Abstract
Androgen insensitivity syndrome (AIS) is a rare X-linked genetic disorder caused by mutations in the androgen receptor (AR) gene. AIS can be divided into partial type (PAIS), mild type (MAIS), and complete type (CAIS) based on the degree of androgen insensitivity. CAIS is characterized by a male genotype and a complete female phenotype. A 10-year-old child presented with a bilateral inguinal mass for 9 years. Physical examination revealed a complete feminine genital appearance and a painless mass in bilateral inguinal area. Pelvic magnetic resonance imaging (MRI) revealed long T1 and T2 elliptic signal nodules in bilateral inguinal area, absence of uterus-ovary signal and a short blind end of the vagina. Chromosomal analyzes manifested a 46, XY karyotype. By analyzing the above clinical data, the preliminary diagnosis of CAIS was confirmed. Then laparoscopic bilateral gonadectomy was performed. The histological examination of resected gonad showed it consisted of dysplastic testicular tissue and no signs of malignancy were observed. Sanger sequencing revealed the presence of a hemizygous mutation c.927 T > G (p. Tyr309*) in exon 1 of the AR gene. This is the first report of a novel nonsense mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Androgen insensitivity syndrome (AIS), formerly known as “testicular feminization syndrome,” is a X-linked genetic disorder caused by mutations in the androgen receptor (AR) gene [1]. It is one of the most common 46, XY, disorders of sex development (DSD) [2]. AIS is characterized by a wide spectrum of femaleness in male genotype (46, XY) individuals and can be divided into partial type (PAIS), mild type (MAIS), and complete type (CAIS) based on the degree of androgen insensitivity. PAIS indicates patients with ambiguous or predominantly male external genitalia; MAIS indicates patients with typical male external genitalia [3]. The features of CAIS are a complete feminine genital appearance, however, absence of uterus and ovaries, a short blind end of the vagina, and poor development of pubic hair and armpit hair [4].
The AR gene, which is located on Xq11–12, includes eight exons and encodes the AR protein. The AR protein belongs to the nuclear receptor superfamily. It consists of 920 amino acid residues, and contains four main functional domains [5]. The N-terminal transactivation domain (NTD) is encoded by exon 1 and is associated with the transcription of target genes. The DNA-binding domain (DBD), encoded by exons 2 and 3, contains the “zinc finger” constructure, which binds particularly with hormone response elements (HREs). The C-terminal ligand-binding domain (LBD) contains specific binding sites for androgens and is encoded by exons 4–8. The hinge region is located between the LBD and DBD, and contains the phosphorylation site for AR [6]. AR protein can form a complex with endogenous androgens, such as testosterone (T) and dihydrotestosterone (DHT), giving rise to different biological messages and regulating the expression of downstream signaling molecules. Ultimately, androgens can mediate the differentiation and development of the normal male phenotype [7].
AIS is a recessive hereditary disease caused by AR gene mutation on chromosome Xq11.12. Previous studies have reported more than 1000 different mutations [8] and approximately 80% have been related to AIS [2]. Compared with MAIS and PAIS, patients with CAIS are less responsive to androgens and need gonadectomy to prevent malignancy [9]. Here, we present a case of CAIS caused by a novel nonsense mutation of the AR gene.
Case Presentation
A 10-year-old child, reared as a female, was referred to our hospital because of a bilateral inguinal mass for 9 years. Physical examination revealed feminine habitus with a height of 145 cm, weight of 32 kg, BMI (body mass index) of 15.22 kg/m2 and enlargement of both breasts. Gynecological examination revealed well-developed labia, small clitoris, normal opening of the vagina and these signs were completely consistent with feminine genital appearance (Fig. 1a). A painless mass was palpable in the bilateral inguinal area. Medical history included congenital heart disease. The patient has a little twin sister whose genotype is 46, XX and whose phenotype is normal female external genitalia. There was no otherwise relevant family history. The patient is a top student at school and is good at playing the piano.
Clinical and imaging features of the patient. a Preoperative photograph shows a feminine genital appearance. b Pelvic MRI shows bilateral inguinal masses (white arrow), c a short blind end of the vagina (white arrow), d absence of uterus and ovaries. e The picture illustrates bilateral gonads above the opening of deep inguinal ring
Ultrasonography examination showed mass echo in the bilateral inguinal area (right 37 × 27 × 14 mm, left 31 × 22 × 8 mm); moreover, uterus-ovary echo was not observed. There was no obvious abnormality in other organs. Pelvic magnetic resonance imaging (MRI) revealed long T1 and T2 elliptic signal nodules of approximately 28 × 22 mm in the bilateral inguinal area (Fig. 1b), a short blind end of the vagina (Fig. 1c) and absence of a uterus-ovary signal (Fig. 1d). The result of hormone test was follicle-stimulating hormone (FSH) 13.5 IU/L, luteinizing hormone (LH) 29.1 IU/L, estradiol 7.0 pg/mL, total testosterone 11 ng/mL, and anti-Müllerian hormone (AMH) > 23 ng/mL (reference 1.66–9.49). Chromosomal karyotyping by G-banding on metaphase chromosomes from peripheral blood lymphocytes manifested a 46, XY, 16qh + karyotype in the patient. Psychological assessment revealed the feminine characteristics of the patient. By analyzing the above clinical data, the preliminary diagnosis of CAIS was confirmed.
