Abstract
Endometrial injury is one of the leading causes of female infertility and is caused by intrauterine surgery, endometrial infection, repeated abortion, or genital tuberculosis. Currently, there is little effective treatment to restore the fertility of patients with severe intrauterine adhesions and thin endometrium. Recent studies have confirmed the promising therapeutic effects of mesenchymal stem cell transplantation on various diseases with definite tissue injury. The aim of this study is to investigate the improvements of menstrual blood-derived endometrial stem cells (MenSCs) transplantation on functional restoration in the endometrium of mouse model. Therefore, ethanol-induced endometrial injury mouse models were randomly divided into two groups: the PBS-treated group, and the MenSCs-treated group. As expected, the endometrial thickness and gland number in the endometrium of MenSCs-treated mice were significantly improved compared to those of PBS-treated mice (P < 0.05), and fibrosis levels were significantly reduced (P < 0.05). Subsequent results revealed that MenSCs treatment significantly promoted angiogenesis in the injured endometrium. Simultaneously, MenSCs enhance the proliferation and antiapoptotic capacity of endometrial cells, which is likely contributed by activating the PI3K/Akt signaling pathway. Further tests also confirmed the chemotaxis of GFP-labeled MenSCs towards the injured uterus. Consequently, MenSCs treatment significantly improved the pregnant mice and the number of embryos in pregnant mice. This study confirmed the superior improvements of MenSCs transplantation on the injured endometrium and uncovered the potential therapeutic mechanism, which provides a promising alternative for patients with serious endometrial injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Endometrial injury is usually attributed to intrauterine surgery, endometrial infection, repeated abortion or genital tuberculosis, and other factors, resulting in the formation of a thin endometrium, partial or complete fibrosis of the uterine cavity, uterine cavity coarctation, distortion, and even disappearance, and finally, it can further develop into intrauterine adhesion (IUA) [1,2,3]. Generally, the aforementioned factors can lead to damage of the endometrial basal layer, which plays an important role in repairing the wound area, and results in clinical manifestations including amenorrhea, recurrent abortion, and infertility, which seriously affect the physical and psychological health of patients [4, 5]. In recent years, the incidence and detection rates of thin endometrium and IUA are on the rise, which has become the secondary leading cause of female infertility [6, 7].
It is generally believed that endometrial thickness ≤ 7 mm will affect the pregnancy rate and live birth rate after fresh or frozen embryo transfer, so endometrial thickness ≤ 7 mm is defined as thin endometrium [8]. There are many clinical treatments for thin endometrium, such as local curettage of endometrium, hormone supplement, drug stimulation, and electrical stimulation, but the improvements are limited and depend more on the individual difference [9]. Simultaneously, the aim of IUA treatment is to reshape the morphology of uterine cavity and restore uterine function, and the main treatment is transcervical resection of adhesions, removal of adhesive tissue, restoration of the anatomical morphology of the uterus, placement of physical barriers to prevent re-adhesion, and estrogen therapy to stimulate endometrial regeneration [10,11,12]. Unfortunately, for patients with severe IUA, few normal endometrial cells exist in the endometrium, and even if the uterine cavity shape is restored, the endometrium is difficult to regenerate, not to mention its functional recovery [13].
Tissue regeneration, especially stem cell-based tissue regeneration, provides new options for the treatment of patients with serious IUA and thin endometrium. In the past decade, both mesenchymal stem cells (MSCs) themselves, which possess the characteristics of superior proliferative capacity, multilineage differentiation potential, and low immunogenicity, and their derivatives (extracellular vesicles and exosomes) have been demonstrated to play important roles in the improvement of injured tissues [14, 15]. Consequently, several types of MSCs have been proven to be closely related to the renewal and regeneration of the endometrium and are promising for use in the repair of endometrial injury, such as bone marrow-derived stem cells (BMSCs), umbilical cord mesenchymal stem cells (UcMSCs), adipose mesenchymal stem cells (ADSCs), and human embryonic stem cells (hESCs) [16,17,18,19]. Currently, it has been demonstrated that the mechanisms of MSCs-based therapy for endometrial regeneration mainly include the following: (1) angiogenesis and proliferation in endometrium induced by MSCs-derived paracrine cytokines; (2) inhibition of the local inflammatory response by indirect (MSCs-derived anti-inflammatory cytokines) and direct methods (immunoregulatory effect of MSCs on immunocytes through contact); (3) transdifferentiating into endometrial-like cells and fusion into the newly formed endometrium by MSCs themselves [20].
Currently, endometrial stem cells existed in human [21], murine [22], bovine [23], and equine [24] uteri have been identified; especially, human menstrual blood-derived endometrial stem cells (MenSCs) have become a promising therapeutic option for endometrial injury without effective treatment due to their comprehensive advantages, mainly including noninvasive protocol for their collection, abundant source material, stable donation, and autotransplantation [25]. Simultaneously, MenSCs transplantation is likely to exhibit superior therapeutic effects for the injured endometrium by virtue of the geographical relationship in which MenSCs originate from the deciduous endometrium. Therefore, in this study, we aimed to investigate the improvement of MenSCs transplantation on the morphological and functional recovery of injured endometrium in ethanol-induced IUA mouse models and the underlying mechanisms.
