Abstract
Adenomyosis is an estrogen-dependent gynecologic disease characterized by the presence of endometrial tissue within the myometrium. Adenomyosis presents with abnormal uterine bleeding, pelvic pains, and infertility. This review aimed to investigate the major estrogen downstream effectors involved in the process of adenomyosis development and their potential use for nonhormonal treatment. A literature search was performed for preclinical and clinical studies published between January 2010 and November 2021 in the PubMed and Google Scholar databases using a combination of specific terms. Adenomyosis presents with a wide spectrum of clinical manifestations from asymptomatic to severe through a complex process involving a series of molecular changes associated with inflammation, invasion, angiogenesis, and fibrosis. Adenomyosis may develop through a multistep process, including the acquisition of (epi)genetic mutations, tissue injury caused at the endometrial–myometrial interface, inside-to-outside invasion (from the endometrial side into the uterine wall), or outside-to-inside invasion (from the serosal side into the uterine wall), and epithelial–mesenchymal transition, tissue repair or remodeling in the myometrium. These processes can be regulated by increased estrogen biosynthesis and progesterone resistance. The expression of estrogen downstream effectors associated with persistent inflammation, fragile and more permeable vessel formation, and tissue injury and remodeling may be correlated with dysmenorrhea, heavy menstrual bleeding, and infertility, respectively. Key estrogen downstream targets (e.g., WNT/β-catenin, transforming growth factor-β, and nuclear factor-κB) may serve as hub genes. We reviewed the molecular mechanisms underlying the development of adenomyosis and summarized potential nonhormonal therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenomyosis is characterized by the presence of ectopic endometrial glands and stroma located within the myometrium [1]. The clinical manifestations of adenomyosis include abnormal uterine bleeding (AUB), pelvic pains, and infertility [2]. Adenomyosis often coexists with endometriosis, and their clinical manifestations are similar; however, adenomyosis is characterized by AUB [2, 3]. Unfortunately, the etiology of adenomyosis is not sufficiently understood. It remains inconclusive whether adenomyosis and endometriosis are two different diseases [2, 4] or are different phenotypes of a single disease [5]. Researchers have accumulated clinical, histological, genetic, genomic, and transcriptomic data to elucidate its etiology [6,7,8,9,10,11,12,13]. As with endometriosis, the dysregulation of estrogen and its downstream signaling pathways may play an important role in disease development and maintenance [14]. Adenomyosis may develop from tissue injury, inflammation, angiogenesis, or tissue remodeling in the myometrium [8, 15], but the underlying regulatory mechanisms at each step remain unknown. This review consists of three sections focusing on (1) the molecular mechanisms underlying the multistep process of adenomyosis development, (2) estrogen and its downstream effectors involved in adenomyosis-related symptoms, and (3) promising nonhormonal treatment strategies.
Search Strategy and Selection Criteria
A computerized literature search was performed to identify relevant studies reported in English. The PubMed and Google Scholar electronic databases were searched for studies published between January 2000 and October 2021 with the following keywords: adenomyosis, estrogen, genetic, injury, invasion, inflammation, angiogenesis, remodeling, pain, bleeding, and infertility. The references of each article were searched to identify potentially relevant studies. The publications of the original studies and review papers were also included. Given the heterogeneity of the research theme, data from the studies were synthesized using a descriptive review design with narrative methods. Figure 1 shows that the first identification phase included records identified through a database search. During the first screening stage, the focus was on the terms in the titles and abstracts. Duplicates were removed during the second screening phase. The titles, abstracts, and full-text articles were read to remove inappropriate papers. The final eligibility phase included an analysis of the full-text articles after excluding those whose detailed data cannot be extracted.
Number of articles identified by searching for keyword combinations. Articles were identified using keyword combinations and the records were identified through database search, records after duplicate removal, records screened, and removal of inappropriate articles by reading full-text articles and full-text articles assessed for eligibility. Keywords: 1, adenomyosis; 2, estrogen; 3, genetic; 4, injury; 5, invasion; 6, inflammation; 7, angiogenesis; 8, remodeling; 9, pain; 10, bleeding; and 11, infertility
Molecular Mechanisms Underlying the Multistep Process of Adenomyosis Development
This section discusses the molecular mechanisms underlying the step-wise process of adenomyosis progression, including the acquisition of (epi)genetic mutations, the cause of tissue injuries at the endometrial–myometrial interface, and the inside-to-outside invasion (from the endometrial side into the uterine wall) or outside-to-inside invasion (from the serosal side into the uterine wall), tissue repair, and remodeling in the myometrium [8, 15]. Adenomyotic cells alter their gene/protein expression pattern to adapt to ever-changing host environments. Table 1 presents the official symbol, official full name, molecular attributes, biological functions, targeted gene and protein expression in adenomyotic cells or lesions, and citation numbers used in the text.
The Acquisition of (epi)Genetic Mutations
The first step is to acquire (epi)mutations on the key genes required to drive adenomyosis initiation (Fig. 2A).
Adenomyosis as a multistep process. Left half: The molecular mechanisms underlying the step-wise process of adenomyosis progression include A the acquisition of (epi)genetic mutations, B tissue injury caused at the endometrial–myometrial interface, C inside-to-outside invasion (from the endometrial side into the uterine wall) or outside-to-inside invasion (from the serosal side into the uterine wall), and D tissue repair and remodeling in the myometrium. Right half: List of typical effector molecules
KRAS, KRAS Proto-oncogene, and GTPase
KRAS is one of the most common mutated genes in endometriosis and adenomyosis [6]. KRAS is mutated in 37.1% of patients with adenomyosis [16]. KRAS mutations are known to activate estrogen receptor signaling (i.e., estrogen dominance) and to reduce progesterone receptor expression (i.e., acquisition of progesterone resistance) [6]. Therefore, the accumulation of genetic alterations in the KRAS gene may act as a driving force for adenomyosis initiation and development.