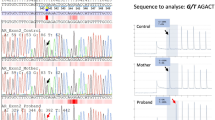
With full discussion and consent of the patient and her parents, laparoscopic bilateral gonadectomy was performed to reduce the risk of malignancy. No uterus or ovaries were found in the pelvic cavity. Bilateral gonads were seen above the opening of deep inguinal ring and removed subsequently (Fig. 1e). Due to normal-appearing female external genitals of the patient, genital reconstruction was not performed. The histological examination of the resected gonad showed dysplastic testicular tissue lacking of ovarian tissue (Fig. 2a), and immunohistochemical analysis showed inhibin-α (mesenchyme +) and Ki-67 (5% +). The tumor makers CD30 ( −), CD117 ( −), PLAP ( −), and SALL-4 ( −) demonstrated no signs of malignancy (Fig. 2b, c, d, e, f, g). Sanger sequencing revealed the presence of a hemizygous mutation c.927 T > G (p. Tyr309*) in exon 1 of the AR gene in this patient (Fig. 3).
After postoperative follow-up for 18 months, the patient recovered well and received hormone replacement therapy for a long time. She had a healthy physique and psychological state as a female, and her grades were excellent at school. Her parents were satisfied with the current status of their daughter.
Discussion and Conclusions
CAIS is a sexual differentiation disorder characterized by male genotype and complete female phenotype [10]. Due to complete androgen insensitivity, the differentiation process of Wolffian ducts into epididymis, seminal vesicles, and vas deferens fails and testis, the target organ of androgen, is undescended and dysplastic, resulting in female external genitalia and a lack of male secondary sexual characteristics [2]. Patient with CAIS do not virilize, even though the concentration of serum testosterone is within or above the basal male testosterone levels [5]. Negative feedback from the anterior pituitary gland leads to elevated basal estradiol and luteinizing hormone (LH) levels due to an impaired androgen response. Conversely, follicle-stimulating hormone (FSH) is usually at a normal concentration, as it is mainly regulated by inhibin [11]. Anti-Müllerian hormone (AMH), a glycoprotein secreted by support cells, is generally at a high level compared to normal male children. The elevated AMH level suppresses the development of the Müllerian ducts and causes their degeneration; hence, the Müllerian ducts cannot develop into the female internal genitalia (uterus, cervix, fallopian tube, and upper vaginal barrel) [6, 12]. CAIS is usually diagnosed in puberty for typical clinical features, including bilateral inguinal hernia, labial swelling and primary amenorrhea [10]. Previous studies have shown that nearly 57% of CAIS patients present with inguinal hernia, especially in the female population [13].
At present, more than 1000 mutation sites of the AR gene have been reported in the Human Gene Mutation Database [14]. These mutations include substitution, translocation, deletion, and insertion, located at various loci of the AR gene [15]. The effects of mutations on the amino acid sequences of polypeptide chains are generally classified as follows: same sense mutation, missense mutation, nonsense mutation, and terminator codon mutation [16]. Among them, missense mutations are the most frequent, and are characterized by mild clinical symptoms [17]. The majority of missense variants cause dysfunctional LBD and DBD [18].
This is the first report of a novel nonsense mutation in exon 1 of the AR gene. The mutation is a single-nucleotide substitution (T to G) at position 927 (c.927 T > G), resulting in the transition of 309 amino acid residues from the tyrosine to the terminator and dysfunction of the encoded protein (Fig. 3). The N-terminal transactivation domain (NTD) is encoded by exon 1 and glutamine, glycine and proline repeats are the unique features of the NTD [17]. The NTD is the transactivating domain and the least conserved domain compared to other domains of the AR gene, which initiates and regulates the transcription of target genes and contributes to the formation of the final three-dimensional structure of the androgen receptor [6]. While the precise biochemical function of NTD remains poorly understood, we can speculate that the novel nonsense mutation in exon 1 that we reported results in non-functional AR protein, which may be responsible for androgen insensitivity.
In conclusion, for female patients with inguinal hernia, we should raise suspicion of CAIS. After the diagnosis of CAIS, surgery, hormone replacement therapy, and psychotherapy may be necessary to enable the patients to achieve a good recovery both physically and mentally. Whole exome sequencing (WES) is helpful for analysis of the genetic mechanism of CAIS, and a novel missense mutation in exon 1 of the AR gene was identified in our case. This novel mutation may enrich the database of mutations by providing new insights into the molecular mechanism of CAIS.
