, Dangat K, Taralekar V, Mahadik S, Joshi S. Fatty acids, antioxidants, and oxidative stress in pre-eclampsia. Int J Gynaecol Obstet. 2008. https://doi.org/10.1016/j.ijgo.2007.08.011.
Anastasakis E, Papantoniou N, Daskalakis G, Mesogitis S, Antsaklis A. Screening for pre-eclampsia by oxidative stress markers and uteroplacental blood flow. J Obstet Gynaecol. 2008. https://doi.org/10.1080/01443610802042852.
Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialdehyde levels in pre-eclampsia. Cell Biochem Funct. 2005. https://doi.org/10.1002/cbf.1152.
Al-Sheikh YA, Al-Zahrani KY. The Status of Biochemical and Molecular Markers of Oxidative Stress in Preeclamptic Saudi Patients. Curr Mol Med. 2018. https://doi.org/10.2174/1566524019666190104105843.
Bernardi FC, Felisberto F, Vuolo F, Petronilho F, Souza DR, Luciano TF, de Souza CT, Ritter C, Dal-Pizzol F. Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control study. Oxid Med Cell Longev. 2012. https://doi.org/10.1155/2012/636419.
Tsukimori K, Yoshitomi T, Morokuma S, Fukushima K, Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens. 2008. https://doi.org/10.1038/ajh.2008.289.
Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta. 2005. https://doi.org/10.1016/j.placenta.2004.04.002.
Zusterzeel PL, Steegers-Theunissen RP, Harren FJ, Stekkinger E, Kateman H, Timmerman BH, Berkelmans R, Nieuwenhuizen A, Peters WH, Raijmakers MT, Steegers EA. Ethene and other biomarkers of oxidative stress in hypertensive disorders of pregnancy. Hypertens Pregnancy. 2002. https://doi.org/10.1081/PRG-120002908.
Zusterzeel PL, Mulder TP, Peters WH, Wiseman SA, Steegers EA. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radic Res. 2000. https://doi.org/10.1080/10715760000301011.
D’Souza V, Rani A, Patil V, Pisal H, Randhir K, Mehendale S, Wagh G, Gupte S, Joshi S. Increased oxidative stress from early pregnancy in women who develop preeclampsia. Clin Exp Hypertens. 2016. https://doi.org/10.3109/10641963.2015.1081226.
Genc H, Uzun H, Benian A, Simsek G, Gzelisgen R, Madazli R, Güralp O. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet. 2011. https://doi.org/10.1007/s00404-011-1865-2.
Basbug M, Demir I, Serin IS, Ozcelik B, Saraymen R, Narin F, Tayyar M. Maternal erythrocyte malondialdehyde level in preeclampsia prediction: a longitudinal study. J Perinat Med. 2003. https://doi.org/10.1515/JPM.2003.072.
Wadhwani NS, Sundrani DP, Wagh GN, Mehendale SS, Tipnis MM, Joshi PC, Kinare AS, Lalwani SK, Mani NS, Chandhiok N, Chandak GR, Gupte SA, Fall CHD, Joshi SR. The REVAMP study: research exploring various aspects and mechanisms in preeclampsia: study protocol. BMC Pregnancy Childbirth. 2019. https://doi.org/10.1186/s12884-019-2450-0.
Wadhwani N, Dangat K, Randhir K, Poddar A, Joshi P, Pisal H, Kadam V, Bakshi R, Chandhiok N, Lalwani S, Mehendale S, Wagh G, Gupte S, Sachdev HS, Fall C, Joshi S. Longitudinal Assessment of Calcium and Magnesium Levels in Women with Preeclampsia. Biol Trace Elem Res. 2022. https://doi.org/10.1007/s12011-022-03440-y.
Karacay O, Sepici-Dincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, Kandemir O, Altan N. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res Clin Pract. 2010. https://doi.org/10.1016/j.diabres.2010.04.015.
Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Lab Physicians. 2018. https://doi.org/10.4103/JLP.JLP_26_18.
Rudra CB, Qiu C, David RM, Bralley JA, Walsh SW, Williams MA. A prospective study of early-pregnancy plasma malondialdehyde concentration and risk of preeclampsia. Clin Biochem. 2006. https://doi.org/10.1016/j.clinbiochem.2006.02.009.