SMARCA4, SWI/SNF-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily a, Member 4, or BRG1
Genes encoding the SWI/SNF chromatin remodeling complex subunits are mutated in approximately 20% of human cancers [17]. SMARCA4 and AT-rich interaction domain 1A (ARID1A) are members of the SWI/SNF family, have helicase and ATPase activities, and regulate the transcription of certain genes by altering the chromatin structure. An adenomyosis-like morphology (invasion of endometrial cells into the myometrium) has been observed in genetically engineered mice with inactivated SMARCA4 [18]. However, no evidence was found that human adenomyosis is caused by loss-of-function mutations in the SMARCA4 gene.
HDAC3 and Histone Deacetylase 3
HDAC3 functions as a potential tumor suppressor and is involved in transcriptional repression. HDAC3 has been shown to promote estrogen receptor (ESR1) mRNA stability [19]. Immunohistochemistry showed that HDAC1 and HDAC3 expression was significantly increased in both the eutopic and the ectopic endometrium in women with adenomyosis [20]. A case series demonstrated that treatment with HDAC inhibitors, such as valproic acid, can improve dysmenorrhea and reduce the size of adenomyotic lesions [21]. However, there is no direct evidence that adenomyosis is characterized by a global hypomethylation of SMARCA4 and HDAC3 genes.
Persistent Tissue Damage that Occurs at the Interface Between The Endometrium and the Myometrium
The second step is a tissue injury process that occurs at the endometrial–myometrial interface (junctional zone), which is secondary to mechanical, traumatic, or iatrogenic damage or occurs spontaneously. Figure 2B shows the key molecules associated with tissue damage, and Table 1 lists the function of each gene. Adenomyosis could sometimes occur after surgical interventions, such as dilation, curettage, normal delivery, and cesarean section [8, 9]. Uterine hyperperistalsis can cause spontaneous tissue damage [8]. The tissue injury and repair (TIAR) hypothesis was first proposed by Leyendecker et al. [8, 9]. The disruption of the junctional zone is required for endometrial cell invasion into the myometrium. Tissue damage involved in TIAR allows adenomyotic cells to adapt and respond to unfavorable microenvironments, such as hypoxia and oxidative stress. Furthermore, hypoxia and oxidative stress at the local wound sites cause accelerated tissue damage, inflammatory process, impaired energy metabolism and nociceptive pain [21, 22], and upregulated expression of hypoxia-related molecules and neuroinflammatory factors through overexpression of hypoxia-inducible factor-1alpha (HIF-1α), HIF-2α, vascular endothelial growth factor (VEGF), tissue factor (TF), oxytocin receptor (OTR), nerve growth factor (NGF), prostaglandin E2 (PGE2), and a variety of interleukins, such as interleukin-1beta (IL-1β) [3]. PGs and OTR induce contraction and peristalsis of the uterine smooth muscle, eliciting repetitive microdamage in the junctional zone [9]. The production of PGs by cyclooxygenase-2 (COX-2) leads to the exacerbation of inflammation, accompanied by tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 overexpression. Inflammation is a defense response induced by tissue injury. The procoagulant TF, the initiator of the extrinsic coagulation pathway, is a coagulation activation marker that responds quickly to vascular endothelial cell injury. Adenomyotic lesions have acquired mechanisms to adapt to the hypoxic state initiated primarily by the activation of HIFs and inflammatory cytokines. The key molecules believed to be associated with tissue injury include molecules related to hypoxia, neuroinflammation, and PGs. Adenomyotic cells may evolve to cope with unfavorable microenvironments, such as hypoxia, oxidative stress, and inflammation caused by tissue damage.
Inside-to-Outside Invasion (from the Endometrial Side into the Uterine Wall) or Outside-to-Inside Invasion (from the Serosal Side into the Uterine Wall)
The third step is the invasion of endometrial cells into the myometrium (Fig. 2C). The ability of endometrial cells to invade either the junctional myometrium or the uterine serosa following tissue injury primarily depends on alterations in the expression of molecules associated with persistent inflammation and angiogenesis [10, 11].
Genes Associated with Invasion: MMP-2, MMP-9, NF-κB, and WNT/β-Catenin
Matrix metalloproteinases (MMPs) are the most widely investigated molecules that facilitate protease-dependent invasion and the progression of cancer and endometriosis by degrading extracellular matrix (ECM) proteins [23,24,25]. MMP family proteins, such as MMP-2 and MMP-9, are also associated with adenomyotic cell invasion and contribute to angiogenesis via tissue remodeling of vascular endothelial cells [23,24,25]. MMP gene expression is regulated by various transcription factors. The nuclear factor-κappaB (NF-κB) transcription factor family is an upstream regulator of various MMP proteins [26]. NF-κB regulates the expression of several molecules and pathways responsible for cell invasion, angiogenesis, proliferation, and antiapoptosis. In both adenomyosis [27] and endometriosis [28], MMPs promote endometrial cell migration and invasion by activating the NF-κB signal pathway. Additionally, the WNT/β-catenin pathway, another important transcription factor responsible for MMP expression, accelerates invasion by activating several target genes including MYC proto-oncogene (Myc), Cyclin D1, and genes related to the ECM [29]. Nuclear and cytoplasmic β-catenin expression was higher in ectopic than in control endometrium [29]. Detailed information on the WNT/β-catenin signaling pathways has been provided in the endometriosis field [30]. Increased estrogen biosynthesis and progesterone resistance promoted the invasive potential of endometrial cells in endometriosis through the activation of the Wnt/β-catenin pathway [30]. Therefore, the promising candidate transcription factors and their downstream effectors involved in adenomyotic cell invasion include NF-κB, WNT/β-catenin, and MMPs.
