Abstract
The ability to use frozen sperm for insemination during in vitro fertilization (IVF) is crucial for patients and for reproductive endocrinologists. However, concerns exist regarding the effects of cryopreservation on sperm quality and IVF outcomes. This study compares outcomes of frozen donor oocyte IVF cycles with intracytoplasmic sperm injection (ICSI) of good quality fresh versus frozen ejaculated sperm. Patients who underwent their first frozen donor oocyte IVF cycle between 2013 and 2019 at Mayo Clinic were identified. The primary outcome was live birth rate (LBR). Secondary outcomes included fertilization rate (FR), blastocyst development rate (BR), and clinical pregnancy rate (CPR). Twenty-six patients used fresh sperm and 19 patients utilized frozen sperm; there were no significant demographic differences between the groups. There were no significant differences noted in CPR, FR, and BR. Although the LBR was not statistically different when frozen versus fresh sperm was utilized (52.6% vs. 61.5%, p = 0.55), there was a distinct trend towards improved outcomes with fresh sperm that may be clinically significant. This data suggests that frozen sperm may be an alternative to a fresh sample, however fresh sperm may ultimately be a better option. This finding should be further explored with studies utilizing a larger sample size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to use a frozen sperm sample for insemination during in vitro fertilization (IVF) cycles is crucial for many patients. In the case of severe male factor infertility, cryopreserving sperm helps avoid the need for repeat testicular extraction procedures [1], allows storage of back-up samples for patients with oligozoospermia, and is important for storage and transportation of donor semen [2]. For patients requiring treatment with gonadotoxic therapies such as chemotherapy or radiation, semen can be frozen for fertility preservation [2]. Additionally, it allows for patient and provider flexibility: procedures for both male and female partners do not need to coincide [1] and male partners are not required to be present on the day of oocyte retrieval or oocyte thaw [2].
However, there are concerns that the process of cryopreserving semen may have consequences for the quality of the sperm. Issues such as reduced post-thaw survival, lower motility, decreased morphology, and impaired fertilization capacity have all been identified in the literature [2]. Furthermore, studies have demonstrated that frozen sperm samples may have higher rates of deoxyribonucleic acid (DNA) fragmentation [3, 4] which has been linked to decreased fertilization, arrested embryo development and increased risk of spontaneous abortion [5], reduced pregnancy rates [6], and reduced live birth rate [7]. Previous studies that have examined outcomes of autologous IVF cycles with fresh versus frozen sperm have shown no significant differences in clinical outcomes [8]. However, using autologous oocytes to study sperm quality may introduce confounding factors due to the female partner’s infertility. The donor oocyte is an ideal model for assessing the contributions of sperm to IVF outcomes as donor oocytes are assumed to be of good quality from young women without infertility [9].
The objective of this study was to compare clinical and laboratory outcomes of frozen donor oocyte IVF cycles in which good quality fresh versus frozen sperm was used for insemination via intracytoplasmic sperm injection (ICSI).
Materials and Methods
This study was deemed exempt from review by the Mayo Clinic Institutional Review Board (Application #19–012,375). In this retrospective cohort analysis, data from all patients that underwent frozen donor oocyte IVF cycles from January 2, 2013, to September 30, 2019, at a single, academic clinic were obtained. Patients were identified from a clinical database maintained within the Mayo Clinic Division of Reproductive Endocrinology and Infertility (REI) that was created based on the Society for Assisted Reproductive Technology (SART) reporting criteria. Patients that used fresh donor oocytes were excluded along with patients who had denied the use of their medical records for research. Patients with semen analyses consisting of a total motile sperm (TMS) count of less than 20 million were excluded to reduce confounding from poor sperm quality. Similarly, patients that used a sperm sample derived directly from the testicles were also excluded. Only the initial transfer of the first cycle that occurred during the study period was used for analysis. No embryos were transferred to a gestational carrier in this study. All patients were required to have a thyroid-stimulating hormone (TSH) level, intrauterine cavity assessment (via sonohysterogram or hysteroscopy), and embryo transfer (mock or live) completed within 12 months prior to a live embryo transfer. ICSI was used for insemination of all oocytes. By protocol, the best quality embryo was transferred first in both fresh and frozen embryo transfers and any supernumerary embryos were cryopreserved for future use. Embryo transfer was completed with transabdominal ultrasound guidance according to standard practice [10, 11].
