Abstract
Vulvovaginal candidiasis (VVC) and recurrent vulvovaginal candidiasis (RVVC) are the most common lower genital tract infections in reproductive women. In recent years, the research on its pathogenesis mainly focuses on vaginal local immunity and IL-17 as key factors in adaptive immunity, attracting much attention. However, the role of IL-17 in local immunity in VVC and RVVC is poorly understood. At the same time, neutrophils are considered the most effective way to control and eliminate candidal infection and have a controversial role in VVC and RVVC. In this study, we built a mouse RVVC model. After analyzing the vaginal lavage solution of RVVC mice with an inflammatory factor antibody chip and ELISA, we found that IL-17 may play a protective role in RVVC. The experiment of constructing RVVC mice with different concentrations of IL-17 using halofuginone and comparing the vaginal fungi load and vaginal epithelial damage verified that IL-17 had a protective effect in RVVC. In addition, in vitro and in vivo studies found that IL-17 can promote neutrophil apoptosis and recruit neutrophils in the vagina. The neutrophils in the vagina can secrete IL-17 in an autocrine manner. These two may be why IL-17 plays a protective role in RVVC. In summary, the study suggests that IL-17-mediated regulation of neutrophil function is involved in host immune response to RVVC, which helps us to further understand the potential mechanism of IL-17 in RVVC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvovaginal candidiasis (VVC) is one of the most common infectious diseases of the reproductive tract, caused primarily by the opportunistic Candida albicans infection. Recurrent vulvovaginal candidiasis (RVVC) refers to four or more episodes of VVC with symptoms confirmed by a mycologist within 1 year [1]. The main symptoms of RVVC include vaginal pain, itching, redness, burning, and recurring symptoms [2], which seriously affect the patient’s psychological status and quality of life. The pathogenesis of RVVC is unclear [3], and previous studies have found that the pathogenesis of RVVC is mainly related to the invasiveness of the strain and the local immunity of the vagina [4].
In a previous study [5], we compared the differences in the invasiveness of VVC and RVVC pathogenic strains. The results showed no differences in fungi species, 25S rDNA-PCR genotyping, Plb activity, and drug susceptibility between VVC and RVVC pathogens. However, Secretory aspartyl proteinases (Sap) activity of RVVC pathogenic strains with severe symptoms and refractory treatment is weaker than VVC pathogenic strains. As an invasion factor secreted by the tip of C. albicans hypha, Sap can invade and adhere to the host cell, destroy the integrity of the host cell epidermis, and increase the permeability of the cell membrane, thus reducing the defense ability of the host cell [6]. At the same time, Sap can stimulate the host to produce immune responses, such as promoting polymorphonuclear neutrophil infiltration and producing pro-inflammatory cytokines such as IL-1 and IL-18 by activating caspase [7]. These results indicate that compared with the invasiveness of C. albicans, the local immune response in the vagina might be the most important factor causing RVVC.
Once the vaginal epithelium is infected with Candida, the innate and adaptive immune responses are immediately activated [8]. The Th17 cell in adaptive immunity has been the research focus in recent years [9]. As a major effector of Th17, IL-17 plays a role in the antifungal effect of skin and mucous membranes by producing antimicrobial peptides and promoting neutrophil infiltration [9]. There are organ differences in the antifungal effect of IL-17 in vivo. In the oral mucosa of C. albicans infection, the antifungal effect of IL-17 has been confirmed [10]. However, the role of IL-17 and neutrophil cells in VVC and RVVC is controversial. Some studies have highlighted that in VVC, the phagocytosis of neutrophils is weakened, and IL-17 has no protective effect on VVC [11]. Other studies [12] have found that IL-17 plays a vital role in the immune response of vaginal mucosa, defending against C. albicans, and low-level local vaginal IL-17 expression may cause recurrent VVC attacks.
When C. albicans infects the host, both the innate and adaptive immunity of the host play an important role through neutrophils [13]. After infection with C. albicans, neutrophils are recruited to the infected site and recognize C. albicans, fighting the fungus through a series of antimicrobial effect mechanisms such as oxidative burst, cytokine release, phagocytosis, and release of antimicrobial peptides [14]. The role of neutrophils in RVVC has been controversial. It is generally believed that the symptoms of mucosal damage in VVC and RVVC are related to the acute inflammation that occurs when neutrophils migrate into the vagina, and the interaction of C. albicans with vaginal epithelial cells causes this neutrophil response. However, vaginal epithelial cells cannot clear C. albicans, so neutrophils have a greater negative impact on vaginitis [3].