Materials and Methods
Animals
Female BALB/c mice (aged 12 weeks, weighing 22–25 g) were purchased from Vital River Laboratories (Beijing, China), and housed in a specific pathogen free environment with a 12-h light–dark cycle. Furthermore, the handling of mice and experimental procedures were performed with approval from the Animal Care Committee of Xinxiang Medical University in accordance with the guidelines established by the Chinese Council on Animal Care. Vaginal smears were collected at 9:00 am, and conventional Papanicolaou staining was performed to ensure that all the mice used to establish ethanol-induced endometrial injury model were in the estrous phase.
MenSCs Preparation and Identification
All MenSCs used in this study were collected from human menstrual blood using density gradient centrifugation and kindly provided by the Zhongyuan Stem Cell Research Institute (Xinxiang, China). Briefly, cryopreserved MenSCs (passage 2, P2) were recovered in 75-cm2 plastic cell culture flasks (1 × 106 cells per flask) and conventionally cultured with growth medium (high-glucose Dulbecco’s modified Eagle medium, HG DMEM) (Corning, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco, USA), penicillin, and streptomycin). Subsequently, the phenotypes of MenSCs were identified by flow cytometric analysis, and the multilineage differentiation potential of MenSCs was assessed with a Tri-Line Induced Differentiation Kit (osteoblast, adipoblast, chondroblast differentiation; Chembio-engine.Co., China). P4 to P6 fresh MenSCs were used for the following experiments.
Ethanol-Induced Endometrial Injury Model and MenSCs Transplantation
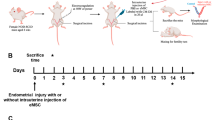
Ethanol-induced endometrial injury mouse models were prepared according to our published report [26]. Briefly, 40 mice were anaesthetized with an anespirator (isoflurane), and 4% phloroglucinol (0.02 mg/g) was injected to relax the cervical muscles. Then, 95% ethanol was gently injected into the uterine horn. After treatment with ethanol for 40 s, 3 mL saline was used to slowly rinse the uterine horn to remove residual ethanol. Subsequently, to assess the improvement of MenSCs transplantation on the morphological and functional recovery of injured endometrium in model mice, the above prepared model mice were randomly divided into two groups: PBS-treated group (n = 20) and MenSCs-treated group (n = 20). The day of modeling was designated day 0, and at day 1, 1 × 106 MenSCs were transplanted into the MenSCs-treated mice through the tail vein (cells suspended in 200 µL PBS); accordingly, at day 8 and day 15, the mice in MenSCs-treated group received another two MenSCs transplantations (1 × 106 cells). The PBS-treated mice received an equal amount of PBS (200 µL) with the same procedure used in the MenSCs-treated mice. At 15 days posttreatment, 10 mice in each group were randomly selected and sacrificed, the uteri were isolated for the following examination, and the remaining 10 mice were subjected to fertility tests.
Hematoxylin Eosin (HE) Staining
The uteri were fixed in 4% paraformaldehyde for 24 h and then conventionally embedded in paraffin. Serial paraffin-embedded Sects. (4 µm) were obtained, sequentially dewaxed in xylene I and xylene II for 20 min each, and rehydrated in a series of ethanol solutions with a decreasing concentration (100% for 10 min, 100% for 10 min, 95% for 5 min, 90% for 5 min, 80% for 5 min, and 70% for 5 min). Then, the sections were rinsed in distilled water (three times, 5 min each), and were stained with an H&E solution according to the manufacturer’s instructions (Servicebio, China). The morphological changes of endometrium were observed under inverted microscope. The transverse section of uterus was taken, the widest part of uterine cavity line was clearly displayed, and the thickness of double-layer endometrium on opposite sides was measured. The gland number in per unit area of endometrium was calculated according to five randomly selected high-power fields of each slide.
Masson Staining
The 4-µm paraffin sections of uteri were dewaxed and rehydrated as described above, and then stained with a Masson staining kit according to the manufacturer’s instructions (Servicebio, China). Briefly, the sections were immersed in Masson A solution overnight, which was followed by a brief wash under running water. Then, the sections were stained in a mixed solution of Masson A and Masson B (1:1) for 1 min, washed under running water, and placed in 1% hydrochloric acid alcohol for 10 s before they were washed again. Subsequently, the sections were immersed in Masson D solution for 6 min and then were stained in Masson E solution for 1 min. Thereafter, the solution was slightly drained, and the sections were placed directly in Masson F solution for 2 to 30 s, and then rinsed in 1% glacial acetic acid for differentiation of the signals. Finally, the sections were dehydrated in absolute ethyl alcohol, clarified in xylene for 5 min, and sealed in Permount mounting medium. Endometrial fibrosis levels were assessed according to five randomly selected fields on each slide, and the fibrotic percentages were calculated using Image-Pro Plus software (version 6.0).