Characteristics of the female patients that were not included in the internal database were captured via retrospective chart review: patient age at the time of transfer, name of commercial oocyte bank, and primary infertility diagnosis. Infertility diagnoses were split into three categories: unsuccessful autologous fertility, premature ovarian insufficiency or “other.” “Other” diagnoses included tubal factor infertility and one patient who desired to use donor oocytes for genetic reasons. For the male partner, chart review yielded the following characteristics: age at the time of semen sample collection used for insemination, total motile sperm count, and sperm morphology. Sperm parameters were determined by the semen analysis that occurred closest to the start of the donor oocyte cycle.
Our primary outcome was live birth rate (LBR). Our secondary outcomes were clinical pregnancy rate (CPR), fertilization rate (FR), and blastocyst development rate (BR). CPR was defined as the presence of fetal cardiac activity on a first trimester ultrasound. FR was calculated as the number of oocytes that fertilized divided by the number of oocytes that survived the thaw and were inseminated by ICSI, multiplied by 100. BR was calculated as the total number of blastocysts that formed divided by the number of embryos that fertilized, multiplied by 100.
Data were analyzed using the SAS version 9.4 software package (SAS Institute, Inc., Cary, NC). Comparisons between groups were evaluated with the chi-square test or Fisher’s exact test for categorical variables; the two-sample t-test for age, morphology, fertilization rate, and blastulation rate; and the Wilcoxon rank sum for TMS and all count variables. All calculated p-values were two-sided and p-values less than 0.05 were considered statistically significant.
Results
Fifty-two transfers were identified during the study period. Seven cases were excluded due to a TMS count of less than 20 million, resulting in 45 patient transfers available for analysis. Nineteen patients (42%) used a frozen ejaculated sperm source whereas 26 patients (58%) used freshly ejaculated sperm. Table 1 describes the baseline characteristics of the fresh and frozen sperm groups. The average age of female patients in the frozen group was 41.5 years versus 39.7 years for the fresh group (p = 0.34). The average age of the male partner was 39.1 years for the frozen cohort and 39.8 years for the fresh group (p = 0.79). There were no statistically significant differences between the groups in terms of oocyte bank used, infertility diagnosis, or sperm parameters. Most patients transferred a single fresh embryo, but 12 patients underwent a double embryo transfer (Table 2). Most (73.3%) embryos were transferred at the blastocyst stage, although 22.2% of patients had cleavage stage embryos transfers and 4.4% of patients had both a blastocyst and a cleavage stage embryo transferred. There were no statistically significant differences in the number of embryos transferred (p = 0.73) or the stage of transfer (p = 0.35) between the fresh and frozen sperm groups.
Within the entire cohort, 7 frozen oocytes were obtained on average from one of two commercially available egg banks and an average of 6.8 oocytes survived the thaw (Table 2). There were no differences noted between the two groups in terms of number of oocytes obtained (p = 0.30) or number that survived the thaw (p = 0.16). The mean number of oocytes that fertilized in the frozen sperm group was 4.8 out of a mean of 6.3 compared to 5.8 out of mean 7.1 in the fresh group, and this difference was not statistically significant (p = 0.28). At the end of the cycle, both the frozen and fresh cohorts had an average of 2.8 blastocysts develop (p = 0.99) and an average of 1.9 versus 2.0 supernumerary blastocyst embryos available for cryopreservation, respectively. Of three patients that used PGT-A, two used fresh sperm with euploidy rates of 66% and 33% and one used frozen sperm with a 20% euploidy rate.
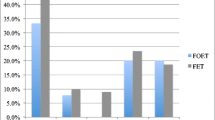
There were no statistically significant differences found in LBR between patients who used frozen sperm versus those who used fresh sperm (52.6% versus 61.5%, p = 0.55; Table 3). The 95% confidence interval for this difference (frozen versus fresh) of − 8.9% is − 38.1 to 20.3%. There also were no differences in significant secondary outcomes (Table 3).
Discussion
This retrospective analysis of the transfers of embryos created with frozen ejaculated sperm versus fresh ejaculated sperm examined differences in laboratory or clinical outcomes in frozen donor oocyte IVF with ICSI cycles. Although there were no significant differences seen in fertilization rate, blastocyst development rate, clinical pregnancy rate, or live birth rate between the two cohorts, the difference in live birth rates was − 9% (− 38.1 to 20.3%) favoring fresh sperm. If this difference were to be confirmed with a larger sample size, this would be clinically significant. In both groups the CPR was above 55% and the LBR was above 50%, which is reassuring that a frozen sample may be an alternative to a fresh sperm sample. This data is clinically important due to increasing use of frozen donor oocytes and the widespread use of frozen semen samples for indications such as clinical flexibility, as back-up in cases of male factor infertility, and for fertility preservation.