Under normal circumstances, maintaining a dynamic balance between the proliferation and apoptosis of neutrophils can promote the body’s resistance to infection and help reduce inflammation. Neutrophil apoptosis is an essential biological pathway for terminating neutrophil-mediated inflammatory responses [15]. Pro-inflammatory cytokines decrease after neutrophil apoptosis; thus, neutrophil apoptosis is a key signal for inflammation resolution [16]. Neutrophil apoptosis also plays an important role in numerous mechanisms of neutrophil resistance to C. albican [13],but the research on neutrophil apoptosis in VVC and RVVC is limited.
In this study, we explored vaginal immunity in RVVC mice and clarified the role of IL-17 in the occurrence and development of RVVC. At the same time, we investigated the role of IL-17 and neutrophils in the local immunity of RVVC and discussed the mechanism of neutrophil apoptosis in RVVC. The study provides a theoretical basis for elucidating the pathogenesis of RVVC and a new direction for diagnosing and treating RVVC.
Materials and Methods
Strains and Growth Conditions
Two strains of C. albicans collected from VVC patients were used in this study. They were both 25SrDNA genotype A and were sensitive to fluconazole, itraconazole, and amphotericin. One strain had weak Sap enzyme activity (Sap=0.731), and the other had strong Sap enzyme activity (Sap=0.242). The method for judging the enzyme activity of Sap was as described by El-Houssaini [17]: the lower the value of Sap, the higher the enzyme activity.
Constructed RVVC Mouse Model
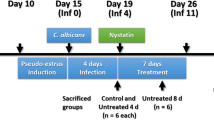
One hundred six female Kunming mice aged 6–8 weeks were purchased from Kunming Medical University and were housed at a density of six per cage and acclimated to a day/night cycle for 3 days. The two strains selected above were used to construct the RVVC mouse model. The mice were randomly divided into 3 groups: the control group (normal saline intervention group, n=6); the strong Sap activity group (Sap=0.242, n=50); the weak Sap activity group (Sap=0.731, n=50). The RVVC model was established according to a previous method [5], and the timeline of the experimental animal treatment and sample collection is shown in Fig. 1. Before infection, the mice received a subcutaneous injection of 0.1mg of estradiol valerate (E2; Aladdin Industrial Co, Shanghai, China) every 2 days for three times in total. C. albicans was prepared into a suspension with a concentration of 5×106 cfu/ml in sterile saline, and 0.02 ml of C. albicans suspension was inoculated into the vaginas of the mice. In the control group, 0.02 ml of PBS solution instead of the fungal suspension was injected intravaginally. Four days after inoculation, vaginal secretions were collected for observation under a light microscope. The presence of hyphae was used to confirm C. albicans infection (VVC model). Next, clotrimazole was used for vaginal treatment, and no hyphae in the vaginal secretion were observed after seven days, which indicated successful treatment. The entire process was repeated three times, and the RVVC model was established after the fourth infection. In each successful infection stage, the vaginal cavity was lavaged with 0.2 ml of phosphate-buffered saline. The lavage fluid was collected and stored at -80°C.
Protein Chip Tests for Differentially Expressed Genes (DEGs)
After the RVVC mice model was successfully constructed, we used the AAM-INF-1 chip provided by RayBiotech to analyze the vaginal lavage fluid of RVVC mice after the second infection (day 26, Fig. 1). Since the vaginal lavage fluid of each mouse was only 200μL, we combined the vaginal lavage fluid of 6 mice in the control group, 10 mice in the strong Sap activity group, and 10 mice in the weak Sap activity group after the second infection in RVVC mice for determination. The membrane chip was sealed, and the antibodies were incubated. The results were obtained by Image Quant LAS 4000, a scanning chemiluminescence imaging analysis system, and the original data were normalized by software and analyzed by means of normalization data. The Edger package (3.30.3) was used to perform a differential analysis of 40 genes. The analysis method was Moderated T-statistics, and the data packet was LIMMA from R/Bioconductor. P-value adjustment (p-value corrected by BH method) and logFC (expression differential multiple, base 2) were used to screen the DEGs, and the selection conditions were as follows: logFC>Log2 (1.2), the difference threshold was 1.2, and the adjusted p-value (or p-value)<0.05. According to the expression amount of these 40 genes, the heatmap was drawn using the heatmap package (1.0.12).
qRT-PCR Verified the DEGs
After the second infection of the RVVC mice, 200μL of vaginal lavage fluid was taken from the strong, weak, and control Sap groups for qRT-PCR analysis, respectively (day 26, Fig. 1). Extraction of the total RNA in the lavage solution was conducted according to the instructions, and OD260 and OD280were measured with a spectrophotometer to detect the RNA purity/concentration. mRNA reverse transcription was performed using the SureScript-First-strand-cDNA-synthesis-kit from Guangzhou Sevier Company. The reverse transcription product cDNA was diluted 5–20 times by adding 0.05ml ddH2O (RNase/DNase-free), and the qRT-PCR reaction was performed. Primers are displayed in Table 1.