Genes Associated with Inflammation: I-6, IL1β, IL-8, IFNα, TNF-α, IFNγ, IL10, IL-22, TGF-β, CCL2, RANTES, NF-κB, LIF, IHH, CRH, UCN, NGF, OTR, SYP, and MAP2
Adenomyosis is characterized by the accumulation of lymphocytes and macrophages in the eutopic endometrium [13]. Adenomyotic lesions release numerous inflammatory mediators, including proinflammatory cytokines (e.g., IL-6, IL-1β, IL-8, TNF-α, interferon-alpha, and IFNγ), antiinflammatory cytokines (e.g., IL10, IL-22, transforming growth factor (TGF)-β), chemokines (e.g., C–C motif chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1) and regulated upon activation, normal T-cell expressed, and presumably secreted [RANTES]), and other mediators [e.g., NF-κB, corticotropin-releasing hormone (CRH), urocortin (UCN), NGF, OTR, synaptophysin (SYP), microtubule-associated protein 2 (MAP2)] [13, 31,32,33,34,35]. These pleiotropic regulators play a key role in the development and maintenance of adenomyotic lesions by affecting the properties of epithelial cells, stromal cells, or adjacent macrophages [10]. Changes in the local levels of inflammatory mediators are implicated in the pathophysiology of adenomyosis, including endometrial cell invasion, crosstalk between macrophages and endometriotic cells, and endometrial receptivity [10, 13, 27, 31, 32, 34, 36]. For example, high expression levels of IL-22, a member of the IL-10 family of cytokines, promote endometrial stromal cell invasiveness by upregulating proangiogenic and proinflammatory factors, such as VEGF, IL-6, and IL-8 [32]. Conversely, several mediators, including leukemia inhibitory factor (LIF), Indian hedgehog (IHH), JunB proto-oncogene, AP-1 transcription factor subunit, IL-6, Fos proto-oncogene, AP-1 transcription factor subunit, IL-10, and suppressor of cytokine signaling 3 (SOCS3), are downregulated in adenomyosis lesions compared with the control [37]. These molecules are more commonly known as decidualization-related genes. The downregulation of these molecules causes implantation defects [37,38,39] (see the “Estrogen and its Downstream Effectors Involved in Adenomyosis-Related Symptoms” section). It remains unknown why changes in gene expression profile occur in women with adenomyosis, but adenomyosis can be characterized by an imbalance in inflammatory and antiinflammatory cytokines expression.
Genes Associated with Angiogenesis: MMP-2, MMP-9, COX-2, VEGF, TF, NF-κB, vWF, CDH1 (E-Cadherin), IL-22, and ANXA2
Many candidate molecules, including MMP-2, MMP-9, COX-2, VEGF, TF, NF-κB, von Willebrand factor (vWF), E-cadherin (CDH1), IL-22, and annexin A2 (ANXA2), are responsible for the angiogenic reactions in adenomyosis [3, 23, 24, 40,41,42,43,44,45,46,47]. MMP-2 and MMP-9 are known to drive multiple signal transduction pathways, contributing not only to the invasion of endometrial cells into the myometrium but also to angiogenesis in vascular endothelial cells of adenomyotic lesions [23]. TF overexpression in adenomyosis mediates VEGF production and angiogenesis by activating protease-activated receptor-2 [40]. IL-22 contributes to adenomyosis progression not only by promoting invasion but also by inducing vascular endothelial cell proliferation, survival, and angiogenesis [41]. E-cadherin, a cell adhesion and antiangiogenic molecule, had lower expression levels in eutopic and ectopic endometrium of adenomyosis compared with normal endometrium [3]. Loss of E-cadherin promotes cell migration and angiogenesis. NF-κB transcription factor activates a subset of downstream targets, including MMP-2, MMP-9, COX-2, VEGF, and TF [24, 42]. Therefore, the activation of NF-κB signal pathways is a hallmark of excessive angiogenesis in adenomyosis. Furthermore, ANXA2, a downstream molecule of the WNT/β-catenin pathway, contributes to several biological functions, including angiogenesis and invasion, in adenomyotic lesions [43]. Adenomyosis-related angiogenesis is associated with the increased expression of NF-κB, COX-2, VEGF, TF, MMP, IL-6, IL-22, and ANXA2 and the decreased expression of IL-10 and E-cadherin [3, 44,45,46,47]. The invasion of endometriotic cells in adenomyosis can be accelerated by inflammation and angiogenesis, a tightly orchestrated process.
Tissue Repair and Remodeling in the Myometrium
In the final step (Fig. 2D), endometrial cells invading the myometrial layer elicit adjacent smooth muscle cell hyperplasia and hypertrophy. The progression of adenomyosis involves chronic injury, persistent activation of myofibroblasts, wound healing response, and tissue repair. Mesenchymal cells and myofibroblasts are involved in wound healing and fibrosis via epithelial–mesenchymal transition (EMT) and fibroblast-to-myofibroblast transdifferentiation (FMT) in response to tissue damage [12, 13, 48, 49]. The EMT/FMT-associated molecular targets include genes that facilitate cell motility, invasion, angiogenesis, antiapoptosis, and fibrogenesis [12, 13]. TGF-β, a key profibrogenic mediator, drives the differentiation and transformation of fibroblasts into myofibroblasts. Several preclinical studies have reported using a mouse adenomyosis model. The EMT during adenomyosis development is induced by TGF-β by promoting β-catenin expression and activation [50]. Shen et al. demonstrated that activated platelet-derived TGF-β plays a critical role in fibrosis development by activating the small mothers against the decapentaplegic homolog (SMAD) signaling pathway, which triggers fibrogenetic gene expression [49]. Estrogen induces EMT by activating TGF-β-dependent ANXA2 signaling [43]. Estrogen and TGF-β induce ECM remodeling and EMT by activating the ANXA2/HIF-1α/VEGF pathway and the ANXA2/signal transducer and activator of transcription 3 (STAT3) pathway, respectively [43, 51]. ANXA2 is a key mediator involved in excessive ECM proteolysis and tissue remodeling. Therefore, functional crosstalk between the estrogen, TGF‐β, and WNT/β-catenin signaling pathways is a major driver of EMT and plays an important role in fibrosis development [52].
