Abstract
Anti-Mullerian hormone (AMH) is one of the direct indicators of follicular pool but no standard cutoff has been defined for diagnosis of polycystic ovary syndrome (PCOS). The present study evaluated the serum AMH levels among different PCOS phenotypes and correlated the AMH levels with clinical, hormonal, and metabolic parameters among Indian PCOS women. Mean serum AMH was 12.39 ± 5.3ng/mL in PCOS cohort and 3.83 ± 1.5 ng/mL in non-PCOS cohort (P < 0.01). Out of 608 PCOS women, 273 (44.9%) women belonged to phenotype A, 230 (37.8%) women were phenotype D. Phenotypes C and B were 12.17% and 5.10% respectively. Among those with the highest AMH group (AMH>20ng/ml; 8.05%), majority belonged to phenotype A. Menstrual cycle length, serum testosterone, fasting total cholesterol levels, and follicle number per ovary had positive correlation with serum anti-Mullerian levels (P < 0.05). AMH cutoff for the diagnosis of PCOS was calculated as ≥ 6.06 ng/mL on ROC analysis with sensitivity and specificity of 91.45% and 90.71% respectively. The study shows high serum AMH levels in PCOS are associated with worse clinical, endocrinological, and metabolic parameters. These levels may be used to counsel patients regarding treatment response, help in individualized management and prediction of reproductive and long-term metabolic outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS) is a multisystem endocrine disorder with a prevalence of 6–20% among women of reproductive age and is the most common cause of anovulatory infertility [1]. After many controversies following the original Rotterdam criteria [2], European Society of Human Reproduction and Embryology (ESHRE), and American Society of Reproductive Medicine (ASRM) developed international PCOS guideline [3]. The guideline has adopted the original Rotterdam criteria and requires the presence of at least two of the following three features: (a) oligo/or anovulation (OA); (b) hyperandrogenism (HA); and (c) polycystic ovarian morphology (PCOM). Four clinical phenotypes have been defined depending upon number and type of criteria present: phenotype A- anovulation (OA), hyperandrogenism (HA), and polycystic ovarian morphology (PCOM) (OA+HA+PCOM); phenotype B- anovulation and hyperandrogenism (HA+OA); phenotype C- hyperandrogenism and polycystic ovarian morphology (HA+PCOM); and phenotype D-anovulation and ovarian morphology (OA+PCOM). Despite this classification, definition of each individual parameter still remains controversial.
International PCOS guideline defined PCOM to be >19 when done using high resolution (>8MHz) machines. Guideline also describes the challenges and controversies in this criterion [3]. Among the three Rotterdam criteria, maximum controversy lies with PCOM criterion. The reason being lack of specific biochemical marker, inter-observer variation, and advancement in resolution of ultrasound machines over time. Many authors have questioned the cutoff of PCOM with high resolution USG machines [4]. Following the publication of this guideline, there are many questions on missing out PCOS by excluding women with AFC of 12–19. Hence, cutoff for antral follicle count (AFC) continues to remain a challenge while diagnosing PCOS. Validation of the guideline would only be possible with sufficient data from different ethnic groups with regards to epidemiological distribution, exact prevalence, and nomograms of AFC among PCOS women and non-PCOS women.
Ultrasound involves expensive equipment and trained personnel which increases the cost. The lack of facility and expertise in peripheral settings might further delay diagnosis. Further, the route of USG (transabdominal or transvaginal) impacts accuracy of scan and transvaginal ultrasound route may not be possible and culturally acceptable among adolescent girls. Multi-follicular appearance on ultrasound and volume >10cc overlaps with PCOM diagnostic cutoffs in these young girls.
AMH is a polypeptide of the transforming growth factor - beta (TGF beta) family, solely secreted by granulosa cells of the pre-antral and small antral ovarian follicles and is considered as a direct indicator of follicular pool [5, 6]. Due to higher number of follicles, AMH levels are considerably high in PCOS women as compared to non-PCOS counterparts [7]. High AMH inhibits the recruitment of primordial follicles from the resting follicular pool and may suppress follicle-stimulating hormone (FSH) action contributing to ovulatory disturbances.