Construction of RVVC Mice with Different Th17 Concentrations
Since C. albicans with strong Sap activity induced strong host immunity, we selected C. albicans with strong Sap activity (Sap=0.242) for infection. Twenty-four mice were randomly divided into 4 groups: control group (Control), halofuginone-0μg group, halofuginone-5μg group, and halofuginone-10μg group. Mice were injected with 0μg, 5μg, and 10μg of halofuginone solution 2 days before infection, on the day of infection, and 2 days after infection, respectively. Mice in the control group were treated with saline instead of C. albicans and halofuginone. The first and second infected mice were intervened with halofugine. After the second infection (day 26, Fig. 1), 1mLof retroorbital blood and 300μL vaginal lavage fluid were collected for the following experiments.
Wright Staining in Vaginal Lavage Fluid of Mice with Different Th17 Concentrations
After the second infection, 100μL vaginal lavage fluid was collected from all 4 groups, centrifuged to prepare cell smears, and Wright’s staining was performed.
Determination of Fungi Load
Sterile cotton swabs were utilized to collect mouse vaginal secretions and stored in an Ep tube containing 1mL 0.9% NaCl. Around 200μL of vaginal secretions were taken and inoculated on pre-prepared Saboro medium plates to detect vaginal fungi quantity.
Histological Analysis
For histological evaluation, the mice were sacrificed, the vagina removed, and immediately fixed in 10% (V/V) neutral buffer formalin for 24h. They were then dehydrated, paraffin-embedded, cut into 3–4 μm thick slices, and stained with HE. Five visual fields in the center and four corners were selected for microscopic observation.
Immunohistochemical (IHC) Staining
After the slices were routinely processed according to the previous method, they were incubated with the corresponding primary antibody IL-17A (ABCAM 1:100) and cleaved caspase-3 (Abcam 1:200). Following this, they were incubated with horseradish peroxide-labeled secondary antibody (Zhong Shan Jin Qiao, China), and finally colorified with a Vectastain ABC peroxidase system and a 3,3'-diaminobenzidine (DAB) kit (Zhong Shan Jin Qiao, China). The average dyeing optical density was calculated using Image-Pro Plus software. Five visual fields in the center and four corners were selected for microscopic observation.
Culture of Mouse Neutrophils
The operation was performed on the animal using a peripheral blood neutrophil separation liquid kit (Beijing Solarbio, China). The mouse blood was spread on top of the separation liquid and centrifuged for 20minutes; the layer between reagents C and A in the centrifuge tube indicated neutrophils. The neutrophils were washed and centrifuged again for use.
TUNEL Staining
Mice vaginal tissues, neutrophils from 200μL vaginal lavage fluid, and 5x106neutrophils from mouse blood were taken and stained using the TUNEL (Roche) staining method. The apoptosis rate was observed and calculated under a fluorescence microscope. Five visual fields in the center and four corners were selected for microscopic observation.
Transwell Assay for Neutrophil Migration
After the isolation of neutrophils, 5×106 neutrophils were cultured in the upper chamber of the Transwellchamber(Beijing Solarbio, China). IL-17 (80ng/ml, Beijing Solarbio, China), IL-17+Ab (1/20, Beijing Solarbio, China), control mouse serum (Ctrl.serum) (1/20), and second infected mouse serum (Infection.serum)(1/20) were added to the lower chamber. The number of cells in the lower chamber was counted after 4 h of culture.
Flow Cytometry Testing
One mL of mouse retroorbital blood was used to detect Th17 content in whole blood. Rat anti-mouse IL-17 FITC conjugate (BD Pharmingen) and rat anti-mouse CD4-PE conjugate (Santa Cruz Biotechnology) were used to label cells. Neutrophils were obtained from 200μL mice vaginal lavage solution, and rat anti-mouse IL-17 FITC conjugate (BD Pharmingen) and rat anti-mouse LY-6G-PE conjugate (BD Pharmingen) were used to label the cells. Mice blood neutrophils were co-cultured with IL-17 (80ng/ml), IL-17+Ab(1/20), control mouse serum (Ctrl. serum) (1/20), and second infected mouse serum (Infection. serum) (1/20) for 48 h. Afterward, 105 cells were taken from each group, and the apoptosis rate was detected by flow cytometry. The proportion of apoptotic neutrophils was stained with Annexin V/PI kit (BD Pharmingen). FACScan cell fluorometer (Becton Dickinson, BD) was used for analysis. CELLQuest software (BD) was used to analyze the obtained data.