Protein Array Assays
Angiogenesis arrays (QAH-ANG-1, RayBiotech, USA) were performed to measure the expression levels of 10 angiogenesis-associated biological factors in MenSC-derived conditioned medium (MenSC-CM) that had been concentrated tenfold by ultrafiltration (n ≥ 6). Signals were visualized using a laser scanner (InnoScan 300 Microarray Scanner, France), and the GAL file with the microarray analysis software was used to quantify the targeted proteins.
Immunohistochemistry
Paraffin sections of uteri were conventionally dewaxed and hydrated and a series of tissue antigen recoveries were performed. Primary antibodies, including anti- platelet endothelial cell adhesion molecule-1 (CD31) antibody, anti-cytokeratin 18 (CK18) antibody, anti-marker of proliferation Ki-67 (Ki67) antibody, and anti-adhesion G protein-coupled receptor E1 (F4/80) antibody (Table S1), were diluted with PBS containing 1% bovine serum albumin. The nonspecific antigens of the specimens were blocked for 30 min using 3% bovine serum albumin. Subsequently, the sections were incubated with primary antibodies overnight at 4 °C and then with secondary antibodies for 40 min at room temperature. Hematoxylin staining was performed after the reaction was paused using diaminobenzidine (DAB) chromogen kit. Images were acquired under microscope. CK18 IHC scores were calculated using the ImageJ plug-in IHC profiler. The numbers of positively stained cells were counted and quantified using Image-Pro Plus based on optical density. Additionally, the vascular density was measured from five randomly selected fields of vision of each section.
Western Blotting
Tissues were lysed in lysis buffer containing protease and phosphatase inhibitors (Beyotime Biotechnology, China). The protein samples (20 µg/lane) were then separated by 10–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and blocked with 5% nonfat milk in TBS containing 0.1% Tween 20. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody, anti-vascular endothelial growth factor (VEGF) antibody, anti-proliferating cell nuclear antigen (PCNA) antibody, anti-leukemia inhibitory factor (LIF) antibody, anti-phosphatidylinositol 3-kinase (PI3K) antibody, anti-phosphorylation phosphatidylinositol 3-kinase (p-PI3K) antibody, anti-protein kinase B (Akt) antibody, anti-phosphorylation protein kinase B (p-Akt) antibody, anti-B-cell lymphoma-2 (Bcl-2) antibody, anti-B-cell leukemia/lymphoma XL (Bcl-xL) antibody, anti-BCL2-associated X (Bax) antibody, anti-p53 upregulated modulator of apoptosis (PUMA) antibody, and anti-green fluorescent protein (GFP) antibody (Table S1) were used for western blotting analysis. The signals were developed using the ECL WB substrate kit and detected using Amersham Imager 600 (GE Healthcare Life Sciences) with Image Lab software.
Fertility Tests
At 15 days posttreatment, 10 mice in each group were randomly selected, and further housed with 8-week-old proven fertile males at a female:male ratio of 1:2 for 2 months, and the detection of the vaginal plug of female mice were recognized as successful mating. Subsequently, the mice were weighed every 2 days to detect the body weight changes, and the number of pregnant mice was recorded. Furthermore, the pregnant mouse was euthanized on approximately the 18th day of gestation, and the bilateral uterus was isolated and imaged, as well as the number of embryos was recorded.
MenSCs Distribution In Vivo
GFP-labeled MenSCs were prepared by conventional lentiviral transfection. Then, ethanol-induced endometrial injury mouse models (n = 9) were used to examine the chemotaxis and accumulation of intravenously injected GFP-labeled MenSCs in the injured endometrium. On days 1, 3 and 5 after GFP-labeled MenSCs transplantation (1 × 106 cells suspended in 200 µL PBS), three mice were selected and sacrificed; then, the lung and uterus were isolated, observed and imaged under a fluorescence microscope. Simultaneously, protein samples of the lung and uterus were extracted for Western blotting to detect the GFP expression, which indirectly reflects the chemotaxis of MenSCs towards injured endometrium.
Statistical Analysis
The data presented as the mean ± SEM were analyzed with Statistical Package for the GraphPad Prism 8.0. The normality and the homogeneity of variance were checked by Shapiro–Wilk tests prior to apply Student’s t-test and one-way ANOVA analysis. To determine statistical significance, Student’s t-test was used for comparisons between two groups; one-way ANOVA followed by Dunnett’s test was used for comparisons between multiple groups. Statistical significance was assumed for P < 0.05.