Abbreviations
- AIS:
-
Androgen insensitivity syndrome
- MAIS:
-
Mild androgen insensitivity syndrome
- PAIS:
-
Partial androgen insensitivity syndrome
- CAIS:
-
Complete androgen insensitivity syndrome
- AR:
-
Androgen receptor
- MRI:
-
Magnetic resonance imaging
- DSD:
-
Disorders of sex development
- NTD:
-
N-terminal transactivation domain
- DBD:
-
DNA-binding domain
- HREs:
-
Hormone response elements
- LBD:
-
Ligand-binding domain
- BMI:
-
Body mass index
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- AMH:
-
Anti-Müllerian hormone
- WES:
-
Whole exon sequencing
References
Terro JJ, El-Helou E, Jammoul K, El Lakkis R, Shibli A, El-Chamaa B, Al-Shami J, Naccour J, Damaj N, Abtar HK. Bilateral inguinal masses or hernias in a female teenager with delayed menarche: think of complete androgen insensitivity syndrome (CAIS), a case report. Int J Surg Case Rep. 2020;76:25–9.
Lanciotti L, Cofini M, Leonardi A, Bertozzi M, Penta L, Esposito S. Different clinical presentations and management in complete androgen insensitivity syndrome (CAIS). Int J Environ Res Public Health. 2019;16(1268):1–20.
Yuan SM, Huang H, Tu CF, Du J, Xu DB, Lin G, Lu GX, Tan YQ. A rare polypyrimidine tract mutation in the androgen receptor gene results in complete androgen insensitivity syndrome. Asian J Androl. 2018;20(3):308–10.
Malcher A, Jedrzejczak P, Stokowy T, Monem S, Nowicka-Bauer K, Zimna A, Czyzyk A, Maciejewska-Jeske M, Meczekalski B, Bednarek-Rajewska K et al. Novel mutations segregating with complete androgen insensitivity syndrome and their molecular characteristics. Int J Mol Sci. 2019;20(5418):1–16.
Mongan NP, Tadokoro-Cuccaro R, Bunch T, Hughes IA. Androgen insensitivity syndrome. Best Pract Res Clin Endocrinol Metab. 2015;29(4):569–80.
Tyutyusheva N, Mancini I, Baroncelli GI, D'Elios S, Peroni D, Meriggiola MC, Bertelloni S. Complete androgen insensitivity syndrome: from bench to bed. Int J Mol Sci 2021;22(1264):1–11.
Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 2018;465:134–42.
Liu XY, Cai Z, Li F, Ji L. A novel missense mutation in the androgen receptor gene causes the complete androgen insensitivity syndrome. J Obstet Gynaecol. 2019;39(5):720–1.
Tadokoro-Cuccaro R, Hughes IA. Androgen insensitivity syndrome. Curr Opin Endocrinol Diabetes Obes. 2014;21(6):499–503.
Oakes MB, Eyvazzadeh AD, Quint E, Smith YR. Complete androgen insensitivity syndrome–a review. J Pediatr Adolesc Gynecol. 2008;21(6):305–10.
Bertelloni S, Dati E, Baroncelli GI, Hiort O. Hormonal management of complete androgen insensitivity syndrome from adolescence onward. Horm Res Paediatr. 2011;76(6):428–33.
Josso N, Rey RA. What Does AMH Tell Us in Pediatric Disorders of Sex Development? Front Endocrinol (Lausanne). 2020;11:619.
Georgiou R, Hall NJ, Stanton M. Screening for complete androgen insensitivity syndrome in girls with inguinal hernia: parental insight. Arch Dis Child. 2013;98(4):316–7.
Wang S, Xu H, An W, Zhu D, Li D. Mutational analysis of the androgen receptor gene in two Chinese families with complete androgen insensitivity syndrome. Exp Ther Med. 2016;11(6):2277–83.
Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380(9851):1419–28.
Hornig NC, Holterhus PM. Molecular basis of androgen insensitivity syndromes. Mol Cell Endocrinol. 2021;523:111146.
Tadokoro-Cuccaro R, Davies J, Mongan NP, Bunch T, Brown RS, Audi L, Watt K, McEwan IJ, Hughes IA. Promoter-dependent activity on androgen receptor N-terminal domain mutations in androgen insensitivity syndrome. Sex Dev. 2014;8(6):339–49.
Poon KS, Tan KM, Loke KY. A novel de novo androgen receptor nonsense mutation in a sex-reversed 46, XY infant. Hum Genome Var. 2021;8(1):35.
Author information
Authors and Affiliations
Contributions
XM and KW performed the operation. KW reviewed the literature. KW, XM, JC, QW, and YW collected the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Written informed consent was obtained from the minor’s legal guardian for the publication of any potentially identifiable images or data included in this article.
Consent for Publication
Written informed consent was obtained from the minor's legal guardian for the publication of any potentially identifiable images or data included in this article.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wang, K., Wang, Q., Chen, J. et al. Case Report: a Novel Nonsense Mutation in the Androgen Receptor Gene Causing the Complete Androgen Insensitivity Syndrome. Reprod. Sci. 29, 2659–2663 (2022). https://doi.org/10.1007/s43032-022-00944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00944-9