Western Blotting Analysis
Total cell protein was extracted using RIPA lysate and was quantified with BCA protein assay kit. The samples were subjected to electrophoresis and membrane transfer. β-actin was used as an internal control, sealed with 5% fat-free milk. The primary antibodies of IL-17A antibody(1:1000,abcam, Cambridge, MA, USA), Fas antibody (1:1000, abcam, Cambridge, MA, USA), FasL antibody (1:1000, CST, Danvers, MA, USA), cleaved caspase-3 antibody (1:2000, abcam, Cambridge, MA, USA), and mouse anti-β-actin antibody (1:5000,abcam, Cambridge, MA, USA) were used to incubate the sample bands, respectively. Secondary antibodies (Goat anti-mouse IgG) were then incubated. ImageJ software was used for gray analysis and relative protein expression calculation after exposure.
Statistical Analysis
SPSS 23.0 was utilized to analyze the data, and graphpad prism 8.0 software was used for statistical mapping. The measurement data are expressed by mean standard deviation (mean±standard deviation, ±s). T-test or analysis of variance (ANOVA) was used to compare the group comparison of measurement data subject to Gaussian Distribution, otherwise the nonparametric rank sum test was used. Showed in the heatmap using the R package ggpubr (Alboukadel Kassambara (2020). ggpubr: 'ggplot2' Based Publication Ready Plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr). P<0.05 was considered statistically significant.
Results
Construction of Mouse RVVC and Protein Chip Tests
In our prior study [5], we developed an RVVC model in mice, where mice are serially infected with C. albicans and treated with antifungal four times (Fig. 1). The percent of animals colonized with C. albicans after each round of infection/treatment was reported as infection rate. This study found that infection with the strong Sap-producing strain was less likely to result in RVVC compared to infection with weak Sap-producing strain. In strong Sap-producing strain groups of mice, the infection rate (37.93% of mice remained infected) was lowest after the 3rd round of infection (Table 2), suggesting that the local immune response during the second infection was protective against subsequent infections. Therefore, for this study, we sought to identify and characterize the immune factors present during the 2nd infection that might be mediating this response.
First we measured inflammatory protein levels in the vaginal lavages collected after the 2nd infection via protein chip array. Analysis of these results indicated that there were five DEGS in the strong Sap group compared with the control group, among which three genes were up-regulated (IL-17, IL-4, IL-1β) and two down-regulated (RANTES, Timp1) (p<0.05). There were three DEGs in the weak Sap group compared with the control group, one was up-regulated (IL-17), and two were down-regulated (Tnfrsf1b, Timp1) (p<0.05) (Table 3). The heatmap showed that the gene expression level of the two experimental groups was higher than that of the control group, and the gene expression level of the strong Sap group was higher than that of the weak Sap group (Fig. 2A). The higher expression of IL-17, IL-4, and IL-1β in the strong Sap group was highlighted by log2 fold change (p<0.05) (Fig. 2B). The qRT-PCR assay exhibited that in both the Sap strong and Sap weak groups, the mRNA expression of Timp1 and RANTES was significantly decreased. In contrast, the mRNA expression of IL-1β, IL-4, and IL-17 was significantly increased (p<0.05). In addition, the mRNA level of IL-4 and IL-17 in the Sap strong group was higher than in the Sap weak group (p<0.05) (Fig. 2C).
Bioinformatics analysis revealed that IL-17 was closely related to RVVC. A. Heatmap of DEGs among Control, Sap strong group and Sap weak group (n=1); B. Expression of DEGs in different experimental groups (n=1); C. qRT-PCR verified the DEGs (n=3). (* p<0.05, vs control group. # p<0.05, strong-Sap group vs weak-Sap group)
Effect of Inhibiting Th17 on Colony Count and Neutrophils in Vaginal Lavage Fluid of RVVC Mice
We used halofuginone (HF), a pharmacological inhibitor of IL-17 T-helper cell (Th17) development [18], to observe the effect of inhibition of Th17 on colony count and neutrophils in vaginal lavage fluid in RVVC mice. As the local immune changes in the vagina were most evident after the second infection, we used the mice after the second infection to conduct the following experiments. The number of Th17 cells in the blood of RVVC mice (HF=0μg) was significantly increased compared with the control group, and HF dose-dependently decreased the number of Th17 cells (p<0.05) (Fig. 3A). The number of colonies formed in the vaginal lavage fluid of infected mice increased significantly and was proportional to the concentration of halofuginone (p<0.05) (Fig. 3B). On the contrary, the neutrophil count in vaginal lavage fluid of the halofuginone 0μg mice group was slightly decreased; however, the difference was not statistically significant compared with the control group (p>0.05). The higher the halofuginone concentration, the lower the number of neutrophils (p<0.05) (Fig. 3C).