Pimentel AM, Pereira NR, Costa CA, Mann GE, Cordeiro VS, de Moura RS, Brunini TM, Mendes-Ribeiro AC, Resende AC. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens Res. 2013. https://doi.org/10.1038/hr.2013.34.
Llurba E, Gratacós E, Martín-Gallán P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med. 2004. https://doi.org/10.1016/j.freeradbiomed.2004.04.035.
Owaki Y, Watanabe K, Iwasaki A, Saitou T, Matsushita H, Wakatsuki A. Placental hypoplasia and maternal organic vascular disorder in pregnant women with gestational hypertension and preeclampsia. J Matern Fetal Neonatal Med. 2021. https://doi.org/10.1080/14767058.2019.1608175.
Yoshida A, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Wakatsuki A. Placental oxidative stress and maternal endothelial function in pregnant women with normotensive fetal growth restriction. J Matern Fetal Neonatal Med. 2018. https://doi.org/10.1080/14767058.2017.1306510.
Fujimaki A, Watanabe K, Mori T, Kimura C, Shinohara K, Wakatsuki A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta. 2011. https://doi.org/10.1016/j.placenta.2011.02.004.
Nishizawa H, Suzuki M, Pryor-Koishi K, Sekiya T, Tada S, Kurahashi H, Udagawa Y. Impact of indoleamine 2,3-dioxygenase on the antioxidant system in the placentas of severely pre-eclamptic patients. Syst Biol Reprod Med. 2011. https://doi.org/10.3109/19396368.2011.587590.
Kimura C, Watanabe K, Iwasaki A, Mori T, Matsushita H, Shinohara K, Wakatsuki A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med. 2013. https://doi.org/10.3109/14767058.2012.733766.
Lindström E, Persson LÅ, Raqib R, El Arifeen S, Basu S, Ekström EC. Associations between oxidative parameters in pregnancy and birth anthropometry in a cohort of women and children in rural Bangladesh: the MINIMat-cohort. Free Radic Res. 2012. https://doi.org/10.3109/10715762.2011.651467.
Tastekin A, Ors R, Demircan B, Saricam Z, Ingec M, Akcay F. Oxidative stress in infants born to preeclamptic mothers. Pediatr Int. 2005. https://doi.org/10.1111/j.1442-200x.2005.02146.x.
Ferreira RC, Fragoso MBT, Tenório MCDS, Martins ASDP, Borbely AU, Moura FA, Goulart MOF, Oliveira ACM. Biomarkers of placental redox imbalance in pregnancies with preeclampsia and consequent perinatal outcomes. Arch Biochem Biophys. 2020. https://doi.org/10.1016/j.abb.2020.108464.
Omar M, Borg HM. Assessment of oxidative stress markers and level of antioxidant in preeclampsia. Ind J Obst Gynae Res. 2019. https://doi.org/10.18231/j.ijogr.2019.062.
Acknowledgements
The authors thank the staff at the participating hospitals and Mother and Child Health department, IRSHA, Bharati Vidyapeeth (Deemed to be University) for their assistance.
Funding
This study was supported by Indian Council of Medical Research, New Delhi, India (grant number: 5/7/1069/13-RCH, dated 31/03/2017).
Author information
Authors and Affiliations
Contributions
Aditi Godhamgaonkar: Writing—original draft, Methodology, Investigation, Writing—review & editing. Kamini Dangat, Kajal Shelke, Divya Shukla, Girija Wagh, Sanjay Lalwani and Sanjay Gupte: Investigation. Karuna Randhir: Formal Analysis. Sadhana Joshi: Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study protocol was approved by the Institutional Ethics Committee, Bharati Vidyapeeth Deemed University (IEC/2015/37, dated 03.10.2015).
Consent to Participate
Written informed consent was obtained from all individual participants included in the study.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of their data.
Conflict of Interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Godhamgaonkar, A., Dangat, K., Randhir, K. et al. Longitudinal Assessment of Oxidative Stress Markers in Women with Preeclampsia. Reprod. Sci. 31, 2731–2740 (2024). https://doi.org/10.1007/s43032-024-01574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-024-01574-z