Despite enormous data on AMH levels in PCOS, it is still not included in the guideline as a diagnostic criterion for PCOS. Unanswered questions include AMH cutoff to diagnose PCOS, its exact correlation with follicular pool and its correlation with other clinical, endocrinological, and metabolic parameters of PCOS. Although many authors from various ethnicities have attempted to study serum levels of AMH, data from India is sparse.
The present study was planned to evaluate serum AMH levels among different PCOS phenotypes and its correlation with clinical, endocrinological, and metabolic parameters among PCOS women.
Materials and Methods
The study was conducted as a single center retrospective case-control study in the Department of Obstetrics & Gynecology at a tertiary care referral hospital. The data was taken from a prospectively maintained database of women attending outpatient gynecology clinic and infertility clinic. Inclusion criteria for the study group were (i) diagnosis of PCOS based on Rotterdam criteria (ii) with complete data. Exclusion criteria for the study group were (i) patients with incomplete data; (ii) women with ovarian surgery, associated endometriosis and those on any hormonal medications, including oral contraceptive pills. Inclusion criteria for the control group were (i) women with tubal or male factor infertility; (ii) complete data. Exclusion criteria for the control group: (i) history of ovarian surgery; (ii) women with endometriosis; on any hormonal medications (iii) women with serum AMH < 1.5 ng/mL or AFC < 7; (iv) women with isolated polycystic ovarian morphology (i.e., AFC > 12). A total of 1300 women were screened, out of which, 1146 women were eligible and recruited for the study. Among them, 608 were diagnosed PCOS and 538 as non-PCOS (flow chart — Fig. 1)
Ethical Approval
Ethical clearance was taken from the Institute Ethics committee (IEC – 551/06/08/2021) before starting the study. Information compiled from the records included demographic data, clinical profile (menstrual cycle length, modified FG score for hirsutism), anthropometric parameters (weight, BMI, waist circumference, waist-hip ratio), baseline hormonal profile (FSH, LH, serum testosterone, AMH, TSH, Prolactin), and ultrasound findings (ovarian volume; antral follicle count, follicular number per ovary (FNPO) done on days 2–5 of menses) and lipid profile. Serum progesterone (days 21–22) was done in women with regular cycles. Criteria used to diagnose PCOS were (i) oligo or anovulation defined as delayed cycles >35 days/ frank amenorrhea or serum progesterone < 3ng/ml on days 21–22; (ii) clinical (mFG score ≥ 5) or biochemical evidence of hyperandrogenism (serum testosterone ≥ 0.56ng/ml); (iii) sonographic evidence of polycystic ovary morphology (PCOM) – AFC ≥12 or ovarian volume ≥ 10cm3. Transvaginal ultrasound of pelvis was done on days 2–5 of a spontaneous or progesterone withdrawal cycle. USG machine used for ovarian measurement was Voluson E8 Expert with 5-9 MHz transvaginal transducer. Phenotypic classification of PCOS women was done according to the number and type of criteria present. For the control group, data was collected from the clinic database of women consulting for tubal or male factor infertility. Baseline hormone profiles and AFC data were taken and used for the study.
Biochemical Analysis
Serum AMH assessment was done using ultrasensitive two-site enzyme linked immunosorbent assay (Ansh labs, USA) and linearity range of assay was 0.06–18 ng/mL. The lowest amount of AMH in a sample that can be detected using the kit is 0.023ng/mL.
Statistical Analysis
Data were analyzed by Stata 14 and presented in mean (SD)/median (IQR) and frequency (%). Continuous variables following normal distribution were compared by independent t test/one-way ANOVA followed by multiple comparisons using Bonferroni test. Variables following non-normal distribution were compared by Wilcoxon rank-sum test/Kruskal Wallis test followed by multiple comparisons using Dunn test with Bonferroni correction. Categorical variables were compared by Chi-square/Fischer exact test. Correlation between continuous variables was assessed by Spearman correlation coefficient. ROC curve analysis was used to find the discriminant ability of AMH for the diagnosis of PCOS and its appropriate cutoff. Different multivariable logistic regression model was generated to see the discriminant power of these models. P values <0.05 was considered statistically significant.