Effect of Th17 inhibition on colony count and neutrophils in vaginal lavage fluid of RVVC mice. A. Flow cytometry showed HF dose-dependently decreased the number of Th17 cells in the blood of RVVC mice (n=6); B. HF intervention increased the colony count in vaginal lavage fluid of RVVC mice (n=6); C. HF intervention reduced the neutrophil count in vaginal lavage fluid of RVVC mice. (Scale bar=50μm) (n=6). (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
HF Dose-Dependently Downregulates Vaginal Epithelial IL-17 Expression and Aggravates Vaginal Epithelial Injury
HE staining showed finger-like protruding vaginal folds of the mice in the control group, and inflammatory cells infiltrated the epithelium. After C. albicans infection, the thickness of the vaginal mucosa of mice in the HF 0μg group was uneven, and inflammatory cell infiltration was observed in the epithelium. With the increase of halofuginone concentration, the infiltration of inflammatory cells in the vaginal epithelium decreased, but the vaginal folds were enlarged, and the damage was aggravated (Fig. 4A). After infection with C. albicans, the boundary of the basal layer of the vaginal epithelial mucosa in mice was unclear. Therefore, we measured IL-17, cleaved caspase-3 and apoptosis across the whole mucosal layer. Immunohistochemical staining showed no difference in the expression of IL-17 in vaginal epithelial cells between the halofuginone 0μg group and the control group (p>0.05). Vaginal epithelial IL-17 expression gradually decreased with increasing halofuginone concentration (p<0.05) (Fig. 4B). In addition, compared with the control group, the apoptosis rate of vaginal epithelial cells and the expression level of cleaved caspase-3 protein in epithelial cells were significantly increased in the infection group, demonstrating that HF treatment promotes apoptosis of mouse epithelial cells (p<0.05) (Fig. 4C–D).
The protective effect of IL-17 on vaginal epithelium. A. HE staining was used to detect the injury of vaginal epithelium (n=6); B. HF dose-dependently down-regulates IL-17 expression in vaginal epithelial (n=6); C. Expression of Cleaved-Caspase3 in vaginal epithelium detected by immunohistochemistry (n=6); D. Detection of apoptotic cells in vaginal epithelium by Tunel staining (n=6). (*p<0.05, **p<0.01, ***p<0.001) (Scale bar=50μm)
The Relationship Between Intravaginal IL-17 and Neutrophils in Mice
Vaginal lavage fluids from control mice and infected mice (HF=0μg) were assessed for IL-17-producing neutrophils via flow cytometry. The result showed that the neutrophils in the vagina of infected mice (HF= 0μg) highly expressed IL-17 compared with the control group (p<0.05) (Fig. 5A). The number of apoptotic neutrophils in vaginal lavage fluid was determined by TUNEL staining, and the results indicated that the number of apoptotic neutrophils in the vagina of infected mice (HF=0μg) increased (p<0.05) (Fig. 5B). Western blotting (WB) detected the protein expression of IL-17, Fas, FasL, and cleaved caspase-3 of neutrophils in the vagina. The results showed that the expression level of these proteins was significantly up-regulated in the infected group (HF=0μg) (p<0.05) (Fig. 5C).
The relationship between IL-17 and vaginal neutrophils in RVVC mice. A. Flow cytometry was used to detect the expression of IL-17 in vaginal neutrophils of RVVC mice (n=3); B. TUNEL staining was used to detect neutrophil apoptosis in vaginal lavage fluid (n=3) (Scale bar=50μm). C. Western blotting was used to detect the expression of IL-17 and apoptosis-related proteins in vaginal lavage fluid (n=3). (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
The Relationship Between IL-17 and Neutrophils In Vitro
To further observe the role of IL-17 in recruiting neutrophils and promoting neutrophil apoptosis in RVVC mice, we isolated neutrophils from the whole blood of healthy mice. The neutrophils were cultured with different mediums: IL-17(80ng/ml), IL-17+Ab(1/20), serum from healthy mice (Ctrl.serum) (1/20), and serum from RVVC mice (infection.serum, HF=0μg)(1/20), respectively. Then, the migration of neutrophils was detected by Transwell assay, the apoptosis of neutrophils was detected by flow cytometry and Tunel staining, and western blotting detected the expression of apoptosis-related protein affected by IL-17. Our experiment found that the content of Th17 in the blood of infected mice was significantly higher than that of the uninfected control group (Fig. 3A). Due to IL-17 being the main effector factor of Th17 cells [19], the IL-17 content in the serum of infected mice was also higher than that of the uninfected control group. Therefore, we used the serum of infected mice and the serum of the control group to represent the high IL-17 and low IL-17 groups, respectively. The results showed that the neutrophil cultured with infection serum had a higher apoptotic rate, migrate cell number, and apoptosis-related protein (FasL, Fas, and cleaved-caspase-3) expression levels than neutrophil cultured with control serum(p<0.05) (Fig. 6A–G). In addition, compared with the neutrophil cultured with IL-17, IL-17neutralization therapy resulted in a lower apoptotic rate, migrate cell number, and apoptosis-related protein expression (p<0.05) (Fig. 6A–G).