The consideration of whether to use frozen or fresh sperm for insemination is of particular interest for clinicians and patients during frozen donor oocyte IVF with ICSI cycles. Although an oocyte thaw must be timed with the intended carrier’s programmed or natural cycle, the parent(s) do not physically need to be present on the day of the oocyte thaw. This is in contrast with fresh autologous cycles where the day of sperm collection needs to coincide with the day of the oocyte retrieval—for insemination purposes and post-procedural care of the partner after anesthesia. Therefore, in frozen donor cycles, a previously frozen sperm sample can be used for the insemination process and avoid additional office appointments. Furthermore, a cryopreserved sample can be organized at the time of the initial semen analysis when frozen donor oocytes are to be used, thereby reducing the number of semen collections required. Given the non-inferior laboratory and clinical outcomes with use of frozen sperm, it is reasonable to offer patients cryopreservation for improved convenience for both fertility practices and their patients.
Similar outcomes with the use of fresh versus frozen sperm have been found in other studies that have analyzed outcomes of autologous IVF cycles. In 1989, Englert et al. used frozen and fresh donor sperm samples for insemination via ICSI in autologous cycles complicated by severe male factor infertility. Although the frozen sample cohort had a significantly lower quality of sperm, lower embryo vitality score, and number of supernumerary embryos for cryopreservation, the cumulative ongoing pregnancy rates were equivalent and, thus the conclusion was that frozen sperm could be used without CPR impairment [12]. A 2007 study examined outcomes of ejaculated partner sperm and found no difference in fertilization, implantation, or pregnancy rates unless the semen samples were abnormal prior to freezing [8]. In cases of oligoasthenozoospermia or asthenozoospermia, fertilization rate was lower in the frozen sample, although ultimately there were no differences in implantation rate or pregnancy outcomes.
The effects of using frozen sperm on IVF outcomes can also be extrapolated from studies in which cryopreserved semen frozen for fertility preservation prior to oncologic treatments was used for insemination. In Meseguer et al., only 30% of patients over a 14-year period in Spain had normal sperm production following oncologic treatment and 70% were found to have parameters most likely necessitating the use of assisted reproductive technology (ART). When the cryopreserved samples of these patients were used for insemination, their clinical outcomes were comparable with a control group of couples with tubal factor infertility, and significantly better than patients who used testicular-extracted sperm due to post-chemotherapy azoospermia [13]. Hourvitz et al. also found non-inferior outcomes in IVF cycles that utilized frozen samples from men with a previous malignancy. The clinical pregnancy rate was 56.8%, similar to the CPR of other patients with male factor infertility in the same clinic and similar to the frozen group’s CPR of 57.9% in this study [14].
This is the first published study, to our knowledge, to examine differences in outcomes between good quality fresh versus frozen ejaculated sperm samples in frozen donor oocyte IVF cycles. All cycles occurred at a single, academic center, thereby minimizing inter-clinic variability. Additionally, only good quality semen specimens as determined by prior semen analysis were included, thereby reducing confounding effects of male factor infertility. However, this study has certain limitations. Most importantly, this study is limited by its small sample size. Despite a nearly 7-year period, this study is underpowered to declare the observed difference of 61.5% versus 52.6% as statistically significant. ICSI was used for all frozen oocyte cycles which may have helped compensate for a decreased fertilization ability of either cohort. Additionally, the quality of the sperm samples was determined by the most recent semen analysis and not based on parameters on the day of insemination. Although it is unlikely that a patient with a previous normal analysis could have had a poor sample on the day of insemination, this is a possibility that could have led to data inaccuracies.
Conclusion
Frozen sperm are commonly used in IVF cycles due to various patient and clinic practices. However, concerns exist regarding whether a frozen sample could have detrimental effects on the IVF cycle outcomes. In this study, the effect of fresh versus frozen ejaculated sperm samples was assessed in frozen donor oocyte egg cycles to control for oocyte quality, and no significant differences were found between the two groups in regard to live birth rate, clinical pregnancy rate, blastocyst development rate, or fertilization rate. This data indicates that frozen sperm may be an adequate substitute for fresh sperm in IVF cycles. However, the 9% difference in LBR between the cohorts could be considered clinically significant, and these findings warrant further investigation within a larger sample population.