Results
A total of 1300 women were screened for the study out of which 154 were excluded due to incomplete data. Final sample size analyzed was 1146. Table 1 describes the baseline characteristics of PCOS (N=608) and nonPCOS groups (N=538). Mean serum AMH levels were 12.39 ± 5.3ng/mL in PCOS cohort and 3.83 ± 1.5 ng/mL in non-PCOS cohort (P < 0.01). Serum LH levels, follicle number ovary (FNPO) were significantly higher in PCOS cohort as compared to control cohort (P<0.01).
Serum AMH and PCOS Phenotypes
PCOS cohort was stratified into four phenotypic groups according to NIH 2012 extension of Rotterdam classification. Out of 608 PCOS women, 273 (44.9%) belonged to phenotype A and 230 (37.8%) were phenotype D. Phenotypes C and B were 12.17% and 5.10% respectively. Figure 2 describes the Box-whisker plot with serum AMH ranges in all PCOS phenotypes and nonPCOS cohort.
To get a better understanding of behavior of PCOS women with rising AMH levels, the whole PCOS cohort was divided into five AMH groups (Table 2). Table 3 describes the distribution of different clinical, endocrine, metabolic parameters, among different AMH groups. Most of the women were in second (5–10 ng/ml) and third groups (>10–15 ng/ml). Forty-nine (8.05%) patients had AMH values > 20 ng/ml. Among those with the highest AMH groups (AMH >20 ng/ml), majority belonged to phenotype A (Table 2).
Relationship of Clinical and Endocrinological Profile with Serum AMH Levels in PCOS Women
Menstrual cycle length was positively correlated with serum AMH levels and correlation was statistically significant (P= 0.0490) (Fig. 3a). Median LH and LH/FSH ratio positively correlated with increasing AMH levels (Fig. 3c and d). Serum testosterone and follicle number per ovary (FNPO) also showed a significant rising trend with serum AMH values (P= 0.0001 and 0.002 respectively) (Fig. 3b).
a) Correlation of cycle length with various serum AMH levels in PCOS, b) correlation of AFC with various serum AMH levels in PCOS, c) correlation of LH/FSH ratio with various serum AMH levels in PCOS, d) correlation of LH with various serum AMH levels in PCOS, e) correlation of Total cholesterol with various serum AMH levels in PCOS, f) correlation of serum testosterone with various serum AMH levels in PCOS
Relationship of Metabolic Profile with Serum AMH in PCOS Women
Fasting glucose did not differ among five AMH groups. Median fasting insulin (IQR) in PCOS cohort was 11.31μU/mL (0.79–141) and had no correlation with AMH levels (P= 0.3333). Median HOMA–IR (IQR) was 2.52 (0.17–28.54) and was comparable among different PCOS phenotypes and different AMH groups. Fasting total cholesterol levels had significant positive trend with serum AMH levels (P= 0.0122) (Fig. 3e.
Relationship of Age and BMI with Serum AMH
Figure 4a describes the trend of serum AMH levels with advancing age in PCOS and non-PCOS women. It was seen that non PCOS women had lower serum AMH levels as they age, but serum AMH levels in PCOS cohort took longer time to decrease. BMI was significantly higher in PCOS cohort, but BMI did not correlate with serum AMH levels.
Diagnostic Cutoff of Serum AMH for PCOS
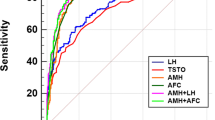
ROC analysis showed AMH levels of ≥ 6.06 ng/mL as diagnostic cutoff for diagnosis of PCOS with sensitivity of 91.45% and specificity of 90.71% respectively (Fig. 4b).