The relationship between IL-17 and blood neutrophils in vitro. A, B. Blood neutrophil apoptosis was detected by flow cytometry (n=3). C. Analysis of migrate cell number (n=3). D, E. TUNEL staining was used to detect blood neutrophil apoptosis under different treatment conditions (n=3) (Scale bar=50μm). F, G. Western blotting was used to detect the expression of apoptosis-related proteins in blood neutrophils (n=3). (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
Discussion
After the vaginal epithelium is infected with C. albicans, pattern recognition receptors on the surface of innate immune cells immediately bind to mannan on the surface of C. albicans; they then activate downstream signals to release cytokines and chemokines [20]. To maximize the information content of a single sample and carry out upstream and downstream molecular analysis of cytokines, we used a cytokine antibody chip to analyze the mouse vaginal lavage solution and a qPCR for verification. As RVVC is an inflammatory disease, this study used the AAM-INF-1chip to discuss the role of inflammatory factors in RVVC. The results suggested that C. albicans with strong Sap can promote vaginal epithelium to secrete more IL-4 and IL-17, as discussed in existing literature [21]: Sap can encourage the host to produce corresponding cytokines. These experimental data, along with our previous study [5], which found that IL-17 and IL-4 were found to be most highly expressed during infection with the strong Sap-expressing strains. At the same time, it was found that the high expression of IL-17 was closely related to the low infection rate, suggest the IL-17 response is important in mediating protection [5].
IL-17 has been confirmed to be involved in various pathological processes such as tissue inflammation, autoimmunity, and host defense [22] and has become a research hotspot for therapeutic targets for many diseases [23, 24]. However, there are still few reports on their roles in VVC and RVVC. To confirm the protective mechanism of IL-17 in RVVC, we used different doses of halofuginone to construct mice with increasing levels of Th17 inhibition and then infected with C. albicans [18]. A strain with strong Sap, which can produce stronger host immunity, was used in the experiment. The study found that the more Th17 inhibition by halofuginone treatment, the less IL-17 was produced in the vaginal epithelial (Fig. 4), and the higher the fungal burden. At the same time, vaginal epithelial HE and immunohistochemical staining found that the mice with low Th17 content had more significant vaginal epithelial damage and excessive apoptosis. It was suggested that Th17 and IL-17 have a protective role in RVVC.
It is known that IL-17 can initiate, recruit, and activate neutrophils to participate in the inflammatory response and act on vaginal epithelial cells [25]. Our study also confirmed that IL-17 increases the chemotatic ability of neutrophils and that this is neutralized by addition of an IL-17 antibody (Fig. 6). At the same time, the serum of VVC mice containing high Th17 levels also had a stronger ability to recruit neutrophils than the serum of the uninfected control group. In vivo experiments found that a higher Th17 response in mice was associated with an increased number of neutrophils but lower numbers of hyphae and spores in the vagina, supports the protective role of IL-17-recruited neutrophils in VVC. We speculate that although neutrophils recruited to the vagina may act on the vaginal epithelium to bring about inflammatory manifestations such as increased vaginal discharge, they also can kill C. albicans or synergize with IL-17 to fight against the C. albicans effect.
Taylor et al. [26] identified a class of neutrophils that produce IL-17 in an autocrine manner to enhance reactive oxygen species production and antifungal activity, mediating fungal killing in vitro and in vivo. Our study found that the IL-17 content of neutrophils in the vagina of RVVC mice increased, suggesting that neutrophils in the vagina can also secrete IL-17. However, this study did not determine whether IL-17 was first produced by Th cells or neutrophils. Furthermore, it was challenging to identify the pathways by which neutrophil-secreted IL-17 secretion is regulated, and these issues warrant further study.