Result
MenSCs Transplantation Promotes the Morphological Recovery of Injured Endometrium
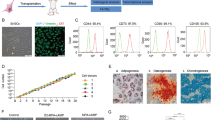
All MenSCs used in our study fulfilled the characteristics of MSCs (Fig. 1), such as typical spindle fibroblast-like morphology cultured in vitro; positive expression for CD44, CD73, CD90, and CD105; negative expression for CD34, CD11b, CD19, and CD45; human leukocyte antigen DR (HLA-DR); and adipogenic, osteogenic, and chondrogenic differentiation potential. Based on ethanol-induced endometrial injury mouse model, we found that both the endometrial thickness and the gland number in the endometrium of MenSCs-treated mice were significantly upregulated in comparison to those in PBS-treated mice (Fig. 2A, C, and D, P < 0.05). The subsequent Masson staining results also showed that compared to the fibrosis levels in the endometrium of PBS-treated mice, the fibrosis levels in the endometrium of MenSCs-treated mice were notably downregulated (Fig. 2B and E, P < 0.05).
The MenSCs used in our study fulfill the standard of typical MSCs. A The morphology of P0 and P3 MenSCs was observed under a microscope, and P3 MenSCs exhibited a typical spindle-like morphology when cultured in a cell flask. B The immunophenotype of P3 MenSCs was detected by flow cytometry, and the results demonstrated that MenSCs positively expressed classical cell surface markers of MSCs (CD44, CD73, CD90, and CD105) and negatively expressed hematopoietic stem cell markers (CD34 and CD45), CD11b, CD19, and HLA-DR. C P3 MenSCs were used for the multilineage differentiation potential assay, including adipogenesis (oil red O staining), osteogenesis (Alizarin red staining), and chondrogenesis (Alcian blue staining)
MenSCs transplantation improved the morphology of injured the endometrium. A Schematic description of the experimental design. B HE staining results showed that compared with the model mice, the morphology of the endometrium was significantly improved 15 days after MenSCs transplantation, and both the endometrial thickness and the number of endometrial glands were significantly increased in MenSCs-treated model mice. n = 5. C Masson staining results showed that MenSCs transplantation significantly reduced endometrial fibrosis in model mice. n = 5. D–F ImageJ was used to quantify the thickness of the endometrium, the number of endometrial glands, and the degree of fibrosis. n = 5. **P < 0.01
MenSCs Transplantation Promotes Angiogenesis in the Injured Endometrium
Angiogenesis is always considered to be essential for regeneration of injured tissues. Therefore, a protein array assay was performed to quantify the associated angiogenic factors in MenSC-conditioned medium (CM). As shown in Fig. 3A and B, MenSCs were capable of secreting high levels of pro-angiogenic factors (angiogenin 2 (ANG-2), hepatocyte growth factor (HGF), and VEGF). Furthermore, we analyzed the number of CD31+ cells and VEGF expression in uterine tissues to investigate the angiogenesis-promoting effect after MenSCs transplantation. As expected, the number of CD31+ cells was significantly increased in the endometrium of MenSCs-treated mice compared to that in PBS-treated mice (Fig. 3C and E, P < 0.05). Subsequent examination also confirmed that the VEGF expression was significantly upregulated in the uterus of MenSCs-treated mice (Fig. 3D and F, P < 0.05).
MenSCs transplantation significantly promotes endometrial angiogenesis. A Representative protein array images and angiogenesis factors are examined and are shown. n = 3. B Fluorescence intensity of the indicated angiogenesis factors. n = 3. C The expression of CD31 in the endometrium was detected by immunohistochemical staining after MenSCs transplantation for 15 days, and the results showed that the number of CD31+ cells was significantly upregulated in the endometrium of MenSCs-treated model mice. n = 3. D The western blotting results confirmed that the expression of VEGF was also significantly upregulated in the uterus of MenSCs-treated model mice. n = 3. E, F Image-Pro Plus software was used to quantify CD31.+ cells and the relative expression of VEGF. n = 3. * P < 0.05, ***P < 0.001
MenSCs Transplantation Promotes the Proliferation and Integrity of Endometrial Cells
CK18 is essential for maintaining epithelial cellular structural integrity and resisting extracellular stresses. As shown in Fig. 4A and C, the CK18+ cells in the endometrium of MenSCs-treated mice were significantly upregulated compared with those in PBS-treated mice (P < 0.05) and were mainly distributed in the uterine luminal epithelium and endometrial glandular tissue. Simultaneously, as a typical proliferation-related marker, Ki67+ cells were significantly increased in the endometrium of MenSCs-treated mice in comparison with that in PBS-treated mice (Fig. 4B, D, and E, P < 0.05), and the increased Ki67+ cells were mainly distributed in the functional layer and epithelium of endometrium (Fig. 4B). In accordance with the above result, PCNA expression in the uteri of MenSCs-treated mice was also significantly upregulated (Fig. 4F and G, P < 0.05).