In addition to endometrial and stromal cells, macrophages are major components of the adenomyosis microenvironment and are essential for disease progression as they contribute to ECM degradation, deposition, and remodeling during EMT [13, 53]. Thus, the crosstalk between macrophages and endometrial cells is required for EMT in adenomyosis [54]. EMT and FMT activation by prolonged TIAR can lead to fibrosis in adenomyotic lesions. Based on these, the key molecules associated with tissue repair and remodeling in the myometrium include WNT/β-catenin, TGF-β, and ANXA2 and their downstream signals SMAD and STAT3.
In summary, the development of adenomyosis involves complex processes, including tissue injury, inflammation, invasion, angiogenesis, tissue repair, remodeling, and fibrosis.
Estrogen and its Downstream Effectors Involved in Adenomyosis-Related Symptoms
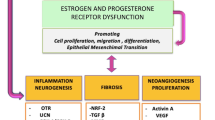
Adenomyosis is an estrogen-dependent disease. 17β-estradiol is produced by the adenomyotic glands through the action of aromatase [14]. The expression of estrogen downstream effectors, including aromatase [55, 56], 17β-hydroxysteroid dehydrogenase type 2, estrone sulfatase [57, 58], ESR [59, 60], progesterone receptor B (PGR-B) [57, 58], NF-κB [45], COX-2 [46, 47], VEGF [45], TF [45], and PGE2 [61], was upregulated in the patients with adenomyosis. The increased expression of these molecules is involved in the dysregulation of estrogen and progesterone signaling pathways [57, 58]. An increased ESR2/ESR1 ratio and PGR deficiency were frequently observed in the ectopic and eutopic endometrium of adenomyosis, indicating estrogen dominance and progesterone resistance [11, 62, 63]. Therefore, many of the genes and molecules involved in the development of adenomyosis are regulated by estrogen and its downstream target molecules.
Figure 3 shows the potential downstream target candidates of estrogen signaling that affect the symptoms associated with adenomyosis. Adenomyosis can cause dysmenorrhea, heavy menstrual bleeding, and infertility through at least three major pathways, namely, inflammation, angiogenesis, and EMT/tissue remodeling pathways. These signaling pathways are often associated with each other.
Pelvic Pain
As shown in Fig. 3 (Route A), estrogen increases the production of PGE2 by upregulating COX-2 expression, which induces aromatase activity and local estrogen biosynthesis. Increased local estrogen production further triggers NGF and OTR expression, which stimulates aromatase expression and leads to estrogen overproduction (66). Estrogen and aromatase establish a positive regulatory feedback loop by upregulating both COX/PGs and OTR/NGF signaling pathways, accelerating estrogen-dependent cell proliferation, angiogenesis, inflammation, tissue damage and repair, and EMT [60]. The expression of COX-2 [64,65,66], OTR [64,65,66,67], NGF [64,65,66], transient receptor potential cation channel subfamily V member 1 (TRPV1) [64,65,66,67], TF [44], ANXA2 [43], VEGF [68, 69], HIF-2α [68, 69], and HDAC2 [20] has been reported to result in increased pelvic pain or dysmenorrhea in animal models and humans. Complex biological processes, such as thrombin formation (e.g., TF), nociceptive processing (e.g., OTR, NGF, and TRPV1), EMT (e.g., ANXA2), angiogenesis (e.g., VEGF and COX-2), hypoxic condition (e.g., HIF-2α and VEGF), and epigenetic factors (e.g., HDAC2), may also be positively associated with dysmenorrhea [20, 22, 43, 44, 68,69,70]. Neuroinflammatory factors, such as NGF, not only drive chronic pain (36) but also facilitate endometrial cell growth, leading to disease exacerbation [11].
AUB
Estrogen stimulates angiogenesis in vascular endothelial cells by regulating the HIF-1α, HIF-2α, VEGF, and TF pathways via NF-κB-dependent transcriptional activation [3, 15, 44, 47, 55, 71] (Fig. 3, route B). The overexpression of multiple mediators associated with fragile and more permeable vessel formation, including aromatase, COX-2, PGs, NF-κB, vWF, HIF-1α, HIF-2α, VEGF, TF, IL-22, IL-10, IL-6, and IL-8, can lead to AUB [3, 15, 44, 47, 55]. Increased estrogen can also lead to heavy menstrual bleeding through persistent angiogenesis and neovascularization.
Infertility
Altered gene expression of EMT-related molecules (e.g., TGF-β, ANXA2, and WNT/β-catenin) and decidualization-related genes (e.g., homeobox A10 (HOXA10), IL-10, CRH, UCN, MAP2, VEGF, HIF-2α, hypoxia, and reactive oxygen species) may be responsible for the pathophysiology of adenomyosis-related infertility (Fig. 3, Route C) [13, 15, 22, 35, 69, 72]. β-catenin overexpression can cause infertility due to estrogen-induced EMT, impaired decidualization, and defective endometrial implantation [73]. Effective anti-TGF-β1 treatment has been shown to improve pregnancy and live birth rate in a mouse adenomyosis model [38]. This suggests that WNT/β-catenin and TGF-β may be directly or indirectly involved in infertility. Additionally, the expression of several decidualization-related molecules (e.g., IL-10, HOXA10, LIF, and IHH) was often altered in adenomyosis. The downregulation of these genes is known to negatively regulate endometrial acceptance, decidualization activation, embryo implantation, and later placentation [74, 75]. The decreased expression of endometrial IL-10 has been reported to contribute to infertility in women with adenomyosis by downregulating HOXA10 expression [76]. LIF, a member of the multifunctional IL-6 family of cytokines, is involved in cell differentiation, angiogenesis, epithelial–mesenchymal transition, and stromal cell decidualization [37]. The downregulation of endometrial LIF expression also contributes to impaired endometrial receptivity and loss of implantation [37]. IHH mediates endometrial receptivity and regulates embryonic implantation [39]. LIF is crucial to the embryo implantation process in mice as it upregulates IHH mRNA. IHH signaling is dysfunctional in the endometrial tissues of women with adenomyosis [39]. Furthermore, adenomyosis-related infertility may also be associated with the localization and extent of a myometrial thickness [5].