Role of Discriminating Power of Different Models in Prediction of PCOS
Logistic regression analysis was done to assess the role of using multiple markers in the diagnosis of PCOS (Table 4). Among all models, model 3 showed minimum parameters to diagnose PCOS with AUC of 0.98. Model 4 used parameters like serum AMH, AFC, serum LH, LH/FSH ratio, and diagnosed PCOS with AUC of 0.9912. Inclusion of BMI to model 4, i.e., model 6 was found to have a predictive value with AUC of 0.9925.
Discussion
The present study evaluated the correlation of various clinical, endocrinological, and metabolic markers with serum AMH levels among Indian PCOS women. The study also reported the diagnostic cutoff value of serum AMH for polycystic ovarian morphology (PCOM) and distribution of serum AMH levels among different clinical PCOS phenotypes.
Ethnic differences have been reported among PCOS women from different backgrounds, and the South Asian population has been reported to have worse metabolic profiles and suboptimal reproductive outcomes compared to Caucasians [8, 9]. Although not clear, overexpression of AMHR2 gene may be related to high AMH levels in PCOS women [10]. Though considered cycle independent, its role as a diagnostic marker for PCOM has several limitations including heterogeneity in the methods used for estimation and different age groups. Present data has shown that serum AMH level of more than 6.06ng/mL using ultrasensitive two-site enzyme-linked immunosorbent assay (Ansh labs, USA) diagnoses PCOS with high sensitivity and specificity.
In a systematic review to evaluate the diagnostic accuracy of AMH for diagnosis of PCOM, threshold cut-off value was 50 pmol/L (7 ng/ml) with area under ROC curve 0.87 in adolescents. The respective threshold cut-off for adult PCOS women was 20–30 pmol/L (2.8–4.2 ng/ml) with area under curve 0.67–0.92. Although this systematic review reported significantly higher serum AMH levels in adolescents and adult PCOM women than those of non-PCOM counterparts in all studies, it reported significant overlap between cases and controls [11]. Table 5 describes different AMH cut-off for PCOM by different authors. It is very important to note that studies done so far are heterogenous with poorly defined populations, non-uniform approaches to AMH cutoffs, challenges with assay selection and technical challenges. An updated meta-analysis was published in April 2022, including 13,509 patients has also concluded that AMH could be a promising marker for the diagnosis of PCOS; however, substantial heterogenicity among study populations and assay used warrants further prospective data from different ethnic population with uniform AMH assay selection. [12]. The present study is done on a larger sample size with single AMH assay in Indian PCOS women. The present study also shows that age related decline of serum AMH levels is subtle and plateaued in PCOS women compared to non-PCOS women.
Antral follicles of size 2–5 mm are shown to have an association with the severity of the menstrual disorder in PCOS, being highest in women with amenorrhea [13]. AMH levels have been studied to be correlated with oligo/anovulation and cycle length among PCOS women. The threshold of prediction of amenorrhea was 11.4ng/ml with area under ROC curve 0.87 (95%CI 0.80–0.92; P value <0.01) with optimal sensitivity (91.7%) and specificity (79.4%) [14]. In the present study, rising AMH levels were associated with longer duration of menstrual cycle, higher follicle number per ovary (FNPO), and higher cholesterol levels.
Consistent with previous studies, LH levels and LH/FSH ratio had positive correlation with serum AMH levels in the present study which is hypothesized to be due to AMH affecting follicular growth by suppressing the expression of the aromatase-dependent LH receptor. The sensitivity of follicles is reduced to FSH which causes defective selection of lead follicle and anovulation [15,16,17]. Owing to the cost involved, free androgen index (FAI) was not calculated in the present study. Serum testosterone levels positively correlated with serum AMH in the present study, similar to previously published studies [16, 18].
The relation of AMH levels to serum insulin levels, BMI and HOMA-IR has been studied by different authors, but results are controversial [19, 20]. Our previously published data reported a significant correlation of serum AMH with HOMA-IR, BMI was found to be significantly high in phenotype A and phenotype B [21]. This was not reported in the present study and may be due to larger sample size of PCOS cohort. Further prospective data with long-term follow-up is needed to understand the exact correlation of AMH with metabolic markers and metabolic health among different PCOS phenotypes.