MenSCs transplantation promotes the proliferation and integrity of endometrial cells. A, B The expression of CK18 and Ki67 in the endometrium was detected by immunohistochemical staining after MenSCs transplantation for 15 days. And the results showed that CK18 expression was significantly upregulated in both endometrial luminal epithelial cells and endometrial glandular epithelial cells in MenSCs-treated model mice; moreover, the number of Ki67+ cells was significantly upregulated in both the endometrial epithelium and stroma in MenSCs-treated model mice. n = 3. C–E Image-Pro Plus software was used to quantify the relative expression of CK18 and the Ki67.+ cells. n = 3. F The western blotting results confirmed that the expression of PCNA was also significantly upregulated in the uterus of MenSCs-treated mice. n = 3. G The relative expression of PCNA was quantified using Image-Pro Plus software. n = 3. *P < 0.05, ***P < 0.001
MenSCs Transplantation Enhances Antiapoptotic Capacity and Activates the PI3K/Akt Signaling Pathway in the Injured Uterus
To gain further insight into the MenSCs-induced proliferation of endometrial cells, we first examined the antiapoptotic capacity of the uterus in MenSCs-treated model mice, and the results showed that the expression of antiapoptotic factors (Bcl-2 and Bcl-xL) was dramatically upregulated in the uterus of MenSCs-treated model mice compared with that in PBS-treated mice (Fig. 5A, D, E, P < 0.05). Meanwhile, the expression of proapoptotic factors (Bax and PUMA) in MenSCs-treated uteri also exhibited a significant downregulation (Fig. 5A, F, G, P < 0.05). Subsequently, the changes in the expression of key factors in PI3K/Akt signaling pathway in the MenSCs-treated uterus were analyzed, which is closely related to cell proliferation and antiapoptosis. As shown in Fig. 5A–C, the phosphorylation of PI3K (p-PI3K) and Akt (p-Akt) was significantly upregulated in the uterus of MenSCs-treated model mice compared with that in PBS-treated mice (P < 0.05).
MenSCs transplantation enhances the antiapoptotic capacity and activates the PI3K/Akt signaling pathway in the injured uterus. A The western blotting results indicated that the expression of antiapoptotic factors (Bcl-2 and Bcl-xL) was dramatically upregulated and that proapoptotic factors (Bax and PUMA) were downregulated in the uterus of MenSCs-treated model mice; moreover, the PI3K/Akt signaling pathway was significantly activated. n = 3. B–G The relative expression of targeted proteins was quantified using Image-Pro Plus software. n = 3. H Schematic diagram: MenSCs treatment enhanced the antiapoptotic capacity of impaired endometrial cells and promoted the proliferation of endogenous endometrial cells by activating the PI3K/Akt signaling pathway. *P < 0.05, **P < 0.01
MenSCs Transplantation Improves the Fertility of Model Mice
LIF play vital roles in reproductive systems. Our results not only showed that the expression of LIF was significantly upregulated in the uterus of MenSCs-treated mice (Fig. 6A, B, P < 0.05), but also confirmed that MenSCs treatment significantly improved the fertility of model mice in terms of both the number of pregnant mice and the number of embryos (Fig. 6C–E, P < 0.05).
MenSCs transplantation improves the fertility of model mice. A The western blotting results confirmed that the expression of LIF was also significantly upregulated in the uterus of MenSCs-treated model mice. B Image-Pro Plus software was used to quantify the relative expression of LIF. C–E After MenSCs treatment, model mice were caged with adult male mice, and then, the number of pregnant mice and embryos was recorded and analyzed. **P < 0.01, ***P < 0.001
MenSCs Tend to Migrate Towards the Injured Uterus
After GFP-labeled MenSCs (Fig. 7A) transplantation for 1, 3, and 5 days, we clearly observed a green influence in the lung tissue, but not in the uterine tissues (data not shown) under a stereo fluorescence microscope (LEICA M205FA, Germany), and the intensity of the green influence in the lung tissue gradually decreased with time (Fig. 7B, C), which is consistent with GFP expression in the lung tissues examined by WB (Fig. 7D and E). Although we could not directly observe the green influence in the uterine tissue, the indirect detection of GFP expression in the uterus showed that the GFP signal gradually increased with time, which strongly suggested that the transplanted MenSCs were capable of migrating towards the injured uterus (Fig. 7D and E), and provided support for MenSCs-derived improvement of the injured endometrium.