Increased estrogen biosynthesis is an upstream regulator of the pathogenic process of adenomyosis development. Estrogen is a key factor that connects the three downstream routes and provides an insight into the adenomyosis-related symptoms. Impaired homeostasis of tissue injury and inflammation [Route A (COX-2 and PGs)], angiogenesis [Route B (NF-κB)], and tissue remodeling [Route C (WNT/β-catenin and TGF-β)] can cause adenomyosis-related symptoms. However, it remains unclear whether these mediators can determine symptom severity and prognosis in adenomyosis.
Promising Nonhormonal Treatment Strategies
This section provides a brief overview of recent preclinical and clinical studies that shed light on future nonhormonal therapy for adenomyosis. The first half of this review suggested that estrogen targets hub genes, including Wnt/β-catenin, TGF-β, and NF-κB, regulates downstream key molecules, and causes symptoms associated with adenomyosis. Early adenomyosis may lack certain clinical symptoms, so molecules that are involved in diverse signaling pathways influencing EMT, tissue remodeling, and fibrosis may be the mainstay of treatment for women with symptomatic adenomyosis.
WNT/β-Catenin
Figure 4 illustrates a simplified view of the WNT/β-catenin pathways leading to the activation of downstream effectors. Since β-catenin aggravates adenomyosis progression by activating TGF-β-induced EMT [50], inhibiting the target molecules involved in EMT (e.g., WNT/β-catenin and TGF-β) may be a promising approach to suppress disease progression. β-catenin forms a complex with casein kinase 1 alpha (CK1α), glycogen synthase kinase 3 beta (GSK‑3β), axin, and the tumor suppressor protein adenomatous polyposis coli [77]. Selective GSK‑3β inhibitors have shown promising preclinical and clinical activities in cancer models [78]. However, no reported in vitro and in vivo studies have been found using selective GSK-3β inhibitors in adenomyosis models. Rac family small GTPase 1 (Rac1) was discovered as a molecule that activates the Wnt/β-catenin signaling [79]. Rac1 is important for cancer invasion, angiogenesis, and aggressiveness, making Rac1 an attractive therapeutic target, especially in oncology [79]. Rhein and F4, small-molecule inhibitors of Rac1, inhibit tumor growth and invasion in breast cancer [79]. The Rac1 inhibitor NSC23766 can enhance the antitumor activity of ovarian cancer cells [80]. Feng et al. investigated the in vivo therapeutic efficacy of Rhein in a mouse adenomyosis model [81]. Rhein given orally significantly reduced adenomyosis weight and volume in a mouse model by suppressing the WNT/β-catenin signaling pathway [81]. Moreover, Rhein markedly impaired β-catenin nuclear translocation and the expression of downstream target genes, resulting in the decreased activation of EMT-associated proteins, including snail family transcriptional repressor (Snail), and the decreased expression of NF-κB and Rac1 [81]. Thus, Rac1 inhibitors may be a potential therapeutic target for adenomyosis.
TGF-β
There are several approaches for inhibiting TGF-β, including antisense oligonucleotides, inhibitors of ligand-receptor interactions, such as anti-TGF-β antibodies, antireceptor antibodies, TGF-β-trapping receptor ectodomain proteins, and small-molecule inhibitors, that target TGF-β receptor kinases [82]. Some of these inhibitors are undergoing clinical trials and present opportunities for cancer (glioma, melanoma, or breast cancer) and noncancer diseases (TGF-β-dependent diseases, such as fibrosis and scarring) treatment [82]. Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone), a small synthetic molecule with TGF-β inhibitory activities, is effective for the treatment of idiopathic pulmonary fibrosis with only mild adverse events [50]. Yoo et al. explored the signaling pathways involved in uterine conditional activation of β-catenin using a mouse adenomyosis model [50]. They found that the signaling pathways altered by pirfenidone include Wnt/β-catenin signaling and TGF-β signaling [50]. Pirfenidone inhibited the EMT process by upregulating epithelial marker E-cadherin expression. TGF-β reduces LIF expression, which plays a key role in decidualization, placental formation, and embryo implantation and impairs pregnancy outcomes [38]. TGF-β1 inhibition by anti-TGF-β1-neutralizing antibodies improved pregnancy outcomes in a tamoxifen-induced adenomyosis mouse model by increasing LIF expression [38]. The genetic deletion of ANXA2, a downstream target of WNT/β-catenin and TGF-β, suppressed endometrial tissue growth and angiogenesis in a nude mouse adenomyosis model by inhibiting the HIF-1α/VEGF signaling pathways [43]. Several studies have focused on the bidirectional crosstalk between TGF-β and Rac1 in the progression of cancer [83] and fibrosis [84]. The combination of TGF-β and Rac1 inhibitors for adenomyosis treatment may maximize therapeutic benefit. New antifibrotic agents targeting signaling pathways mediated by WNT/β-catenin and TGF-β may have the potential for the nonhormonal treatment of patients with adenomyosis.
NF-κB
We present an overview of the preclinical studies on the therapeutic potential for adenomyosis of some inhibitors targeting the NF-κB signaling pathway. The NF-κB family transcription factor is considered a master regulator of the inflammatory processes in response to tissue injury, pathogens, inflammation, and innate immunity and regulates many genes that influence adenomyosis development [85]. NF-κB inhibitors include proteasome inhibitors, IκB kinase inhibitors, and natural and synthetic compounds [85]. These inhibitors have been developed for clinical applications in cancer therapy, whereas natural compounds, such as polyphenols, terpene, alkaloids, and phenols, can provide additional opportunities for adenomyosis treatment [85]. Andrographolide is a terpene isolated from a herb and is known for its antiinflammatory, antioxidant, and anticancer properties (Right half of Fig. 4) [86]. This herb may be useful in ameliorating symptomatic osteoarthritis, upper respiratory tract infection, and progressive multiple sclerosis [86]. Andrographolide has been reported to inhibit COX-2, VEGF, and TF expression in adenomyotic stromal cells by suppressing TNF-α-induced NF-κB activation [45]. Additionally, berberine, an isoquinoline derivative alkaloid, inhibits NF-κB transcriptional activity and ESR-dependent signaling pathways in adenomyotic stromal cells and cancer cells [87, 88]. Berberine has been shown to inhibit cancer cell proliferation and invasion [88]. Berberine promoted apoptosis and cell cycle arrest and inhibited the proliferation of adenomyotic stromal cells by suppressing IL-6, IL-8, TGF-β, VEGF, and MMP-2 expression [87].