Adoption of universal blood marker to represent antral follicle count excludes the need for ultrasound machines and interobserver variation. Studies have shown a strong association between AMH with AFC and hence its role as ovarian reserve marker is promising [22]. Our study has several limitations. It is a retrospective study and ultrasound was done by different observers which may affect the results of FNPO and ovarian volume. Free androgen index (FAI) and serum triglycerides would be better indicators of metabolic health which were removed from analysis due to incomplete data. Most of the patients in PCOS cohort were infertile which may be the reason for negligible numbers of phenotypes B and C. This must have missed the single phenotypes and those who conceived spontaneously despite having PCOS.
Substantially large sample size from Indian background was taken which is one of the principle strengths of the study. The study was well structured and PCOS women were categorized into five AMH groups for better understanding. A large control cohort was taken to avoid bias. There is large uniformity of cases with controls which almost simulates the general population.
We conclude that role of serum AMH as a surrogate marker of PCOM is highly promising as it correlates with FNPO and ovarian volume. AMH cutoff for the diagnosis of PCOS ≥ 6.06 ng/mL with sensitivity and specificity of 91.45% and 90.71% respectively. High serum AMH levels in PCOS are associated with worse clinical, endocrinological and metabolic parameters. These levels may be used to counsel patients regarding treatment response, help in individualized management and prediction of reproductive and long-term metabolic outcomes.
There is a need for further prospective studies to evaluate serum AMH levels among PCOS women at community levels which would give us a reliable epidemiological data in Asian ethnicity.
References
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. https://doi.org/10.1016/S0140-6736(07)61345-2.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004.
Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–9. https://doi.org/10.1093/humrep/der297 Epub 2011 Sep 16.
Kim JJ, Hwang KR, Chae SJ, Yoon SH, Choi YM. Impact of the newly recommended antral follicle count cutoff for polycystic ovary in adult women with polycystic ovary syndrome. Hum Reprod. 2020;35(3):652–9. https://doi.org/10.1093/humrep/deaa012.
Durlinger ALL, Visser JA, Themmen APN. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124(5):601–9. https://doi.org/10.1530/rep.0.1240601.
Seifer DB, Maclaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–46. https://doi.org/10.1016/j.fertnstert.2007.02.014.
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. https://doi.org/10.1016/j.fertnstert.2016.05.003.
Kim JJ, Choi YM. Phenotype and genotype of polycystic ovary syndrome in Asia: Ethnic differences. J Obstet Gynaecol Res. 2019;45(12):2330–7. https://doi.org/10.1111/jog.14132.
Yue CY, Lu LK, Li M, Zhang QL, Ying CM. Threshold value of anti-Mullerian hormone for the diagnosis of polycystic ovary syndrome in Chinese women. PLoS One. 2018;13(8):e0203129. https://doi.org/10.1371/journal.pone.0203129 PMID: 30153296; PMCID: PMC6112651.
Sathyapalan T, Al-Qaissi A, Kilpatrick ES, Dargham SR, Atkin SL. Anti-Müllerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;88(2):258–62. https://doi.org/10.1111/cen.13517.
Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R, Norman RJ, Andersen M, Franks S, Hoeger K, Hutchison S, Oberfield S, Shah D, Hohmann F, Ottey S, Dabadghao P, Laven JSE. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30(7):467–78. https://doi.org/10.1016/j.tem.2019.04.006 Epub 2019 May 31.
Anand S, Kumar A, Prasad A, Trivedi K. Updated meta-analysis on the diagnostic accuracy of serum anti-Mullerian hormone in poly cystic ovary syndrome involving 13 509 subjects. J Obstet Gynaecol Res. 2022;48(8):2162–74. https://doi.org/10.1111/jog.15233 Epub 2022 Apr 8.
Dewailly D, Catteau-Jonard S, Reyss AC, Maunoury-Lefebvre C, Poncelet E, Pigny P. The excess in 2-5 mm follicles seen at ovarian ultrasonography is tightly associated to the follicular arrest of the polycystic ovary syndrome. Hum Reprod. 2007;22(6):1562–6. https://doi.org/10.1093/humrep/dem060.
Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B. Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(1):59.e1–8. https://doi.org/10.1016/j.ajog.2014.02.026.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, La Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–85. https://doi.org/10.1093/humupd/dmt062 Epub 2014 Jan 14. Erratum in: Hum Reprod Update. 2014 Sep-Oct;20(5):804.
Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–17. https://doi.org/10.1093/humupd/dmh010.
Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24(11):2917–23. https://doi.org/10.1093/humrep/dep225.
Skałba P, Cygal A, Madej P, et al. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158(2):254–9. https://doi.org/10.1016/j.ejogrb.2011.06.006.
Jun TJ, Jelani AM, Omar J, Rahim RA, Yaacob NM. Serum anti-Müllerian hormone in polycystic ovary syndrome and its relationship with insulin resistance, lipid profile and adiponectin. Indian J Endocrinol Metab. 2020;24(2):191. https://doi.org/10.4103/ijem.IJEM_305_19.
Cui Y, Shi Y, Cui L, Han T, Gao X, Chen ZJ. Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril. 2014;102(1):230–236.e2. https://doi.org/10.1016/j.fertnstert.2014.03.032.
Gupta M, Yadav R, Mahey R, Agrawal A, Upadhyay A, Malhotra N, Bhatla N. Correlation of body mass index (BMI), anti-Mullerian hormone (AMH), and insulin resistance among different polycystic ovary syndrome (PCOS) phenotypes - a cross-sectional study. Gynecol Endocrinol. 2019;35(11):970–3. https://doi.org/10.1080/09513590.2019.1613640 Epub 2019 May 12.
Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701. https://doi.org/10.1093/humupd/dmu020.
Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL, Cohen A, Hougaard DM, Nyboe AA. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod. 2014;29(4):791–801. https://doi.org/10.1093/humrep/det469 Epub 2014 Jan 16.
Ramezani Tehrani F, Rahmati M, Mahboobifard F, Firouzi F, Hashemi N, Azizi F. Age-specific cut-off levels of anti-Müllerian hormone can be used as diagnostic markers for polycystic ovary syndrome. Reprod Biol Endocrinol. 2021;19(1):76. https://doi.org/10.1186/s12958-021-00755-8.
Bell RJ, Islam RM, Skiba MA, Herbert D, Martinez Garcia A, Davis SR. Substituting serum anti-Müllerian hormone for polycystic ovary morphology increases the number of women diagnosed with polycystic ovary syndrome: a community-based cross-sectional study. Hum Reprod. 2021;37(1):109–18. https://doi.org/10.1093/humrep/deab232 PMID: 34741176.
Gürsu T, Eraslan A, Angun B. Comparison of body mass index, anti-müllerian hormone and insulin resistance parameters among different phenotypes of polycystic ovary syndrome. Gynecol Obstet Clin Med. 2022;2(4):164–170. https://doi.org/10.1016/j.gocm.2022.10.002.
Acknowledgements
Reeta Mahey and Neena Malhotra had proposed and planned the idea. Rohitha Cheluvaraju has drafted the idea. Priyanka Prabhakar, Keerthana Rajasekaran, and Deeksha Patkar have helped in data compilation. Ashish Upadhyay has done the statistical analysis. Monika Rajput revised it critically for important intellectual content. All the authors appraised and approved the final manuscript.
Availability of Material
Not applicable
Code Availability
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Ethical clearance was taken from the Institute Ethics committee (IEC – 551/06/08/2021) before starting the study.
Consent to Participate and Publication
Informed consent was obtained from all individual participants included in the study for participation and publishing purposes.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malhotra, N., Mahey, R., Cheluvaraju, R. et al. Serum Anti-Mullerian Hormone (AMH) Levels Among Different PCOS Phenotypes and Its Correlation with Clinical, Endocrine, and Metabolic Markers of PCOS. Reprod. Sci. 30, 2554–2562 (2023). https://doi.org/10.1007/s43032-023-01195-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01195-y