MenSCs tend to migrate towards the injured uterus. A GFP-labeled MenSCs were prepared by conventional lentiviral transfection and observed under an inverted fluorescence microscope. n = 3. B After receiving GFP-labeled MenSCs for 1, 3, and 5 days from the tail vein, the lung tissues of mice were isolated and observed under a stereographic fluorescence microscope. n = 3. C ImageJ was used to relatively quantify the green fluorescence intensity of GFP-transfected MenSCs in the lungs of mice. n = 3. D GFP expression in the uterus and lungs of mice was detected by western blotting, which can indirectly indicate the chemotaxis of MenSCs towards the above tissues. n = 3. E The relative expression of GFP was quantified by Image-Pro Plus software. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
With the increase in various intrauterine operations (such as dilation and curettage, mediastinotomy, etc.), the number of patients with IUA or thin endometrium is also increasing, and these diseases seriously affect female fertility. Although a variety of treatments have been developed in the recent decades, the therapeutic effects are limited, especially for patients with serious IUA, resulting in the loss of fertility [27]. Therefore, it is urgent to explore new treatments to protect the patients with IUA or thin endometrium against fertility lose. In the past decade, stem cell-based therapies have gained great attention in repairing injured endometrial tissues and recovering endometrial function, and many reports have demonstrated improvements in the injured endometrium after MSCs transplantation both in basic and clinical studies, mainly focused on BMSCs- [28], UcMSCs- [17], and ADSCs-based therapies for endometrial regeneration [18].
In addition to the commonly used types of MSCs, MenSCs isolated from the deciduous endometrium in menstrual blood samples not only fulfill the conventional characteristics of MSCs but also exhibit its unique advantages, such as superior accessibility (women of childbearing age), noninvasive isolation procedures (menstrual cup), and stable donation (periodically collected from the same donor). Because of the abovementioned advantages, MenSCs have gradually become a promising option for MSCs-based therapies for various diseases, and MenSCs transplantation has been successfully performed for treatments of multiple sclerosis, myocardial infarction, and Parkinson’s disease, which provides solid support for MenSCs-based therapies for patients with serious endometrial injuries. A recent report showed that the endometrium of MenSCs-treated mice was thickened, more glands were observed, the degree of fibrosis was alleviated, and the expression of CK, VIM, PR, and ER in the transplantation regions was observed, suggesting that MenSCs treatment was capable of regenerating the injured endometrium in vivo [29]. Moreover, a previous study also demonstrated that MenSCs treatment could prevent or reverse mifepristone-induced impairment of endometrial stromal cells by activating the Akt and p38 MAPK signaling pathways [30].
Consistent with previous studies, our results preliminarily confirmed that MenSCs treatment not only significantly increased the endometrial thickness and gland number, but also reduced the degree of fibrosis in the endometrium and improved the fertility of model mice, which was successfully established by a minimally invasive transcervical ethanol perfusion technique [26]. Furthermore, our subsequent detection demonstrated that endometrial regeneration in the model mouse after MenSCs treatment is likely contributed by MenSCs-induced angiogenesis and cell proliferation in the injured endometrium, and finally leads to fertility improvement in MenSCs-treated mice.
MSCs can secrete a variety of angiogenic factors, such as HGF, VEGF, bFGF, and ANG, which are deeply involved in tissue angiogenesis, cell proliferation, and migration. As expected, our previous findings have demonstrated that MenSCs-derived conditioned medium contains abundant angiogenic factors, which play critical roles in promoting the regeneration of injured endometrium [31]. A subsequent report further confirmed that MenSCs-CM could upregulate the expression of eNOS, VEGFA, VEGFR1, VEGFR2, and Tie2 in HUVECs, and promote cell proliferation, migration, and angiogenesis by activating the Akt and ERK pathways [32]. Moreover, Hu et al. reported that MenSCs transplantation can significantly upregulate the expression of vimentin, VEGF, and keratin in the endometrium of model mice, thus repairing the endometrial lesions [33]. Consistently, our results not only demonstrated that MenSCs were capable of secreting abundant angiogenesis-related factors, but also confirmed the angiogenesis-promoting effect in the injured endometrium of mice after MenSCs transplantation, which manifested as significantly increased CD31+ cells and the upregulation of VEGF in the endometrium.
Simultaneously, replenishment of new cells is essential for the functional restoration of injured endometrium. Although MSCs have the potential to transdifferentiate into various cells, especially in vitro, there is wide agreement that MSCs transplantation is likely to enhance the antiapoptotic capacity of impaired cells and activate the proliferation of endogenous cells, rather than transdifferentiating into cells of injured tissue required. MenSCs treatment significantly promoted the proliferation of EM-E6/E7/hTERT cells in a time-dependent manner [34]. Furthermore, as mentioned above, MenSCs treatment not only significantly restored the proliferation and migration ability of injured endometrial stromal cells induced by mifepristone but also inhibited the apoptosis of endometrial stromal cells, which was partly contributed by upregulating the expression of p-Akt, p-p38, VEGF, and β-catenin [30]. Our further findings also demonstrated that not only the number of Ki67+ cells and PCNA expression was significantly upregulated in the endometrium of MenSCs-treated mice, but also the antiapoptotic capacity of impaired endometrial cells was enhanced. Subsequent studies further confirmed the activation of the PI3K/Akt signaling pathway in the uterus of MenSCs-treated mice, which is closely related to cell metabolism, such as proliferation, differentiation, and apoptosis. Bax, Bcl-xL, and Bcl-2 belong to the Bcl-2 family, which plays critical roles in the mitochondrial-related apoptotic pathway, and activated PI3K/Akt signaling pathway can upregulate the expression of Bcl-2 and reduce the release of cytochrome C, thereby inhibiting the activity of Bax and its induced cell apoptosis [35]. Therefore, we reasonably postulated that MenSCs treatment could enhance the antiapoptotic capacity of impaired endometrial cells and promote the proliferation of endogenous endometrial cells by activating the PI3K/Akt signaling pathway (Fig. 5H).