The neutrophil is one of the key players in the human innate immune system. Neutrophils form the first cellular protection against invasive pathogens. They interact with each other and trigger inflammatory cascade reactions, resulting in various local and systemic effects, such as cell recruitment and increased cytokine production. These conditions are conducive to the fight against microorganisms but also dangerous for the host. Therefore, it is important to keep inflammation within a safe framework, and neutrophil apoptosis is one of the known existing mechanisms to limit inflammatory response [27]. Wang et al. [28] found that in Streptococcus pneumoniae otitis media, IL-17 can recruit neutrophils and promote their apoptosis to participate in bacterial clearance. Sun’s research suggested that IL-17 can play an important role in liver injury through neutrophil infiltration and apoptosis [29]. However, the studies of neutrophil apoptosis in VVC and RVVC are limited. Our study found that compared with control mice, the content of IL-17 in neutrophils in the vagina of infected mice increased, and the apoptosis of neutrophils was enhanced too. Our data suggests that IL-17 in RVVC can promote the apoptosis of neutrophils in the vagina. To confirm this finding, we cultured mouse neutrophils in vitro and co-cultured them with IL-17, IL-17 antibody, control mouse serum, and C. albicans-infected mouse serum, respectively. The purpose of using the serum of infected mice and control mice is that the IL-17 content in the serum of infected mice is significantly higher than that of the control group, thus representing the high IL-17 group and the low IL-17 group. The results showed that the addition of recombinant IL-17 or serum of infected mice increases neutrophil apoptosis. While we cannot rule out additional factors in infected serum that may be promoting neutrophil apoptosis, these data support the role of IL-17 in enhancing neutrophil apoptosis. We speculated that IL-17 could eliminate the recruited neutrophils by enhancing neutrophil apoptosis to protect the mice’s vaginal tissue.
Pietrella et al. also treated VVC mice with halofuginone and observed similar phenotypes, such as increased fungal burden and decreased IL-17. Their experiment also found that both vaginal epithelial cells and neutrophils in mice can secrete IL-17 [12]. In comparison, our study found that the decrease of Th17 in the blood of mice was accompanied by the aggravation of vaginal epithelial injury, which confirmed the protective effect of Th17 and IL-17 in RVVC. At the same time, we further investigated the dose-dependent relationship between IL-17 and neutrophils in the vagina by constructing RVVC mice with different Th17 concentrations and determined the role of IL-17 in promoting neutrophil apoptosis in RVVC mice.
A limitation of our research was that it was only carried out on mice. Fidel conducted an experiment on exogenous fungal infection in human volunteers, and the conclusion supports that the mouse model can replace female vaginal candidiasis [30]. However, different opinions remain. As we know, C. albicans is a normal component of the human vaginal microbiota, and Lactobacillus plays a role in women’s vaginal health, protecting them from Candidal infection. On the contrary, C. albicans does not exist in the mouse vagina, and the vaginal microbiota of mice is mainly composed of Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and Cyanobacteria [31, 32]. At the same time, there is immune resistance and immune tolerance (46) in the human vaginal tract due to prior exposure to Candida species, while mice without any innate immunity to Candida are very sensitive to exogenous fungal infection [31]. These factors limit the study of the murine RVVC model.
In conclusion, IL-17 is a key protective factor in murine RVVC, and IL-17-mediated neutrophil apoptosis may contribute to the pathological process of RVVC mice. Further research, specifically including infected women, is urgently needed to verify these findings.
Data Availability
The authors declare that all data and materials used in this research are available for consultation.
References
Rodriguez-Cerdeira C, Gregorio MC, Molares-Vila A, Lopez-Barcenas A, Fabbrocini G, Bardhi B, et al. Biofilms and vulvovaginal candidiasis. Colloids Surf B Biointerfaces. 2019;174:110–25.
Yano J, Peters BM, Noverr MC, Fidel PL Jr. Novel mechanism behind the immunopathogenesis of vulvovaginal candidiasis: “neutrophil anergy.” Infect Immun. 2018;86(3):e00684-17. https://doi.org/10.1128/IAI.00684-17.
Ardizzoni A, Wheeler RT, Pericolini E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front Microbiol. 2021;12:692491.
Kalia N, Singh J, Sharma S, Kaur M. SNPs in 3'-UTR region of MBL2 increases susceptibility to recurrent vulvovaginal infections by altering sMBL levels. Immunobiology. 2019;224(1):42–9.
Shao MK, Qi WJ, Hou MY, Luo DD. Analysis of pathogenic factors of Candida albicans and the effect of vaginal immunization on recurrent vulvovaginal candidiasis in mice. J Obstet Gynaecol Res. 2022;48(3):857–65.
Gabrielli E, Sabbatini S, Roselletti E, Kasper L, Perito S, Hube B, et al. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence. 2016;7(7):819–25.