Other Candidate Molecules
In vitro and in vivo animal experiments revealed the inhibitory effects of some drugs against adenomyosis. Specific MMP inhibitors suppress adenomyosis progression. Mori et al. demonstrated that a small-molecule MMP inhibitor, ONO-4817, attenuated lesion progression in a xenograft mouse adenomyosis model [25]. The association between MMP and the invasive potential of endometriotic cells has been demonstrated in adenomyosis [23,24,25], whereas one study reported contradictory results [89]. Adenomyosis is characterized by persistent tissue remodeling, and platelet activation may promote adenomyosis-related inflammation and fibrosis [90, 91]. Inhibitors of platelet activation may be novel therapeutic candidates against adenomyosis. Experimental animal models have demonstrated that treatment with antiplatelet drugs, thromboxane A2 synthase inhibitors, effectively suppresses endometrial cell invasion into the myometrium [90, 91]. Valproic acid has gained attention because epigenetic modifications are thought to contribute to the pathogenesis of adenomyosis. Valproic acid is an antiepileptic drug with HDAC inhibitory activity [21, 90]. Animal studies showed that valproic acid has a favorable profile for treating adenomyosis [21]. A comparative case series have shown that treatment with valproic acid for three months improved dysmenorrhea and reduced the size of adenomyotic lesions [21]. These findings suggest that HDACs may be involved in the pathogenesis of adenomyosis. Some inhibitors have been reported to act as potential therapeutic targets for adenomyosis in preclinical and clinical studies.
The blockades of downstream effectors of the WNT/β-catenin, TGF-β, and NF-κB signaling pathways may be promising strategies for the nonhormonal treatment of adenomyosis.
Conclusion
In this review, we summarized the molecular mechanisms underlying the step-wise development of adenomyosis and the potential downstream effectors of estrogen action affecting clinical symptoms. The multistep processes involve a series of molecular changes associated with tissue injury, inflammation, invasion, angiogenesis, and fibrosis. Some key molecules (i.e., WNT/β-catenin, TGF-β, and NF-κB) play a key role in adenomyosis development and may serve as significant hub genes for its clinical manifestations. These genes are potential therapeutic targets. Some inhibitors or compounds have the potential to suppress inflammation, angiogenesis, and EMT in adenomyotic lesions by targeting downstream key players of these hub genes. Although nonhormonal therapeutics for adenomyosis are limited, a better understanding of the misregulated signaling pathways can pave the way for individualized treatment management. In this review, we focused on the genes associated with the multistep process of estrogen downstream effectors that significantly change in adenomyosis. However, to identify and validate adenomyosis-related genes, bioinformatics and functional enrichment analysis should be performed based on Gene Ontology resources via microarray data sets. Hence, future research is required to assess whether such key genes are potential therapeutic targets for adenomyosis.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author.
References
Senturk LM, Imamoglu M. Adenomyosis: what is new? Womens Health (Lond). 2015;11(5):717–24.
Lacheta J. Uterine adenomyosis: pathogenesis, diagnostics, symptomatology and treatment. Ceska Gynekol. 2019;84(3):240–6.
Harmsen MJ, Wong CFC, Mijatovic V, Griffioen AW, Groenman F, Hehenkamp WJK, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update. 2019;25(5):647–71.
Xiaoyu L, Weiyuan Z, Ping J, Anxia W, Liane Z. Serum differential proteomic analysis of endometriosis and adenomyosis by iTRAQ technique. Eur J Obstet Gynecol Reprod Biol. 2014;182:62–5.
Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H. Relationship between adenomyosis and endometriosis; different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol. 2020;253:191–7.
Bulun SE, Yildiz S, Adli M, Wei JJ. Adenomyosis pathogenesis: insights from next-generation sequencing. Hum Reprod Update. 2021;27(6):1086–97.
Kobayashi H, Matsubara S, Imanaka S. Relationship between magnetic resonance imaging-based classification of adenomyosis and disease severity. J Obstet Gynaecol Res. 2021;47(7):2251–60.
Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–38.
Leyendecker G, Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm Mol Biol Clin Investig. 2011;5(2):125–42.
Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014;20(3):386–402.
Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond). 2012;8(3):301–12.
Kobayashi H, Kishi Y, Matsubara S. Mechanisms underlying adenomyosis-related fibrogenesis. Gynecol Obstet Invest. 2020;85(1):1–12.
Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;27(1):108–29.
Bulun SE, Gurates B, Fang Z, Tamura M, Sebastian S, Zhou J, et al. Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol. 2002;55(1):21–33.
Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2–03):129–43.
Inoue S, Hirota Y, Ueno T, Fukui Y, Yoshida E, Hayashi T, et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat Commun. 2019;10(1):5785.
Mullen J, Kato S, Sicklick JK, Kurzrock R. Targeting ARID1A mutations in cancer. Cancer Treat Rev. 2021;100: 102287.
Reske JJ, Wilson MR, Holladay J, Wegener M, Adams M, Chandler RL. SWI/SNF inactivation in the endometrial epithelium leads to loss of epithelial integrity. Hum Mol Genet. 2020;29(20):3412–30.
Oie S, Matsuzaki K, Yokoyama W, Murayama A, Yanagisawa J. HDAC3 regulates stability of estrogen receptor alpha mRNA. Biochem Biophys Res Commun. 2013;432(2):236–41.