Based on MenSCs-derived angiogenesis and cell proliferation in the injured endometrium, we finally evaluated fertility in MenSCs-treated mice. As expected, the endometrial receptivity of model mice was significantly improved, and the subsequent fertility test confirmed that both the fertility rate and fetus quantity in the MenSCs-treated mice were markedly increased. Previous to our study, Tan et al. showed that MenSCs transplantation was capable of effectively promoting fertility restoration in IUA rats [36]; and recently, several clinical trials also demonstrated that autologous MenSCs transplantation significantly increased endometrial thickness and pregnancy potential in patients with refractory IUA, and 8 out of 19 patients (42.1%) achieved clinical pregnancy in these clinical trials [37, 38]. Moreover, a phase I/II Clinical Trial launched at 2018 (TRN: IRCT20180619040147N2) confirmed the improvement of pregnancy rate and live birth rate in poor ovarian responders by intraovarian administration of autologous MenSCs, and resulted in 5 live births in MenSCs-treated group and one birth in control group [39]. Consequently, the results of this study further confirmed the safety and efficacy of MenSCs-based therapy for IUA, which is likely to be contributed by activating the PI3K/Akt signaling pathway. And these harvested data not only provide solid support for MenSCs transplantation for improving the symptoms of IUA, but also pave the way for the clinical application of MenSCs in other refractory diseases.
Additionally, studies have confirmed that MSCs are capable of migrating towards injured tissues due to the upregulated expression of chemokines (such as CXCR4 and SDF-1) released around impaired and inflammatory tissues [40,41,42,43]. Moreover, acute kidney injury (AKI) animals showed distinct accumulation of infused MSCs in the areas corresponding to the location of the kidneys, whereas normal animals showed a diffuse, whole-body distribution with greater accumulation in the lungs in some animals at 24 h after injection. There was no statistically significant difference of fluorescence intensity between the 24 and 72 h time point [42]. Furthermore, Alcayaga-Miranda et al. found that the migration ability of MenSCs was superior to that of BMSCs, which may be related to the greater expression of adhesion molecule CD49a [44]. In this study, GFP-labeled MenSCs were injected into model mice via the tail vein, and green fluorescence was easily observed in the lung but not in the uterus. Therefore, the indirect method of WB was performed, and the results showed that GFP expression was significantly increased in the endometrium of MenSCs-treated mice, which confirmed that the transplanted MenSCs can migrate to the injured uterus, and provided direct evidence of MenSCs-derived angiogenesis and cell proliferation in the injured endometrium.
Conclusions
In summary, our results demonstrated that MenSCs treatment could recover endometrial function in ethanol-induced endometrial injury, not only through angiogenesis in the injured endometrium, but also through enhancing the antiapoptotic capacity of impaired endometrial cells and promoting the proliferation of endogenous endometrial cells, which is likely implemented by activating the PI3K/Akt signaling pathway. Consequently, our study provides foundation for MenSCs transplantation as a promising therapeutic approach for endometrial regeneration in the clinic.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code Availability
Not applicable.
References
Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother. 2018;102:333–43.
Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N. Pathophysiologic features of “thin” endometrium. Fertil Steril. 2009;91(4):998–1004.
Kou L, Jiang X, Xiao S, Zhao YZ, Yao Q, Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J Control Release. 2020;318:25–37.
Yokomizo R, Fujiki Y, Kishigami H, Kishi H, Kiyono T, Nakayama S, et al. Endometrial regeneration with endometrial epithelium: homologous orchestration with endometrial stroma as a feeder. Stem Cell Res Ther. 2021;12(1):130.
Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–78.
Hooker A, Fraenk D, Brolmann H, Huirne J. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur J Contracept Reprod Health Care. 2016;21(4):329–35.
Mentula M, Mannisto J, Gissler M, Heikinheimo O, Niinimaki M. Intrauterine adhesions following an induced termination of pregnancy: a nationwide cohort study. BJOG. 2018;125(11):1424–31.
Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9(1):3–8.
Lebovitz O, Orvieto R. Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–14.
Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12(12):CD009461.
Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215(3):267-75 e7.
Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21(1):44–54.
Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191–8.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–38.