Pericolini E, Gabrielli E, Amacker M, Kasper L, Cassone A. Secretory Aspartyl Proteinases Cause Vaginitis and Can Mediate Vaginitis Caused by Candida albicans in Mice. mBio. 2015;6(3):e00724.
Rosati D, Bruno M, Jaeger M, Ten Oever J. Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms. 2020;8(2):144.
Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43(6):1040–51.
Jiang L, Fang M, Tao R, Yong X, Wu T. Recombinant human interleukin 17A enhances the anti-Candida effect of human oral mucosal epithelial cells by inhibiting Candida albicans growth and inducing antimicrobial peptides secretion. J Oral Pathol Med. 2020;49(4):320–7.
Peters BM, Coleman BM, HME W, Barker KS, FEY A, Cipolla E, et al. The interleukin (IL) 17R/IL-22R signaling axis is dispensable for vulvovaginal candidiasis regardless of estrogen status. J Infect Dis. 2020;221(9):1554–63.
Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, et al. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One. 2011;6(7):e22770.
Klaile E, Prada Salcedo JP, Klassert TE, Besemer M, Bothe AK, Durotin A, et al. Antibody ligation of CEACAM1, CEACAM3, and CEACAM6, differentially enhance the cytokine release of human neutrophils in responses to Candida albicans. Cell Immunol. 2021;371:104459.
Luo S, Skerka C, Kurzai O, Zipfel PF. Complement and innate immune evasion strategies of the human pathogenic fungus Candida albicans. Mol Immunol. 2013;56(3):161–9.
Kobayashi SD, Voyich JM, Buhl CL. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: Cell fate is regulated at the level of gene expression. Proc Natl Acad Sci U S A. 2002;99(10):6901–6.
Maimon N, Zamir ZZ, Kalkar P, Zeytuni-Timor O, Ariel A. The pro-apoptotic ARTS protein induces neutrophil apoptosis, efferocytosis, and macrophage reprogramming to promote resolution of inflammation. Apoptosis. 2020;25(7-8):558–73.
El-Houssaini HH, Elnabawy OM, Nasser HA, Elkhatib WF. Correlation between antifungal resistance and virulence factors in Candida albicans recovered from vaginal specimens. Microb Pathog. 2019;128:13–9.
Pines M, Spector I. Halofuginone - the multifaceted molecule. Molecules. 2015;20(1):573–94.
Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2(5):403–11.
Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33(1):257–90.
Cassone A, Vecchiarelli A, Hube B. Aspartyl proteinases of eukaryotic microbial pathogens: from eating to heating. PLoS Pathog. 2016;12(12):e1005992.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76.
Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394.
Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res. 2019;42(7):549–59.
Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23(5):613–9.
Taylor PR, Roy S, Leal SM Jr, Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15(2):143–51.
Kalimo H, Zoppo G, Paetau A. Polymorphonuclear neutrophil infiltration into ischemic infarctions: myth or truth? Acta Neuropathol. 2013;125(3):313–6.
Wang W, Zhou A, Zhang X, Xiang Y, Huang Y, Wang L, et al. Interleukin 17A promotes pneumococcal clearance by recruiting neutrophils and inducing apoptosis through a p38 mitogen-activated protein kinase-dependent mechanism in acute otitis media. Infect Immun. 2014;82(6):2368–77.
Sun C, Hideki F, Kono H, et al. Interleukin 17A plays a role in lipopolysaccharide/D-galactosamine-induced fulminant hepatic injury in mice. J Surg Res. 2015;199(2):487–93.
Fidel PL, Barousse M, Espinosa T, Ficarra M, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72(5):2939.
Cassone A, Sobel JD. Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect Immun. 2016;84(5):1255–61.
Petrova MI, Elke L, Shweta M, Nicole I, Sarah L. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81.
Code Availability
Not applicable
Funding
This research was supported by a grant from the National Natural Science Foundation of China (Grant No.82260302; Grant No. 81660248) and the Yunnan Provincial Department of Education Scientific Research Fund (Grant No.2022J0259).
Author information
Authors and Affiliations
Contributions
S-MK, Q-WJ, H-MY, and L-SN conducted the experiments. Q-WJ planned and supervised the experiments. S-MK wrote the paper. All authors gave intellectual input to the study and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experiment was approved by the Institutional Animal Care and Use Committee (IACUC) at Kunming Medical University (No.kmmu2020178).
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, M., Hou, M., Li, S. et al. The Mechanism of IL-17 Regulating Neutrophils Participating in Host Immunity of RVVC Mice. Reprod. Sci. 30, 3610–3622 (2023). https://doi.org/10.1007/s43032-023-01291-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01291-z