Liu X, Nie J, Guo SW. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest. 2012;74(1):50–5.
Liu Xishi, Yuan Lei, Guo SW. Valproic acid as a therapy for adenomyosis: a comparative case series. Reprod Sci. 2010;17(10):904–12.
Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod Biomed Online. 2012;24(1):35–46.
Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest. 2006;62(4):229–35.
Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and -9 in endometrial cells. Fertil Steril. 2015;103(4):1089-1097.e2.
Mori T, Yamasaki S, Masui F, Matsuda M, Sasabe H, Zhou YF. Suppression of the development of experimentally induced uterine adenomyosis by a novel matrix metalloproteinase inhibitor, ONO-4817, in mice. Exp Biol Med (Maywood). 2001;226(5):429–33.
DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400.
Park H, Kim SH, Cho YM, Ihm HJ, Oh YS, Hong SH, et al. Increased expression of nuclear factor kappa-B p65 subunit in adenomyosis. Obstet Gynecol Sci. 2016;59(2):123–9.
Yu J, Chen LH, Zhang B, Zheng QM. The modulation of endometriosis by lncRNA MALAT1 via NF-kappaB/iNOS. Eur Rev Med Pharmacol Sci. 2019;23(10):4073–80.
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY, Ahn JY, et al. β-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231(2):210–22.
Klemmt PAB, Starzinski-Powitz A. Molecular and Cellular Pathogenesis of Endometriosis. Curr Womens Health Rev. 2018;14(2):106–16.
Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS, Ho HN. Decreased suppression of interleukin-6 after treatment with medroxyprogesterone acetate and danazol in endometrial stromal cells of women with adenomyosis. Fertil Steril. 2006;86(5):1459–65.
Wang Q, Wang L, Shao J, Wang Y, Jin LP, Li DJ, et al. L-22 enhances the invasiveness of endometrial stromal cells of adenomyosis in an autocrine manner. Int J Clin Exp Pathol. 2014;7(9):5762–71.
Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol. 2002;47(4):251–5.
Carrarelli P, Yen CF, Funghi L, Arcuri F, Tosti C, Bifulco G, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci. 2017;24(3):369–75.
Makrigiannakis A, Vrekoussis T, Zoumakis E, Hatzidakis V, Vlachou E, Salakos N, et al. Endometrial CRH and implantation: from bench to bedside. Hormones (Athens). 2018;17(3):293–7.
AlAshqar A, Reschke L, Kirschen GW, Borahay MA. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies. Biol Reprod. 2021;105(1):7–31.
Prašnikar E, Kunej T, Knez J, Repnik K, Potočnik U, Kovačič B. Determining the molecular background of endometrial receptivity in adenomyosis. Biomolecules. 2020;10(9):1311.
Kay N, Huang CY, Shiu LY, Yu YC, Chang Y, Schatz F, et al. TGF-beta1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28(3):877–87.
Zhou Y, Peng Y, Xia Q, Yan D, Zhang H, Zhang L, et al. Decreased Indian hedgehog signaling activates autophagy in endometriosis and adenomyosis. Reproduction. 2021;161(2):99–109.
van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106(46):19497–502.
Shang WQ, Yu JJ, Zhu L, Zhou WJ, Chang KK, Wang Q, et al. Blocking IL-22, a potential treatment strategy for adenomyosis by inhibiting crosstalk between vascular endothelial and endometrial stromal cells. Am J Transl Res. 2015;7(10):1782–97.
Exacoustos C, Morosetti G, Conway F, Camilli S, Martire FG, Lazzeri L, et al. New sonographic classification of adenomyosis: do type and degree of adenomyosis correlate to severity of symptoms? J Minim Invasive Gynecol. 2020;27(6):1308–15.
Zhou S, Yi T, Liu R, Bian C, Qi X, He X, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics. 2012;11(7):M112.017988.
Liu X, Nie J, Guo SW. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum Reprod. 2011;26(2):337–45.
Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide. Fertil Steril. 2013;100(2):568–77.
Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4(4):312–22.
Smith OP, Jabbour HN, Critchley HO. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22(5):1450–6.
García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–9.
Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2016;31(2):355–69.
Yoo JY, Ku BJ, Kim TH, Il Ahn J, Ahn JY, Yang WS, Lim JM, Taketo MM, Shin JH, Jeong JW. beta-catenin activates TGF-beta-induced epithelial-mesenchymal transition in adenomyosis. Exp Mol Med. 2020;52(10):1754–65.
Wang T, Yuan J, Zhang J, Tian R, Ji W, Zhou Y, et al. Anxa2 binds to STAT3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget. 2015;6(31):30975–92.
Kadota T, Fujita Y, Araya J, Watanabe N, Fujimoto S, Kawamoto H, et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-beta-WNT crosstalk. J Extracell Vesicles. 2021;10(10):e12124.
An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction. 2017;154(1):79–92.
An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Interaction of macrophages and endometrial cells induces epithelial-mesenchymal transition-like processes in adenomyosis. Biol Reprod. 2017;96(1):46–57.
Maia H Jr, Haddad C, Pinheiro N, Casoy J. The effect of oral contraceptives on aromatase and Cox-2 expression in the endometrium of patients with idiopathic menorrhagia or adenomyosis. Int J Womens Health. 2013;5:293–9.
Maia H Jr, Haddad C, Casoy J. Correlation between aromatase expression in the eutopic endometrium of symptomatic patients and the presence of endometriosis. Int J Women’s Health. 2012;4:61–5.
Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83:149–55.
Ezaki K, Motoyama H, Sasaki H. Immunohistologic localization of estrone sulfatase in uterine endometrium and adenomyosis. Obstet Gynecol. 2001;98:815–9.
Urabe M, Yamamoto T, Kitawaki J, Honjo H, Okada H. Estrogen biosynthesis in human uterine adenomyosis. Acta Endocrinol (Copenh). 1989;121(2):259–64.