References
Kalsi J, Thum MY, Muneer A, Pryor J, Abdullah H, Minhas S. Analysis of the outcome of intracytoplasmic sperm injection using fresh or frozen sperm. BJU Int. 2011;107(7):1124–8. https://doi.org/10.1111/j.1464-410X.2010.09545.x.
Eastick J, Venetis C, Cooke S, Storr A, Susetio D, Chapman M. Is early embryo development as observed by time-lapse microscopy dependent on whether fresh or frozen sperm was used for ICSI? A cohort study. J Assist Reprod Genet. 2017;34(6):733–40. https://doi.org/10.1007/s10815-017-0928-0.
Le MT, Nguyen TTT, Nguyen TT, Nguyen TV, Nguyen TAT, Nguyen QHV, et al. Does conventional freezing affect sperm DNA fragmentation? Clin Exp Reprod Med. 2019;46(2):67–75. https://doi.org/10.5653/cerm.2019.46.2.67.
Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76(5):892–900. https://doi.org/10.1016/s0015-0282(01)02834-5.
Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Francois GJ. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87(1):93–100. https://doi.org/10.1016/j.fertnstert.2006.05.057.
Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online. 2003;7(4):477–84. https://doi.org/10.1016/s1472-6483(10)61893-7.
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015;30(2):120–7. https://doi.org/10.1016/j.rbmo.2014.10.018.
Borges E Jr, Rossi LM. Locambo de Freitas CV, Guilherme P, Bonetti TC, Iaconelli A et al Fertilization and pregnancy outcome after intracytoplasmic injection with fresh or cryopreserved ejaculated spermatozoa. Fertil Steril. 2007;87(2):316–20. https://doi.org/10.1016/j.fertnstert.2006.06.032.
Capelouto SM, Nagy ZP, Shapiro DB, Archer SR, Ellis DP, Smith AK, et al. Impact of male partner characteristics and semen parameters on in vitro fertilization and obstetric outcomes in a frozen oocyte donor model. Fertil Steril. 2018;110(5):859–69. https://doi.org/10.1016/j.fertnstert.2018.06.003.
Practice Committee of the American Society for Reproductive Medicine. Electronic address Aao, Practice Committee of the American Society for Reproductive M. Performing the embryo transfer: a guideline. Fertil Steril. 2017;107(4):882–96. https://doi.org/10.1016/j.fertnstert.2017.01.025.
Cozzolino M, Vitagliano A, Di Giovanni MV, Lagana AS, Vitale SG. Blaganje M et al Ultrasound-guided embryo transfer: summary of the evidence and new perspectives A systematic review and meta-analysis. Reprod Biomed Online. 2018;36(5):524–42.
Englert Y, Delvigne A, Vekemans M, Lejeune B, Henlisz A, de Maertelaer G, et al. Is fresh or frozen semen to be used in in vitro fertilization with donor sperm? Fertil Steril. 1989;51(4):661–4. https://doi.org/10.1016/s0015-0282(16)60617-9.
Meseguer M, Molina N, Garcia-Velasco JA, Remohi J, Pellicer A, Garrido N. Sperm cryopreservation in oncological patients: a 14-year follow-up study. Fertil Steril. 2006;85(3):640–5. https://doi.org/10.1016/j.fertnstert.2005.08.022.
Hourvitz A, Goldschlag DE, Davis OK, Gosden LV, Palermo GD, Rosenwaks Z. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril. 2008;90(3):557–63. https://doi.org/10.1016/j.fertnstert.2007.03.002.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Colleen Miller and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Mayo Clinic who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRB of Mayo Clinic.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This data was presented, in part, at the American Society of Reproductive Medicine Virtual Scientific Congress on October 18, 2020.
Rights and permissions
About this article
Cite this article
Miller, C.M., Duong, S., Weaver, A.L. et al. Outcomes of Frozen Oocyte Donor In Vitro Fertilization (IVF) Cycles Using Fresh Versus Frozen Sperm. Reprod. Sci. 29, 1226–1231 (2022). https://doi.org/10.1007/s43032-021-00796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00796-9