Yu J, Jiang L, Gao Y, Sun Q, Liu B, Hu Y, et al. Interaction between BMSCs and EPCs promotes IUA angiogenesis via modulating PI3K/Akt/Cox2 axis. Am J Transl Res. 2018;10(12):4280–9.
Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192.
Shao X, Ai G, Wang L, Qin J, Li Y, Jiang H, et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367–74.
Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257.
Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125.
Hennes A, Devroe J, De Clercq K, Ciprietti M, Held K, Luyten K, et al. Protease secretions by the invading blastocyst induce calcium oscillations in endometrial epithelial cells via the protease-activated receptor 2. Reprod Biol Endocrinol. 2023;21(1):37.
Singh P, Bhartiya D. Mouse uterine stem cells are affected by endocrine disruption and initiate uteropathies. Reproduction. 2023;165(3):249–68.
Calle A, Lopez-Martin S, Monguio-Tortajada M, Borras FE, Yanez-Mo M, Ramirez MA. Bovine endometrial MSC: mesenchymal to epithelial transition during luteolysis and tropism to implantation niche for immunomodulation. Stem Cell Res Ther. 2019;10(1):23.
Navarrete F, Saravia F, Cisterna G, Rojas F, Silva PP, Rodriguez-Alvarez L, et al. Assessment of the anti-inflammatory and engraftment potential of horse endometrial and adipose mesenchymal stem cells in an in vivo model of post breeding induced endometritis. Theriogenology. 2020;155:33–42.
Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, et al. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22(3):1627–39.
Zhang S, Sun Y, Jiang D, Chen T, Liu R, Li X, et al. Construction and Optimization of an Endometrial Injury Model in Mice by Transcervical Ethanol Perfusion. Reprod Sci. 2021;28(3):693–702.
Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. 2014;125(2):121–4.
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96.
Zheng SX, Wang J, Wang XL, Ali A, Wu LM, Liu YS. Feasibility analysis of treating severe intrauterine adhesions by transplanting menstrual blood-derived stem cells. Int J Mol Med. 2018;41(4):2201–12.
Zhu H, Jiang Y, Pan Y, Shi L, Zhang S. Human menstrual blood-derived stem cells promote the repair of impaired endometrial stromal cells by activating the p38 MAPK and AKT signaling pathways. Reprod Biol. 2018;18(3):274–81.
Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, et al. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389–402.
Hu J, Song K, Zhang J, Zhang Y, Tan BZ. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol Med Rep. 2019;19(2):813–20.
Chen X, Wu Y, Wang Y, Chen L, Zheng W, Zhou S, et al. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477.
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–98.
Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101.
Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10(1):61.
Ma H, Liu M, Li Y, Wang W, Yang K, Lu L, et al. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J Obstet Gynaecol Res. 2020;46(11):2347–55.
Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–9.
Zafardoust S, Kazemnejad S, Darzi M, Fathi-Kazerooni M, Rastegari H, Mohammadzadeh A. Improvement of Pregnancy rate and live birth rate in poor ovarian responders by intraovarian administration of autologous menstrual blood derived-mesenchymal stromal cells: phase I/II clinical trial. Stem Cell Rev Rep. 2020;16(4):755–63.
Weidt C, Niggemann B, Kasenda B, Drell TL, Zanker KS, Dittmar T. Stem cell migration: a quintessential stepping stone to successful therapy. Curr Stem Cell Res Ther. 2007;2(1):89–103.
Wang S, Wang Y, Xu B, Qin T, Lv Y, Yan H, et al. Biodistribution of 89Zr-oxine-labeled human bone marrow-derived mesenchymal stem cells by micro-PET/computed tomography imaging in Sprague-Dawley rats. Nucl Med Commun. 2022;43(7):834–46.
Togel F, Yang Y, Zhang P, Hu Z, Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295(1):F315–21.
Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95.
Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Diaz C, Fernandez A, Figueroa FE, et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):32.
Acknowledgements
We acknowledge all the authors for their contribution to the study.
Funding
We would like to acknowledge the Henan Province Foundation of China (212102310611 and 22A320043) and Xinxiang Foundation of China (GG2021029) for the financial support.
Author information
Authors and Affiliations
Contributions
J. L. and Y. L. conceived and designed the experiments. S. Z. and Y. P. analyzed the data and prepared the manuscript. R. Z. and Y. L. performed the experiments. H. C. provided assistance with cell culture and data analysis, and discussed the results and commented on the manuscript. S. Z. wrote the manuscript and prepared the figures; Y. L. revised the manuscript, and gave approval to the final version as the corresponding author. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The present study was approved by the ethical committee of Xinxiang Medical University, Henan, China.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zhang, R., Yin, X. et al. MenSCs Transplantation Improve the Viability of Injured Endometrial Cells Through Activating PI3K/Akt Pathway. Reprod. Sci. 30, 3325–3338 (2023). https://doi.org/10.1007/s43032-023-01282-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01282-0