Zeng YY, Guan YG, Li KY. Role of estrogen, estrogen receptors, and aromatase in the pathogenesis of uterine adenomyosis. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(3):383–7.
Zhao L, Zhou S, Zou L, Zhao X. The expression and functionality of stromal caveolin 1 in human adenomyosis. Hum Reprod. 2013;28(5):1324–38.
Donnez J, Stratopoulou CA, Dolmans MM. Uterine adenomyosis: from disease pathogenesis to a new medical approach using GnRH antagonists. Int J Environ Res Public Health. 2021;18(19):9941.
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95(7):2228-2235.el.
Li Y, Zou S, Xia X, Zhang S. Human adenomyosis endometrium stromal cells secreting more nerve growth factor: impact and effect. Reprod Sci. 2015;22(9):1073–82.
Guo SW, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99(1):231–40.
Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202(4):346.e1-8.
Duckitt K. Menorrhagia. BMJ. Clin Evid. 2015;2015:0805.
Orazov MR, Nosenko EN, Radzinsky VE, Khamoshina MB, Lebedeva MG, Sounov MA. Proangiogenic features in chronic pelvic pain caused by adenomyosis. Gynecol Endocrinol. 2016;32:7–10.
Guo S, Zhang D, Lu X, Zhang Q, Gu R, Sun B, et al. Hypoxia and its possible relationship with endometrial receptivity in adenomyosis: a preliminary study. Reprod Biol Endocrinol. 2021;19(1):7.
Quiñonez-Flores CM, González-Chávez SA, Pacheco-Tena C. Hypoxia and its implications in rheumatoid arthritis. J Biomed Sci. 2016;23(1):62.
Huang TS, Chen YJ, Chou TY, Chen CY, Li HY, Huang BS, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014;18(7):1358–71.
Karel C, Michal J, Radovan P, Pavel V, Jana Ž, Jan V, et al. Adenomyosis - its possible effect on endometrial function and receptivity. Ceska Gynekol. 2021;86(3):205–9.
Patterson AL, Pirochta J, Tufano SY, Teixeira JM. Gain-of-function beta-catenin in the uterine mesenchyme leads to impaired implantation and decidualization. J Endocrinol. 2017;233(1):119–30.
Peng Y, Jin Z, Liu H, Xu C. Impaired decidualization of human endometrial stromal cells from women with adenomyosis. Biol Reprod. 2021;104(5):1034–44.
Ashary N, Laheri S, Modi D. Homeobox genes in endometrium: from development to decidualization. Int J Dev Biol. 2020;64(1-2–3):227–37.
Wang J, Huang C, Jiang R, Du Y, Zhou J, Jiang Y, et al. Decreased endometrial il-10 impairs endometrial receptivity by downregulating HOXA10 expression in women with adenomyosis. Biomed Res Int. 2018;2018:2549789.
Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4:a011163.
Odia Y, Cavalcante L, Safran H, Powell SF, Munster PN, Ma WW, et al. Malignant glioma subset from actuate 1801: phase I/II study of 9-ING-41, GSK-3β inhibitor, monotherapy or combined with chemotherapy for refractory malignancies. Neurooncol Adv. 2022;74(1):vdac012.
Li X, Liu Y, Zhao Y, Tian W, Zhai L, Pang H, et al. Rhein derivative 4F inhibits the malignant phenotype of breast cancer by downregulating Rac1 protein. Front Pharmacol. 2020;11:754.
Zhou G, Peng F, Zhong Y, Chen Y, Tang M, Li D. Rhein suppresses matrix metalloproteinase production by regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian carcinoma cells. Int J Oncol. 2017;50(3):933–41.
Feng T, Wei S, Wang Y, Fu X, Shi L, Qu L, Fan X. Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-catenin signaling pathway. Biomed Pharmacother. 2017;94:231–7.
Massagué J. TGFbeta in Cancer. Cell. 2008;134(2):215–30.
Melzer C, Hass R, von der Ohe J, Lehnert H, Ungefroren H. The role of TGF-beta and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun Signal. 2017;15(1):19.
Patel S, Tang J, Overstreet JM, Anorga S, Lian F, Arnouk A, et al. Rac-GTPase promotes fibrotic TGF-beta1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways. FASEB J. 2019;33(9):9797–810.
Rasmi RR, Sakthivel KM, Guruvayoorappan C. NF-kappaB inhibitors in treatment and prevention of lung cancer. Biomed Pharmacother. 2020;130:110569.
Burgos RA, Alarcón P, Quiroga J, Manosalva C, Hancke J. Andrographolide, an anti-inflammatory multitarget drug: all roads lead to cellular metabolism. Molecules. 2020;26(1):5.
Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng Z. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: imply the possible treatment of adenomyosis. J Pharmacol Sci. 2018;137(1):5–11.
Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19(8):12349–67.
Yang JH, Wu MY, Chen MJ, Chen SU, Yang YS, Ho HN. Increased matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 secretion but unaffected invasiveness of endometrial stromal cells in adenomyosis. Fertil Steril. 2009;91(5 Suppl):2193–8.
Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405.
Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol. 2016;14(1):66.
Zhang X, Li M, Zuo K, Li D, Ye M, Ding L, et al. Upregulated miR-155 in papillary thyroid carcinoma promotes tumor growth by targeting APC and activating Wnt/beta-catenin signaling. J Clin Endocrinol Metab. 2013;98(8):E1305-1313.
Vasquez YM, Mazur EC, Li X, Kommagani R, Jiang L, Chen R, et al. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol. 2015;29(3):421–33.
Acknowledgements
I thank Mrs. Toyomi Kobayashi for creating the figure.
Author information
Authors and Affiliations
Contributions
HK: conception and design, acquisition of data, analysis and interpretation of data, and writing the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Patient Consent for Publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, H. Molecular Targets for Nonhormonal Treatment Based on a Multistep Process of Adenomyosis Development. Reprod. Sci. 30, 743–760 (2023). https://doi.org/10.1007/s43032-022-01036-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01036-4